Summary
A low count of CD4+ and CD8+ lymphocytes is a hallmark laboratory finding in the coronavirus disease 2019 (COVID‐19). Using flow cytometry, we observed significantly higher CD95 (Fas) and PD‐1 expression on both CD4+ T and CD8+ T cells in 42 COVID‐19 patients when compared to controls. Higher CD95 expression in CD4+ cells correlated with lower CD4+ counts. A higher expression of CD95 in CD4+ and CD8+ lymphocytes correlated with a lower percentage of naive events. Our results might suggest a shift to antigen‐activated T cells, expressing molecules increasing their propensity to apoptosis and exhaustion during COVID‐19 infection.
Keywords: COVID‐19, CD95 (Fas), PD‐1, apoptosis, exhaustion
Severe acute respiratory syndrome CoV‐2 (SARS‐CoV‐2) has been identified as a novel representative of genus Betacoronavirus causing an outbreak of unusual viral pneumonia in Wuhan, China, and that progressively became pandemic. 1 The clinical spectrum of coronavirus disease 2019 (COVID‐19) appears to be wide, from an asymptomatic infection or a mild upper‐respiratory‐tract illness in a large group of patients to a severe interstitial pneumonia with Acute Respiratory Distress Syndrome (ARDS) and even death. In symptomatic patients the disease is characterized by a marked increase of cytokines, such as IL‐6, and high levels of inflammatory parameters including C‐reactive protein. Patients with the most severe clinical presentations are elderly and have co‐morbidities. 2
Lymphopenia has been described as a hallmark finding in the 2003 outbreak of coronavirus‐associated SARS and is also the most frequent haematological abnormality in COVID‐19 patients. 3 Baseline lymphocyte count was found to be lower in patients with COVID‐19 critical illness and non‐survivors. Of note, patients at higher risk of ARDS showed a lower count of CD4+ and CD8+ lymphocytes. 4 Moreover, the expression of exhaustion markers, such as PD‐1 and TIGIT, in CD8+ T cells was higher in patients with a severe clinical course. 5
Fas (CD95), a cell surface receptor of the tumour necrosis factor superfamily, has long been viewed as a death receptor that mediates apoptosis to maintain immune homeostasis. 6 CD95 is widely expressed in memory and effector T cells upon contact with antigen, while naive T cells are typically negative. 7 , 8
Programmed cell death 1 (PD‐1, CD279), an antigen of effector T cells, is considered an exhaustion marker, also expressed during antigen‐mediated T‐cell activation. 9 Upregulation of PD‐1 is observed during acute infections and after infection with persistent virus, including HIV, HBV and HCV. In particular, PD‐1 expression in HIV‐specific CD4+ and CD8+ lymphocytes is associated with T‐cell exhaustion and disease progression. 10
In our study we assessed the expression of the surface markers CD95 and PD‐1 in peripheral blood T lymphocytes in COVID‐19 patients, also correlating this phenotype with the T‐cell maturational pattern.
Materials and methods
Multiparametric flow cytometry was performed on EDTA peripheral blood samples collected from 42 new consecutive cases of COVID‐19 at admission to Fondazione Agostino Gemelli Academic Hospital. All cases were diagnosed by SARS‐Cov‐2 nucleic acid testing of throat swab specimens using the reverse transcription polymerase chain reaction (RT‐PCR). Informed consent was obtained from all patients. The study protocol was approved by the Ethical Committee of the Fondazione Policlinico Agostino Gemelli IRCCS (protocol number 0017456/20).
The percentages and absolute counts of CD3+, CD4+, and CD8+ lymphocytes were obtained with a single‐platform method using the standard antibody cocktail TETRA‐1 with an AQUIOS cytometer (Beckman Coulter, Milano, Italy). We next performed extracellular staining with the following monoclonal antibodies: CD45RAFITC, CCR7‐PE, CD3‐PerCP‐Cy5.5, CD95‐APC, CD4‐APCH7, PD‐1‐BV450, CD45‐BV500 (Becton Dickinson Biosciences San Jose, CA) and CD8‐PECy7 (Beckman Coulter, Milano, Italy). Data were acquired with a FACSCanto II cytometer and analyzed with FACSDiva Software (Becton Diskinson Bioscieces San Jose, CA). A minimum of 30000 CD3+ events per tube were recorded. The CD4+ and CD8+ T cells were selected among the CD3+ population. The percentages of CD95 and PD‐1 expression were analyzed in CD4+ and CD8+ cells. To set a gate on positive CD95 or PD‐1 events we used a negative control tube lacking antibodies against CD95 and PD‐1. CD4+ and CD8+ T‐cell maturational subsets were defined as: Naive (CD45RA+CCR7+), Central Memory (CM; CD45RA−CCR7+), Effector Memory (EM; CD45RA−CCR7−), Terminal Effector Memory (TEMRA; CD45RA+CCR7−).
Correlation between parametric continuous variables was analyzed using the Pearson correlation coefficient. The Wilcoxon–Mann–Whitney test was used for two‐sample comparisons (controls versus patients). Data were summarized as medians and ranges. P values <0·05 were considered statistically significant. Statistics were carried out with the NCSS10 Software (version 10.0.19, USA).
Results
We studied 42 patients with a wide age distribution range (median age 73, range 29–93); 46% were males. The most frequent symptoms were fever, dyspnea and cough. Median time from symptom onset to sample collection was four days (range 1–10). No patients had been treated before blood sampling nor had recently received corticosteroids. None had concomitant viral infections (HIV, HBV, HCV). All patients were hospitalized and five were subsequently transferred to the intensive care unit (ICU) (median age 71, range 62–87); one ICU and five non‐ICU patients died (median age 83, range 71–93). As lymphocyte phenotypes may be influenced by immunosenescence we divided the patients into two groups: <65 years (median age 56, range 29–65, n = 15) and ≥65 years (median age 82, range 67–93, n = 27). We compared the younger patients both to an age‐matched group of 19 healthy controls (median age 56, range 28–65) and to the older patients. We also compared the older patients to an age‐matched group of 20 inpatients (median age 79, range 67–92) with no clinical history of cancer, infections or rheumatological diseases.
According to previous reports absolute counts of CD4+ and CD8+ were significantly lower in young patients than in healthy controls and comparable between young and old patients. The CD4/CD8 ratio was not inverted in patients (Table I). 3 , 4 In CD4+ T cells CD95 expression was significantly higher in patients than in controls and comparable between young and old patients (Fig 1, Table I). Moreover, higher CD95 expression correlated with lower CD4+ absolute count (R 0·14, P = 0·02). In CD8+ T cells we observed a homogeneous and higher CD95 expression in patients compared to a heterogeneous expression in controls (Fig 1, Table I). In patients this expression increased with age (R 0·28, P = 0·0003).
Table I.
Phenotypic characteristics of peripheral blood T lymphocytes in COVID‐19 patients.
| Phenotypic findings | Controls ≤65 years (n = 19) | Patients ≤65 years (n = 15) | Patients >65 years (n = 27) | Inpatients >65 years (n = 20) | P * | P † | P ‡ |
|---|---|---|---|---|---|---|---|
| Lymphocytes, × 106/l | 1 950 (1 200–2 950) | 1 180 (350–22 050) | 1 100 (200–2 800) | 1 490 (670–2 818) | <0·000 1 | 0·9 | 0·02 |
| CD3+, × 106/l | 1 400 (827–2 474) | 814 (201–1 456) | 660 (84–2 200) | 1 265 (515–2 235) | <0·000 1 | 0·7 | 0·001 |
| CD4+, × 106/l | 920 (536–1 929) | 480 (122–759) | 450 (66–1 300) | 650 (123–1 273) | <0·000 1 | 0·9 | 0·02 |
| CD8+, × 106/l | 490 (194–754) | 240 (68–513) | 200 (25–924) | 323 (193–1 092) | 0·000 12 | 0·9 | 0·01 |
| Ratio CD4/CD8 | 2 (1·6–2·2) | 1·6 (1·4–21) | 1·7 (0·7–14) | 1·9 (0·9–4·9) | 0·3 | 0·8 | 0·43 |
| CD4+ CD95+ % | 60 (41–72) | 69 (46–92) | 68 (44–92) | 55 (33–78) | 0·005 | 0·6 | 0·006 |
| CD8+ CD95+ % | 57 (34–80) | 77 (51–94) | 90 (63–98) | 75 (34–93) | 0·002 7 | 0·008 | 0·000 08 |
| CD4+ PD‐1+ % | 18 (14–28) | 28 (17–49) | 30 (11–49) | 17 (8–32) | 0·008 | 0·8 | 0·02 |
| CD8+ PD‐1+ % | 24 (10–32) | 33 (16–50) | 26 (12–43) | 18 (7–30) | 0·02 | 0·07 | 0·067 |
P values of Mann–Whitney tests <0·05 are considered significant.
P values comparing patients ≤65 years versus age‐matched healthy controls.
P values comparing patients ≤65 years versus patients >65 years.
P values comparing patients >65 years versus age‐matched inpatients >65 years.
Fig 1.
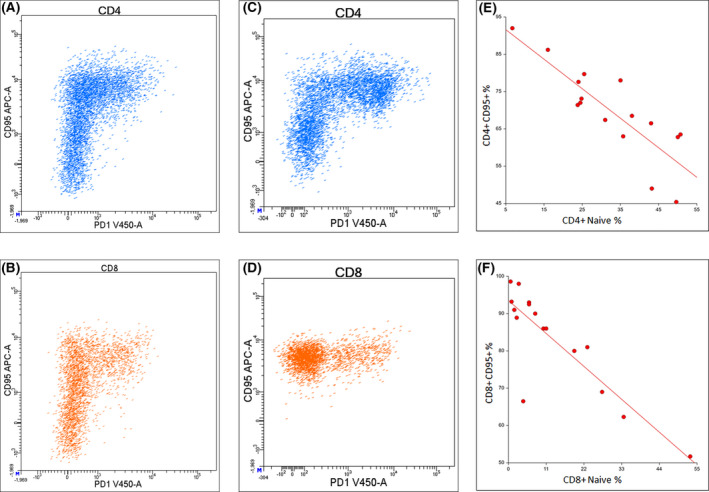
Expression of CD95 and PD‐1 in CD4+ and CD8+ circulating T lymphocytes in one healthy control (A and C) and one COVID‐19 patient (B and D). Correlation between the percentage of CD95 expression and the percentage of naive CD4+ (E) and CD8+ (F) circulating T lymphocytes.
PD‐1 expression in CD4+ and CD8+ T lymphocytes was increased significantly more in young patients than in controls, with a comparable expression between young and old patients (Fig 1, Table I). Specifically, PD‐1 was expressed in CD95+ lymphocytes and a positive correlation between CD95 and PD‐1 percentages was observed among CD4+ cells (R 0·28, P = 0·02).
Likewise, significantly lower counts of CD3+, CD4+, CD8+ lymphocytes and higher CD95 and PD‐1 expression were observed in old patients compared to inpatients, with a trend for PD‐1 expression in CD8+ cells (P = 0·065) (Table I).
In a subgroup of 16 patients (median age 61, range 29–91) we analyzed the T‐cell maturation pattern. In CD4+ lymphocytes a higher CD95 expression correlated both with a lower percentage of Naive (Fig 1) and with a higher percentage of EM cells (R 0·70, P < 0·000 01 and R 0·82, P < 0·000 01, respectively). In CD8+ T cells a higher CD95 expression correlated with a lower percentage of Naïve (R 0·81, P = 0·000 14) (Fig 1) and with a higher percentage of TEMRA cells (R 0·65, P = 0·006 31). No correlation was observed between T‐cell maturational subsets and PD‐1 expression.
Finally, in ten patients developing severe illness (ICU and/or death) compared to others we observed a significant lower CD4+ absolute count (median value 202 × 106/l, range 66–731, versus 507 × 106/l, range 104–1 316, P = 0·01) and a higher CD95 expression (median value 80%, range 54–92, versus 68%, range 44–92, P = 0·02).
Discussion
In 42 Caucasian COVID‐19 patients, we confirmed a significant lymphopenia and reduction of CD4+ and CD8+ T cells at the time of hospital admission both in young and old patients. CD4+ and CD8+ lymphopenia has been described as an unfavourable prognostic factor. 2 , 4 Therefore, we can suppose that T lymphopenia in COVID‐19 infection could be a negative prognostic factor independently of age. Furthermore, we report an increased CD95 and PD‐1 expression in circulating CD4+ and CD8+ lymphocytes in patients, regardless of age. Both antigens are known to be upregulated upon T‐cell activation, and can signal a propensity for apoptosis (CD95) or T‐cell exhaustion (PD‐1). We observed an increased CD95 expression in CD8+ T cells with older age according to previously described higher susceptibility to CD95‐induced apoptosis in elderly individuals. 11 The upregulation of CD95 in CD4+ T cells combined with CD4+ lymphopenia has been reported during other viral infections such as HIV, respiratory syncytial virus and measles. 12 , 13 , 14 In our patients we observed a direct correlation between CD95 expression and a lower CD4+ absolute count, suggesting a comparable mechanism.
Our data on increased PD‐1 expression are in line with data by Zheng et al. showing an exhausted T‐cell phenotype in patients with severe COVID‐19 infection. 5
The increased CD95 and PD‐1 expression in T lymphocytes in COVID‐19 might suggest a shift from Naive T cells to Memory T cells. This hypothesis can be supported by the observation of an inverse correlation between percentage of CD95 expression and percentage of Naive population both in CD4+ and CD8+ T cells. Moreover, CD95 expression directly correlated with a Memory phenotype, in particular Effector Memory in CD4+ and Terminal Effector Memory in CD8+ lymphocytes.
As previously described, T‐cell adaptive immune responses are necessary to temper the early overactivation of the innate immune response. 15 Our results suggest that reduced numbers and potentially impaired functional status of the adaptive immune response might contribute to the overactivation of the innate immune response and to the severity of COVID‐19 infection.
More clinical data as well as a larger number of patients are needed to assess the clinical impact of our findings and better understand the functional and prognostic role of PD‐1 and CD95 in COVID‐19. Determination of cytokines involved in the regulation of immune response, such as IL‐6, TNF‐alfa, IL‐10, IL‐2, might also be helpful. Longitudinal assessment of phenotypic characteristics throughout treatment might allow monitoring for disease evolution and treatment response.
In conclusion, apoptosis via CD95 could be a possible mechanism for COVID‐19‐induced lymphopenia and our data provide new insights into the functional competence of T lymphocytes in COVID‐19 infection.
Funding informations
This research received no external funding.
Authors contributions
SB, EMe, PC, SH, VDS and EMa designed the research study, performed research, analyzed data and wrote paper. FM, SD, MLS, MF performed flow cytometric analysis. MF, RM, AC, SS, AG, MS, and FR contributed patients and were involved in critical revision of the report. All authors approved the final manuscript.
Conflict of interest
The authors have no competing interests.
References
- 1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China 2019. N Engl J Med. 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu C, Chen X, Cai Y, Xia J, Zhoun X, Xu S, et al. Risk factors associated with ARDS and death in patients with coronavirus disease pneumonia in Wuhan, China. JAMA. 2020;180(7):934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fan BE, Chong VCL, Chan SSW, Lim GH, Lim KGE, Tan GB, et al. Hematologic parameters in patients with COVID‐19 infection. Am J Hematol. 2020;95:131–4. [DOI] [PubMed] [Google Scholar]
- 4. Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID‐19) infected patients. Br J Haematol. 2020;189(3):428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zheng H‐Y, Zhang Mi, Yang C‐X, Zhang N, Wang X‐C, Yang X‐P, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID‐19 patients. Cell Mol Immunol. 2020;17(5):541–3. 10.1038/s41423-020-0401-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guégan JP, Legembre P. Nonapoptotic function of FAS/CD95 in the immune response. FEBS J. 2018;285:809–27. [DOI] [PubMed] [Google Scholar]
- 7. Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who’s who of T‐cell differentiation: human memory T‐cell subsets. Eur J Immunol. 2013;43:2797–809. [DOI] [PubMed] [Google Scholar]
- 8. Van den Broek T, Borghans JAM, Van Wijk F. The full spectrum of human naïve T cells. Nat Rev Immunol. 2018;18:363–73. [DOI] [PubMed] [Google Scholar]
- 9. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153–67. [DOI] [PubMed] [Google Scholar]
- 10. Schonrich G, Raftery MJ. The PD‐1/PD‐L1 axis and virus infections: a delicate balance. Front Cell Infect Microbiol. 2019;13(9):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Phelouzat MA, Laforge T, Arbogat A, Quadri RA, Boutet S, Proust JJ. Susceptibility to apoptosis of T lymphocytes from elderly humans is associated with increased in vivo expression of functional Fas receptors. Mech Ageing Dev. 1997;96:35–46. [DOI] [PubMed] [Google Scholar]
- 12. Slyker JA, Rowland‐Jones SL, Dong T, Reilly M, Richardson B, Emery VC, et al. Acute Cytomegalovirus Infection in associated with increased frequencies of activated and apoptosis‐vulnerable T cells in HIV‐1 infected infants. J Virol. 2012;86:11373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roe MFE, Bloxham DM, White DK, Ross‐Russell RTC, Tasker RTC, O’Donnel DR. Lymphocyte apoptosis in acute respiratory syncytial virus bronchiolitis. Clin Exp Immunol. 2004;137:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okada H, Kobune F, Sato TA, Kohama T, Takeuchi Y, Abe T, et al. Extensive lymphopenia due to apoptosis of uninfected lymphocytes in acute measles patients. Adv. Virol. 2000;145:905–20. [DOI] [PubMed] [Google Scholar]
- 15. Kim KD, Zhao J, Auh S, Yang X, Du P, Tang H, et al. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13:1248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]


