Abstract
The outbreak of 2019 novel coronavirus disease (COVID‐19) has posed a grave threat to the global public health. The COVID‐19‐induced infection is closely related to coagulation dysfunction in the affected patients. This paper attempts to conduct a meta‐analysis and systematically review the blood coagulation indicators in patients with severe COVID‐19. A meta‐analysis of eligible studies was performed to compare the blood coagulation indicators in patients with severe and nonsevere COVID‐19. PubMed, Embase, Web of Science, and the Cochrane Library were searched for studies published between 1 December 2019 and 7 May 2020. A total of 13 studies with 1341 adult patients were enrolled in this analysis. Platelet (weighted mean difference [WMD] = −24.83, 95% confidence interval [CI]: −34.12 to −15.54; P < .001), d‐dimer (WMD = 0.19, 95% CI: 0.09‐0.29; P < .001), and fibrinogen (WMD = 1.02, 95% CI: 0.50‐1.54; P < .001) were significantly associated with the severity in patients with COVID‐19. The meta‐analysis revealed that no correlation was evident between an increased severity risk of COVID‐19 and activated partial thromboplastin time (WMD = −1.56, 95% CI: −5.77 to 2.64; P = .468) or prothrombin time (WMD = 0.19, 95% CI: −0.13 to 0.51; P = .243). The single arm meta‐analysis showed that compared with the nonsevere group, the severe group had a lower pooled platelet (165.12 [95% CI: 157.38‐172.85] vs 190.09 [95% CI: 179.45‐200.74]), higher d‐dimer (0.49 [95% CI: 0.33‐0.64] vs 0.27 [95% CI: 0.20‐0.34]), and higher fibrinogen (4.34 [95% CI: 1.98‐6.70] vs 3.19 [95% CI: 1.13‐5.24]). Coagulation dysfunction is closely related to the severity of patients with COVID‐19, in which low platelet, high d‐dimer, and fibrinogen upon admission may serve as risk indicators for increased aggression of the disease. These findings are of great clinical value for timely and effective treatment of the COVID‐19 cases.
Keywords: 2019 novel coronavirus disease, coagulation, meta‐analysis, severity
Highlights
Coagulation dysfunction affects the prognosis of COVID‐19 patients.
Lower platelet, higher d‐dimer and fibrinogen indicate increased severity risk in COVID‐19 patients.
No difference in PT and APTT is evident between severe and non‐severe COVID‐19 patients upon admission.
Abbreviations
- APTT
activated partial thromboplastin time
- ARDS
acute respiratory distress syndrome
- ATS
American Thoracic Society
- COVID‐19
2019 novel coronavirus disease
- DIC
disseminated intravascular coagulation
- ICU
intensive care unit
- NOS
Newcastle‐Ottawa Scale
- PE
pulmonary thromboembolism
- PT
prothrombin time
- RR
respiration rate
- SARS
severe acute respiratory syndrome
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SD
standard deviation
- VTE
venous thromboembolism
- WMD
weighted mean difference
- 95% CI
95% confidence interval
1. INTRODUCTION
The global outbreak of 2019 novel coronavirus disease (COVID‐19) has posed enormous impacts on the public health, with severe and critically ill patients accounting for 20% of all COVID‐19 patients 1 and the fatality rate of critically ill patients amounting to 49%. 2 Severe patients are more likely to develop acute respiratory distress syndrome (ARDS) and multiple organ dysfunction syndrome, which may affect the prognosis of patients with COVID‐19. 3 Therefore, an early screening of severe and nonsevere patients is critical to reduce the mortality rate of patients with COVID‐19.
Of note, abnormal coagulation function has been prevalent in 20% of the patients with COVID‐19. 4 Moreover, the prevalence of disseminated intravascular coagulation (DIC) in COVID‐19 cases is higher than that of severe acute respiratory syndrome (SARS) patients. 5 A recent study reports that the mortality of COVID‐19‐induced DIC is 71.4%. 6 Hence, coagulation dysfunction is closely related to the severity of COVID‐19 cases and can endanger patients' lives. 7 However, a close examination indicates that uncertainties and controversies still persist and await further research efforts to shed new light on them.
This meta‐analysis first followed a strict definition of “severity” and focused on the coagulation blood indicators, including platelet, d‐dimer, prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen. It attempted to explore the difference in coagulation dysfunction between severe and nonsevere adult COVID‐19 patients, so as to screen the severe patients and to timely adjust the therapeutic regimen to improve the prognosis.
2. METHODS
2.1. Data sources and search strategy
This meta‐analysis followed the PRISMA recommendations and was registered with PROSPERO—The International Prospective Register of Systematic Reviews (registration No. CRD42020186941). PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science were systematically searched for papers published between 1 December 2019 and 7 May 2020. The language restriction was English and the search terms were “COVID‐19” or “2019‐nCoV” or “SARS‐CoV‐2” or “Novel Coronavirus‐Infected Pneumonia” or “2019 novel coronavirus” or “coronavirus 2019” and “severe” or “severity.” The search strategy has been provided in the Supporting Information Material (File S1). The classification criteria for severity observed the “The American Thoracic Society (ATS) guidelines for community‐acquired pneumonia,” 8 “WHO COVID‐19 Clinical Guidelines,” 9 or “COVID‐19 Diagnosis and Treatment Protocol” of China. 10 ATS guidelines for community‐acquired pneumonia, 8 in which the severe type is defined according to either one major criterion (including septic shock in need of vasopressors and respiratory failure requiring mechanical ventilation) or three or more minor criteria (including respiration rate [RR] > 30/min, PaO2/FiO2 < 250 mm Hg, multilobar infiltrates, confusion/disorientation, uremia, leukopenia, thrombocytopenia, hypothermia, and hypotension requiring aggressive fluid resuscitation). WHO's interim guidelines for COVID‐19, 9 in which the severe type includes severe pneumonia (for adolescents or adults: fever or suspected respiratory infection, plus one of the following: RR > 30/min; severe respiratory distress; or SpO2 ≤ 93% on room air), ARDS (PaO2/FiO2 ≤ 300mm Hg with positive end expiratory pressure or continuous positive airway pressure ≥5 cm H2O, or nonventilated; SpO2/FiO2 ≤ 315 or else), sepsis, and septic shock. The diagnostic and treatment guidelines for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) issued by Chinese National Health Committee, 10 in which the severe type is designated as one meeting any of the following indices: (a) respiratory distress with RR ≥ 30/min; (b) oxygen saturation at rest ≤93%; (c) PaO2/FiO2 ≤ 300 mm Hg; (d) respiratory failure requiring mechanical ventilation; (e) shock; and (f) requiring intensive care unit (ICU) admission requirement due to multiple organ failure.
2.2. Article eligibility criteria
After the removal of duplicates, two investigators (CHC and HC) independently evaluated study eligibility and inclusion by assessing the titles, abstracts, and the retrieved full texts. Disagreements were settled by consensus. The inclusion criteria were as follows: (a) confirmed cases of COVID‐19; (b) distinction of severe and nonsevere patients on the basis of clear and widely used criteria; (c) a minimum of 20 participants in the study. The exclusion criteria were as follows: (a) review articles, case reports, nonhuman studies and other forms; (b) scarce report of blood coagulation indicators; (c) studies of pediatric patients or pregnant women; (d) study subjects including patients less than 18 years old. Eligible studies were evaluated for the blood coagulation measurable in identifying the severity of the patients with COVID‐19.
2.3. Data extraction and quality assessment
Two reviewers (YH and HHM) independently extracted prespecified data elements (first author, year of publication, country, sample size, age, sex, value of coagulation indicators) from each study. Median and range were used to estimate mean and standard deviation (SD). The percentiles were converted to SD according to the following formula: SD ≈ Norm interquartile range = (P75 − P25) × 0.7413 (P75: 75th percentile and P25: 25th percentile). 11 Study quality was assessed using the Newcastle‐Ottawa Scale (NOS) checklist. Disagreements were settled by consensus. A high‐quality study was defined as a study with a score ≥7. 12
2.4. Data synthesis and analysis
All the statistical analyses were performed with the STATA software (version 12.0; STATA Corporation, College Station, TX). Weighted mean difference (WMD) with 95% confidence interval (CI) was calculated for each blood coagulation indicator. I 2 was employed to evaluate statistical heterogeneity with I 2 values of <25%, 25%‐75%, and >75%, respectively, indicating low, moderate, and high heterogeneity. The choice of the proper‐effect models was based on the analysis results: the fixed‐effect model was used for I 2 ≤ 50% and the random‐effect model for I 2 > 50%. The single‐arm meta‐analysis of proportions with 95% CIs was conducted for the value of blood coagulation indicators. Subgroup analysis was performed according to sample size, if adapting the random‐effect model. In addition, a further sensitivity analysis was performed to test the stability of the results. The Begg test was performed to assess publication bias if a coagulation indicator was retrieved from 10 or more studies. The statistical significance was set at P < .05.
3. RESULTS
3.1. Study selection
The review progress is summarized in Figure 1. A total of 7192 articles were identified basing on the search strategy, and 1453 duplications were removed. Then, 173 were potentially relevant to the review question after screening of titles and abstracts and 160 studies were further excluded according to the aforementioned criteria. Finally, 13 studies 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 with 1341 patients and 371 severe COVID‐19 adults were enrolled in the meta‐analysis.
Figure 1.
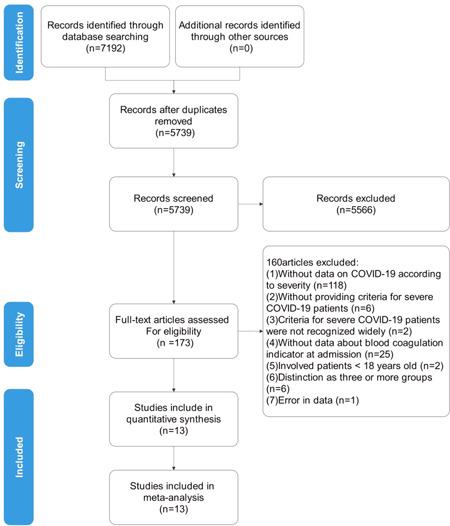
Flow‐chart of study selection
3.2. Study characteristics and quality assessment
All these studies were from China and published in 2020, with different sample sizes ranging from 21 to 204 patients, with a clear severity distinction (all the studies used the Chinese definition). The features of the 13 enrolled studies are summarized in Table 1. The quality assessment was based on NOS, with quality score ranging from 6 to 8. The quality results are shown in Table 1 and the assessment of each literature in the NOS scale is depicted in Table S1.
Table 1.
Description of included studies
| Study | Country | Year | Cases (severe/nonsevere) | Age, y (severe/nonsevere) | Sex (male [%]) (severe/nonsevere) | The value of blood coagulation indicator (severe/nonsevere) | The definition of “severity” | Quality score | |
|---|---|---|---|---|---|---|---|---|---|
| Qu 13 | China | 2020 | 3/27 | 60.0 (5.3)/49.4 (14.9) | NR | PLT | 169.67 ± 48.95/192.26 ± 58.12 | China | 7 |
| Gao 14 | China | 2020 | 15/28 | 45.2 (7.7)/43.0 (14.0) | 9 (60.0)/17 (60.7) | D‐D | 0.49 (0.29‐0.91)/0.21 (0.19‐0.27) | China | 8 |
| APTT | 27.29 ± 6.09/30.42 ± 5.31 | ||||||||
| PT | 11.26 ± 1.42/12.03 ± 1.21 | ||||||||
| FIB | 3.84 ± 1.00/3.11 ± 0.83 | ||||||||
| Wan 15 | China | 2020 | 40/95 | 56 (52‐73)/44 (33‐49) | 21 (52.5)/52 (54.7) | PLT | 147 (118‐213)/170 (136‐234) | China | 7 |
| D‐D | 0.6 (0.4‐1.1)/0.3 (0.2‐0.5) | ||||||||
| APTT | 29.7 (22.62‐39.4)/26.6 (24.5‐28.8) | ||||||||
| Xie 16 | China | 2020 | 28/51 | 62.5(50.5‐67.8)/59.0 (46.0‐66.0) | 18 (64.3)/26 (51.0) | D‐D | 0.7 (0.43‐2.36)/0.67 (0.31‐1.34) | China | 6 |
| Zheng 17 | China | 2020 | 30/131 | 57 (46.5‐66.0)/40 (31‐51) | 14 (46.7)/66 (50.4) | PLT | 160 (133‐214)/171 (137‐221) | China | 7 |
| Zhang 18 | China | 2020 | 58/82 | 64 (25‐87)/51.5 (26‐78) | 33 (56.9)/38 (46.3) | D‐D | 0.4 (0.2‐2.4)/0.2 (0.1‐0.3) | China | 7 |
| Chen 19 | China | 2020 | 11/10 | 61.0 (56.5–66.0)/52.0 (42.8–56.0) | 10 (90.9)/7 (70.0) | PLT | 157 (134‐184.5)/175.6 (148.3‐194) | China | 7 |
| D‐D | 2.6 (0.6‐18.7)/0.3 (0.3‐0.4) | ||||||||
| APTT | 33.7 (32.1‐38.4)/44 (42.6‐47.6) | ||||||||
| PT | 14.3 (13.6‐14.6)/13.4 (12.8‐13.7) | ||||||||
| Liu 20 | China | 2020 | 13/27 | 59.7 ± 10.1/43.2 ± 12.3 | 7 (53.8)/8 (29.6) | PLT | 186.6 ± 68.1/181.4 ± 70.7 | China | 7 |
| D‐D | 0.9 (0.7‐1.5)/0.4 (0.2‐0.8) | ||||||||
| APTT | 39.5 ± 4.2/39.5 ± 4.6 | ||||||||
| PT | 13.4 ± 0.6/13.1 ± 0.6 | ||||||||
| FIB | 6.3 ± 1.3/4.5 ± 1.4 | ||||||||
| He 21 | China | 2020 | 69/135 | 61 (52‐74)/43 (31‐53) | 37 (53.62)/42 (31.11) | PLT | 171 (138‐217)/200 (167‐261) | China | 7 |
| D‐D | 0.95 (0.41‐3.10)/0.32 (0.20‐0.70) | ||||||||
| PT | 11.8 (11.2‐12.7)/11.6 (11.0‐12.3) | ||||||||
| Chen 22 | China | 2020 | 43/102 | 52.8 ± 15.5/45.3 ± 13.6 | 23 (53.5)/56 (54.9) | PLT | 192 (142‐259)/204.5 (175‐254) | China | 7 |
| D‐D | 0.32 (0.21‐0.49)/0.24 (0.16‐0.39) | ||||||||
| APTT | 31.2 (28.5‐32.8)/29.2 (27.63‐31.85) | ||||||||
| PT | 11.9 (11.45‐12.5)/11.85 (11.3‐12.4) | ||||||||
| Zhu 23 | China | 2020 | 16/111 | 57.50 ± 11.70/49.95 ± 15.52 | 9 (56.25)/73 (65.77) | PLT | 155.00 (125.75‐206.00)/205.00 (165.00‐246.00) | China | 7 |
| D‐D | 0.16 (0.07‐0.28)/0.10 (0.08‐0.16) | ||||||||
| FIB | 5.74 (4.05‐6.68)/4.23 (3.63‐5.50) | ||||||||
| Fu 24 | China | 2020 | 16/59 | 51.8 ± 12.8/45.1 ± 14.0 | 10 (62.5)/35 (59.32) | D‐D | 0.32 (0.07‐1.22)/0.19 (0.07‐1.18) | China | 7 |
| FIB | 1.57 ± 0.39/0.94 ± 0.12 | ||||||||
| Zheng 25 | China | 2020 | 29/112 | 55 (47‐63)/45 (37‐55) | 16 (55.2)/58 (51.7) | PLT | 158 (125‐222)/203 (169‐246) | China | 8 |
Note: China: The diagnostic and treatment guidelines for SARS‐CoV‐2 issued by Chinese National Health Committee. Data are mean ± SD, median (interquartile range).
Abbreviations: APTT, activated partial thromboplastin time; D‐D, d‐dimer; FIB, fibrinogen; NR, not reported; PLT, platelet; PT, prothrombin time
3.3. Meta‐analysis of coagulation indicators
Five coagulation indicators (platelet, d‐dimer, APTT, PT, and fibrinogen) of the enrolled studies were analyzed and the results are shown in Figure 2.
Figure 2.
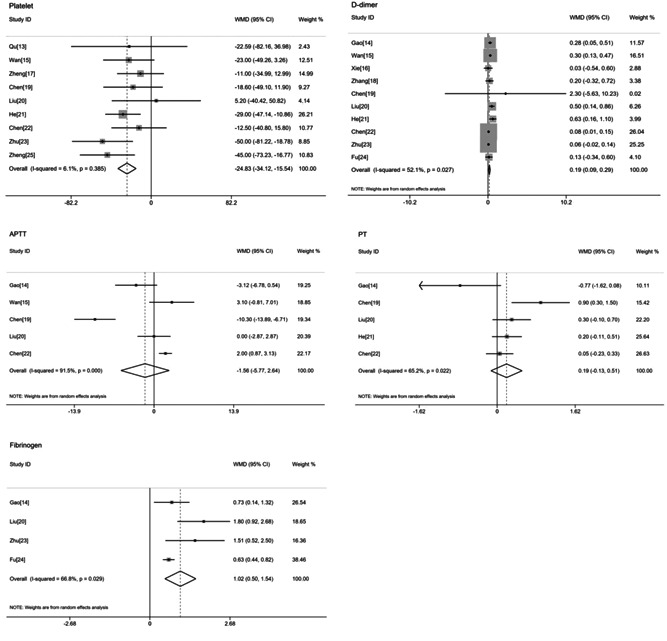
Forrest plots of the meta‐analyzed association of every blood coagulation indicator with the risk of severe 2019 novel coronavirus disease (COVID‐19). APTT, activated partial thromboplastin time; PT, prothrombin time; WMD, weighted mean difference
3.3.1. Platelet
Nine studies with 750 nonsevere and 385 severe COVID‐19 patients were eligible for the meta‐analysis. The fixed‐effect model demonstrated that the severe group had a markedly lower platelet than the nonsevere group (WMD = −24.83, 95% CI: −34.12 to −15.54; P < .001) without evident heterogeneity (I 2 = 6.1%; Figure 2). According to the single‐arm meta‐analysis, the pooled platelet in the severe group was 165.12 (95% CI: 157.38‐172.85) and that of the nonsevere group was 190.09 (95% CI: 179.45‐200.74; Figure 3).
Figure 3.

(A) Platelet; (B) d‐dimer; (C) fibrinogen. Forest plot of the value of blood coagulation indicators in severe and nonsevere COVID‐19 patients, respectively. COVID‐19, 2019 novel coronavirus disease; ES, effect size
3.3.2. D‐dimer
Ten studies assessed coagulation function using d‐dimer in comparing 309 severe and 700 nonsevere COVID‐19 patients. The value of d‐dimer was higher in the severe group when compared with the nonsevere group (WMD = 0.19, 95% CI: 0.09‐0.29; P < .001), with a moderate heterogeneity (I 2 = 52.1%) in the random‐effect model (Figure 2). Results of the single‐arm meta‐analysis showed that for severe patients, the pooled d‐dimer was 0.49 (95% CI: 0.33‐0.64) and that of the nonsevere group was 0.27 (95% CI: 0.20‐0.34; Figure 3).
3.3.3. APTT
Five studies analyzed APTT, involving 262 nonsevere and 122 severe COVID‐19 patients. The pooled WMD was −1.56 (95% CI: −5.77 to 2.64; P = .465) with a substantial heterogeneity (I 2 = 91.5%; Figure 2). Despite the high heterogeneity, the result should be interpreted with caution, for the 95% CI of WMD ranged from −5.77 to 2.64.
3.3.4. PT
For PT, five studies with 302 nonsevere and 151 severe COVID‐19 patients were eligible for meta‐analysis. In four studies, PT was higher in the severe group than in the nonsevere one, but the difference was not significant (WMD = 0.19, 95% CI: −0.13 to 0.51; P = .243, I 2 = 65.2%; Figure 2).
3.3.5. Fibrinogen
A total of four studies with 225 nonsevere and 60 severe COVID‐19 patients were included in the meta‐analysis. The analysis of the random‐effect model showed that compared with the nonsevere group, the severe group had a higher fibrinogen (WMD = 1.02, 95% CI: 0.50‐1.54; P < .001) with a moderate heterogeneity (I 2 = 66.8%; Figure 2). The single‐arm meta‐analysis showed that the pooled fibrinogen was 4.34 (95% CI: 1.98‐6.7) in the severe group but 3.19 (95% CI: 1.13‐5.24) in the nonsevere group (Figure 3).
3.4. Subgroup analysis
Subgroup analysis based on the sample size showed that there was an association between the d‐dimer levels and the severity of COVID‐19 in both sample size <100 subgroup and sample size ≥100 subgroup (sample size <100: WMD = 0.28, 95% CI: 0.12‐0.44; I 2 = 0%; sample size ≥100: WMD = 0.16, 95% CI: 0.03‐0.28; I 2 = 74.1%; Figure 4). APTT increased in the severe COVID‐19 in the subgroup with sample size ≥100 (WMD = 2.08, 95% CI: 1.00‐3.17; I 2 = 0%; Figure 4). There was no association between the PT levels and the severity of COVID‐19 in two subgroups (Figure 4). Fibrinogen increased in the severe COVID‐19 despite the subgroup based on sample size (Figure 4).
Figure 4.
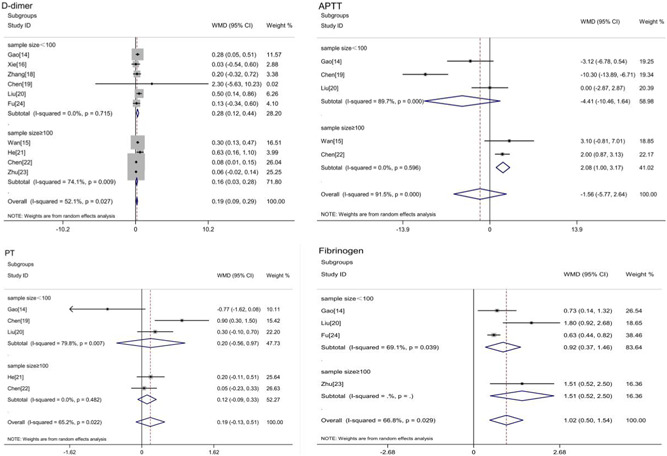
Subgroup analysis according to sample size of coagulation indicators in severe or nonsevere COVID‐19. COVID‐19, 2019 novel coronavirus disease
3.5. Sensitivity analysis and publication bias
As shown in Figure S1, from the results of the sensitivity analysis, the combined results did not change with the exclusion of any of the studies. Thus, sensitivity analysis suggested that these meta‐analyses (d‐dimer, APTT, PT, and fibrinogen) were steady. Because only d‐dimer was retrieved from 10 studies (≥10), a funnel plot regarding the d‐dimer showed that the P‐value of the Begg test was .858. The Begg test of “d‐dimer” suggested that no stable evidence of publication bias was present in the meta‐analysis (Figure 5).
Figure 5.
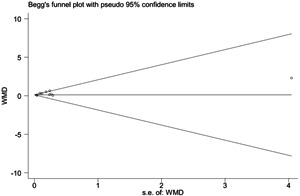
Begg's funnel plot for d‐dimer in comparing severe and nonsevere COVID‐19
4. DISCUSSION
To assess the differences in coagulation dysfunction between severe and nonsevere adult COVID‐19 patients, the current meta‐analysis adopted a well‐established definition of disease severity and systematically evaluated five coagulation indicators (platelet, d‐dimer, APTT, PT, and fibrinogen). The results showed that significant differences in platelet, d‐dimer, and fibrinogen were evident between severe and nonsevere patients though without significant differences in PT and APTT. The findings suggest that lower platelet, higher d‐dimer and fibrinogen are risk factors for increased severity of COVID‐19.
Previous studies suggest that influenza‐associated pneumonia is associated with thrombotic events. 26 Similarly, COVID‐19 can induce thromboembolic complications, especially in severe patients. 27 In severe cases, COVID‐19 triggers a cytokine storm, which activates the coagulation cascade, resulting in the thrombotic phenomena. 28 Nevertheless, the mechanism of COVID‐19‐related coagulation disorder is far more complicated to be explained by inflammation. 29 , 30 A previous study proposed that the immunity‐related pulmonary intravascular coagulation disease differs from sepsis‐induced coagulation dysfunction and DIC, with the latter characterized by platelet reduction and PT prolongation. 31 So COVID‐19‐related coagulation dysfunction may have its own characteristics.
Thrombocytopenia is considered as a dysregulated host response in critical sepsis patients, 32 which usually occurs after a viral infection (such as influenza and HIV). 33 In SARS‐related diseases, the virus has been suspected to cause platelet consumption in the lungs by damaging epithelial cells 34 and infect hematopoietic stem cells and megakaryocytes. 35 Similarly, COVID‐19 is homologous to SARS. A recent study by Yang et al 36 showed that 20.7% of patients with COVID‐19 had a low platelet (<125 × 109/L) and 5% of them had a very low platelet (range, 0‐50 × 109/L) with 92.1% mortality. 36 However, the incidence of thrombocytopenia was much lower in a study by Zheng et al 37 (8.5% vs 20.7%), which may be explained by the fact that the study enrolled fewer severe cases. Still strangely, a study by Panigada et al 38 found that platelet was normal or increased by thromboelastography in a small lCU COVID‐19 sample. Two meta‐analysis studies 39 , 40 also touched upon platelet in screening severe patients—one found no significant difference in platelet between the severe and mild patients, with only five studies and many severity criteria 39 ; the other reported that thrombocytopenia was associated with the severity of COVID‐19, but with a high heterogeneity (I 2 = 92%). 40 Compared with the two meta‐analysis studies, the current meta‐analysis has a clearer definition of “severity,” focuses on “adult patients,” and enrolls more studies. Therefore, the heterogeneity is low and the results are more accurate.
COVID‐19 is associated with thromboembolism events. Studies have shown that 30% of patients with COVID‐19 were complicated by pulmonary thromboembolism 41 and that the incidence of venous thromboembolism (VTE) was 47% in patients who were admitted to ICU after 14 days. 42 Pulmonary microvascular dysfunction may be an important cause of hypoxemia in patients with COVID‐19. Meanwhile, autopsy revealed that pulmonary embolism was the direct cause of death in 4 of 12 patients with COVID‐19. 43 Hence, thrombogenesis is significant in COVID‐19 cases, given that d‐dimer plays a key role in thromboembolism events. Our meta‐analysis is consistent with the listed findings: d‐dimer was elevated in the severe COVID‐19 patients. Elevated d‐dimer reflects hypercoagulable state and VTE events, which is associated with ARDs and death of COVID‐19 patients. 44 Long‐term bedridden condition, obesity, smoking, and advanced age not only are the risk factors for COVID‐19 severity but also lead to increased d‐dimer. 45 , 46 In addition, liver involvement in the severity of COVID‐19 cases 47 may lead to insufficient synthesis of coagulation factors, resulting in hyperplasminolysis and increased d‐dimer. 48
Fibrinogen is an essential part of the blood coagulation cascade. In sepsis, decreased fibrinogen is associated with an increased mortality, 49 which is related to consumptive coagulopathy. The elevated fibrinogen has been documented in infectious diseases, acute stroke, and myocardial infarction. 50 The current study is the first meta‐analysis to summarize increased fibrinogen levels in severe COVID‐19 adults. In addition, fibrinogen is an acute‐phase protein, which is induced by interleukin 6 and associated with inflammatory responses. 51 Once infection occurs, hepatic synthesis of fibrinogen increases 2 to 10 times. 52 Possibly different from septic coagulation disorder, early severe COVID‐19 patients present hypercoagulability rather than consumptive coagulopathy. 53 , 54
Prolonged PT and APTT are linked to anticoagulant, coagulation factor deficiency, and fibrinolysis, which have been used as laboratory tools to predict bleeding. 55 , 56 The performance of PT and APTT was contradictory in the initial research of severe COVID‐19 cases. Some studies showed shortened PT and APTT in severe COVID‐19 patients 14 while another study reported prolonged PT and APTT. 22 Our meta‐analysis found no difference in PT and APTT between the severe and nonsevere groups upon admission. Probably, PT and APTT may fail in an early recognition and be influenced by many factors (eg, anticoagulant). However, this finding should be interpreted with caution because of the high heterogeneity and much less included literature. More clinical trials are urgently needed to investigate the relation between PT/APTT and COVID‐19 severity.
5. LIMITATIONS
The following limitations should be mentioned: first, patients from the studies were all from China. There may exist racial differences in blood coagulation. 57 Further studies on coagulation from other races are highly awaited. Second, blood indicators were included upon admission, without considering previous use of antiplatelet or anticoagulant drugs. Finally, not all five indicators were included in some studies, so we hope that more studies related to blood coagulation will expand the sample size in the future.
6. CONCLUSIONS
Coagulation dysfunction is closely related to the severity of COVID‐19 cases and affects the prognosis of COVID‐19 patients. Low platelet, high d‐dimer and fibrinogen may serve as risk indicators for the progression of COVID‐19 severity in the early screening of severe and nonsevere COVID‐19 patients. Further exploration of coagulation function is crucial for prophylactic and anticoagulation therapy in severe COVID‐19 cases.
AUTHOR CONTRIBUTIONS
JL and HY designed the study and drafted and revised the manuscript. HC, CH, HH, and SZ acquired the data. CL organized the figures. KL and SS contributed equally to this study, provided supervision, and critically revised the manuscript. The authors approved the final version of the manuscript and agreed to be accountable for all aspects of the study.
Supporting information
Supplementary information
Supplementary information
Supplementary information
Lin J, Yan H, Chen H, et al. COVID‐19 and coagulation dysfunction in adults: A systematic review and meta‐analysis. J Med Virol. 2021;93:934–944. 10.1002/jmv.26346
Contributor Information
Songjing Shi, Email: serena10@126.com.
Kaiyang Lin, Email: lky7411@sina.com.
DATA AVAILABILITY STATEMENT
All data are fully available online without restriction.
REFERENCES
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. [DOI] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mattiuzzi C, Lippi G. Which lessons shall we learn from the 2019 novel coronavirus outbreak. Ann Transl Med. 2020;8(3):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong RS, Wu A, To KF, et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326(7403):1358‐1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of Covid‐19 in China. NEJM. 2020;382(19):1861‐1862. [DOI] [PubMed] [Google Scholar]
- 8. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community‐acquired pneumonia. Am J Respir Crit Care Med. 2019;200(7):e45‐e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . Clinical management of severe acute respiratory infection (SARI) when COVID‐19 disease is suspected: Interim guidance V 1.2. 13 March 2020. Available at https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 10.National Health Commission of the people's Republic of China. The Guidelines for the Diagnosis and Treatment of New Coronavirus Pneumonia (5th ed.). www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440.shtml
- 11. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stang A. Critical evaluation of the Newcastle‐Ottawa Scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. [DOI] [PubMed] [Google Scholar]
- 13. Qu R, Ling Y, Zhang YH, et al. Platelet‐to‐lymphocyte ratio is associated with prognosis in patients with coronavirus disease‐19. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID‐19. J Med Virol. 2020;92:791‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID‐19 patients in northeast Chongqing. J Med Virol. 2020;92:797‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non‐ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. 2020;40:1321‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng F, Tang W, Li H, Huang YX, Xie YL, Zhou ZG. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID‐19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24(6):3404‐3410. [DOI] [PubMed] [Google Scholar]
- 18. Zhang J, Dong X, Cao Y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75:1730‐1741. [DOI] [PubMed] [Google Scholar]
- 19. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS‐CoV‐2 infected patients. EBioMedicine. 2020:102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He R, Lu Z, Zhang L, et al. The clinical course and its correlated immune status in COVID‐19 pneumonia. J Clin Virol. 2020;127:104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Q, Zheng Z, Zhang C, et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID‐19) in Taizhou, Zhejiang, China. Infection. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu Z, Cai T, Fan L, et al. Clinical value of immune‐inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fu J, Kong J, Wang W, et al. The clinical implication of dynamic neutrophil to lymphocyte ratio and d‐dimer in COVID‐19: a retrospective study in Suzhou China. Thromb Res. 2020;192:3‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng Y, Zhang Y, Chi H, et al. The hemocyte counts as a potential biomarker for predicting disease progression in COVID‐19: a retrospective study. Clin Chem Lab Med. 2020;58(7):1106‐1115. [DOI] [PubMed] [Google Scholar]
- 26. Ishiguro T, Matsuo K, Fujii S, Takayanagi N. Acute thrombotic vascular events complicating influenza‐associated pneumonia. Respir Med Case Rep. 2019;28:100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spiezia L, Boscolo A, Poletto F, et al. COVID‐19‐related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120:998‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID‐19. JCI Insight, 5(11):e138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Campbell CM, Kahwash R. Will complement inhibition be the new target in treating COVID‐19 related systemic thrombosis. Circulation. 2020;141:1739‐1741. [DOI] [PubMed] [Google Scholar]
- 31. Levi M, Schultz M, van der Poll T. Sepsis and thrombosis. Semin Thromb Hemost. 2013;39(5):559‐66. [DOI] [PubMed] [Google Scholar]
- 32. Claushuis TA, van Vught LA, Scicluna BP, et al. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood. 2016;127(24):3062‐3072. [DOI] [PubMed] [Google Scholar]
- 33. Assinger A. Platelets and infection—an emerging role of platelets in viral infection. Front Immunol. 2014;5:649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang M, Ng MH, Li CK. Thrombocytopenia in patients with severe acute respiratory syndrome (review). Hematology. 2005;10(2):101‐105. [DOI] [PubMed] [Google Scholar]
- 35. Goeijenbier M, van Wissen M, van de Weg C. Viral infections and mechanisms of thrombosis and bleeding. J Med Virol. 2012;84(10):1680‐1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang X, Yang Q, Wang Y. Thrombocytopenia and its association with mortality in patients with COVID‐19. J Thromb Haemost. 2020;18(6):1469‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zheng Y, Zhang Y, Chi H, et al. The hemocyte counts as a potential biomarker for predicting disease progression in COVID‐19: a retrospective study. Clin Chem Lab Med. 2020;58(7):1106‐1115. [DOI] [PubMed] [Google Scholar]
- 38. Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID‐19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738‐1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xiong M, Liang X, Wei YD. Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis. Br J Haematol. 2020:1050‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a meta‐analysis. Clin Chim Acta. 2020;506:145‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leonard‐Lorant I, Delabranche X, Severac F. Acute pulmonary embolism in COVID‐19 patients on CT angiography and relationship to d‐dimer levels. Radiology. 2020:201561. 10.1148/radiol.2020201561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020. 10.1111/jth.14888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wichmann D, Sperhake JP, Lutgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann Intern Med. 2020:M20‐2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang L, Yan X, Fan Q, et al. D‐dimer levels on admission to predict in‐hospital mortality in patients with Covid‐19. J Thromb Haemost. 2020;18(6):1324‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Klovaite J, Benn M, Nordestgaard BG. Obesity as a causal risk factor for deep venous thrombosis: a Mendelian randomization study. J Intern Med. 2015;277(5):573‐584. [DOI] [PubMed] [Google Scholar]
- 46. Benedikter BJ, Bouwman FG, Heinzmann A, et al. Proteomic analysis reveals procoagulant properties of cigarette smoke‐induced extracellular vesicles. J Extracell Vesicles. 2019;8(1):1585163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Parohan M, Yaghoubi S, Seraj A. Liver injury is associated with severe coronavirus disease 2019 (COVID‐19) infection: a systematic review and meta‐analysis of retrospective studies. Hepatol Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kruskal JB, Robson SC, Franks JJ, Kirsch RE. Elevated fibrin‐related and fibrinogen‐related antigens in patients with liver disease. Hepatology. 1992;16(4):920‐923. [DOI] [PubMed] [Google Scholar]
- 49. Matsubara T, Yamakawa K, Umemura Y, et al. Significance of plasma fibrinogen level and antithrombin activity in sepsis: a multicenter cohort study using a cubic spline model. Thromb Res. 2019;181:17‐23. [DOI] [PubMed] [Google Scholar]
- 50. Kamath S, Lip GY. Fibrinogen: biochemistry, epidemiology and determinants. QJM. 2003;96(10):711‐729. [DOI] [PubMed] [Google Scholar]
- 51. Kerr R, Stirling D, Ludlam CA. Interleukin 6 and haemostasis. Br J Haematol. 2001;115(1):3‐12. [DOI] [PubMed] [Google Scholar]
- 52. Dowton SB, Colten HR. Acute phase reactants in inflammation and infection. Semin Hematol. 1988;25(2):84‐90. [PubMed] [Google Scholar]
- 53. Spiezia L, Boscolo A, Poletto F. COVID‐19‐related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120(6):998‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pavoni V, Gianesello L, Pazzi M, Stera C, Meconi T, Frigieri FC. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID‐19 pneumonia. J Thromb Thrombolysis. 2020;50:281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tripodi A. Thrombin generation assay and its application in the clinical laboratory. Clin Chem. 2016;62(5):699‐707. [DOI] [PubMed] [Google Scholar]
- 56. Kamal AH, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc. 2007;82(7):864‐873. [DOI] [PubMed] [Google Scholar]
- 57. Fogarty H, Townsend L, Cheallaigh CN, et al. COVID‐19 coagulopathy in Caucasian patients. Br J Haematol. 2020;189(6):1044‐1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary information
Supplementary information
Data Availability Statement
All data are fully available online without restriction.


