Abstract
Objective
To provide guidance on the management of multisystem inflammatory syndrome in children (MIS‐C), a condition characterized by fever, inflammation, and multiorgan dysfunction that manifests late in the course of severe acute respiratory syndrome coronavirus 2 (SARS–CoV‐2) infection, and to provide recommendations for children with hyperinflammation during coronavirus disease 2019 (COVID‐19), the acute, infectious phase of SARS–CoV‐2 infection.
Methods
A multidisciplinary task force was convened by the American College of Rheumatology (ACR) to provide guidance on the management of MIS‐C associated with SARS–CoV‐2 and hyperinflammation in COVID‐19. The task force was composed of 9 pediatric rheumatologists, 2 adult rheumatologists, 2 pediatric cardiologists, 2 pediatric infectious disease specialists, and 1 pediatric critical care physician. Preliminary statements addressing clinical questions related to MIS‐C and hyperinflammation in COVID‐19 were developed based on evidence reports. Consensus was built through a modified Delphi process that involved 2 rounds of anonymous voting and 2 webinars. A 9‐point scale was used to determine the appropriateness of each statement (median scores of 1–3 for inappropriate, 4–6 for uncertain, and 7–9 for appropriate), and consensus was rated as low, moderate, or high based on dispersion of the votes along the numeric scale. Approved guidance statements were those that were classified as appropriate with moderate or high levels of consensus, as prespecified prior to voting.
Results
The ACR task force approved a total of 128 guidance statements addressing the management of MIS‐C and hyperinflammation in pediatric COVID‐19. These statements were refined into 40 final clinical guidance statements, accompanied by a flow diagram depicting the diagnostic pathway for MIS‐C.
Conclusion
Our understanding of SARS–CoV‐2–related syndromes in the pediatric population continues to evolve. The guidance provided in this “living document” reflects currently available evidence, coupled with expert opinion, and will be revised as further evidence becomes available.
Due to the rapidly expanding information and evolving evidence related to COVID‐19, which may lead to modification of some guidance statements over time, it is anticipated that updated versions of this article will be published, with the version number included in the title. Readers should ensure that they are consulting the most current version.
Guidance developed and/or endorsed by the American College of Rheumatology (ACR) is intended to inform particular patterns of practice and not to dictate the care of a particular patient. The ACR considers adherence to this guidance to be voluntary, with the ultimate determination regarding its application to be made by the physician in light of each patient’s individual circumstances. Guidance statements are intended to promote beneficial or desirable outcomes but cannot guarantee any specific outcome. Guidance developed or endorsed by the ACR is subject to periodic revision as warranted by the evolution of medical knowledge, technology, and practice.
The American College of Rheumatology is an independent, professional medical and scientific society which does not guarantee, warrant, or endorse any commercial product or service.
INTRODUCTION
Since its initial description in December 2019 in Wuhan, China, coronavirus disease 2019 (COVID‐19), caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS–CoV‐2), has rapidly evolved into a worldwide pandemic affecting millions of lives (1). Unlike adults, the vast majority of children with COVID‐19 have mild symptoms. However, there are children who have significant respiratory disease, and some children may develop a hyperinflammatory response similar to what has been observed in adults with COVID‐19. Furthermore, in late April 2020, reports emerged of children with a different clinical syndrome resembling Kawasaki disease (KD) and toxic shock syndrome; these patients frequently had evidence of prior exposure to SARS–CoV‐2 (2, 3). Subsequent to these initial reports from Italy and the United Kingdom, multiple case series from Europe and the United States have surfaced describing a similar phenomenon (4, 5, 6, 7, 8, 9, 10). While this constellation of symptoms has been given many names, for the purposes of this discussion we refer to it as “multisystem inflammatory syndrome in children” (MIS‐C).
For a number of reasons, there is an urgent need to provide guidance to healthcare providers in the evaluation of patients in whom MIS‐C is a diagnostic consideration. These reasons include the fact that 1) case definitions for MIS‐C are variable, 2) clinical descriptions of MIS‐C are limited to case series, 3) clinical features of MIS‐C may also be seen in other infections and malignant entities and in other rheumatic diseases in childhood, 4) suggested treatment strategies have relied on extrapolation from other inflammatory or rheumatic conditions with similar clinical presentations, and 5) myocardial dysfunction may present insidiously but is a major source of morbidity and mortality in MIS‐C. In addition, pediatric rheumatologists are often asked to recommend immunomodulatory therapy for patients developing hyperinflammation as a result of acute SARS–CoV‐2 infection.
Therefore, the American College of Rheumatology (ACR) convened the MIS‐C and COVID‐19–Related Hyperinflammation Task Force on May 22, 2020, which was charged by ACR leadership to provide guidance to clinicians in the evaluation and management of MIS‐C and COVID‐19–related hyperinflammatory syndromes in children. Clinical guidance generated from this effort is intended to aid in the care of individual patients, but it is not meant to supplant clinical decision‐making. Modifications to treatment plans, particularly in patients with complex conditions, are highly disease‐, patient‐, geography‐, and time‐specific, and therefore must be individualized as part of a shared decision‐making process.
METHODS
Task force
Panelists were selected by the task force leadership (LAH and JJM) based on their clinical expertise in rheumatology, infectious diseases, cardiology, cytokine storm–related syndromes, and KD, as well as experience in managing MIS‐C and hyperinflammation in acute SARS–CoV‐2 infection. The multidisciplinary task force was composed of clinicians from the United States and Canada and included 9 pediatric rheumatologists, 2 adult rheumatologists, 2 pediatric cardiologists, 2 pediatric infectious disease specialists, and 1 pediatric critical care physician. All individuals who were approached to develop this guidance agreed to participate.
Prior to the first meeting, task force members were subdivided into 4 work groups to address the following clinical topics related to MIS‐C and hyperinflammation in COVID‐19: 1) diagnostic evaluation of MIS‐C (led by SKL); 2) cardiac management of MIS‐C (led by KGF); 3) treatment of MIS‐C (led by MG); and 4) management of hyperinflammation in COVID‐19 (led by SWC). During the first webinar on May 22, 2020, participants agreed with the importance of addressing these 4 overarching topics and the structure of the work groups. The first webinar was used to confirm the target audience for the guidance, which focuses on clinicians in North America managing inflammatory syndromes in children related to recent or concurrent infections with SARS–CoV‐2. Notably, the task force deliberately did not attempt to create a new case definition for MIS‐C, as several already exist (4, 5, 6) (Table 1). Instead, the task force elected to leverage consensus building to identify the most appropriate diagnostic and therapeutic steps that providers should consider at the present time. All panelists agreed to develop consensus through a modified Delphi process, which involved 2 rounds of asynchronous, anonymous voting and 2 webinars to discuss voting results.
Table 1.
Case definitions of MIS‐C*
| Criteria | RCPCH† | CDC | WHO‡ |
|---|---|---|---|
| Age | All children (age not defined) | <21 years | 0–19 years |
| Fever | Persistent fever (≥38.5°C) | Temperature ≥38.0°C for ≥24 hours or subjective fever for ≥24 hours | Fever for ≥3 days |
| Clinical symptoms |
Both of the following:
|
Both of the following:
|
At least 2 of the following:
|
| Inflammation |
All 3 of the following:
|
Laboratory evidence of inflammation including, but not limited to, 1 or more of the following:
|
Elevated inflammation markers, including any of the following:
|
| Link to SARS–CoV‐2 | Positive or negative by PCR |
Current or recent findings of the following:
|
Evidence of COVID‐19 by the following:
|
| Exclusion | Other infections | No alternative diagnosis | No obvious microbial cause |
Case definitions of multisystem inflammatory syndrome in children (MIS‐C) are adapted from recommendations from the World Health Organization (WHO) (4) and Centers for Disease Control and Prevention (CDC) (6) for MIS‐C, as well as the Royal College of Paediatrics and Child Health (RCPCH) for pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus 2 (SARS–CoV‐2) (5). For laboratory parameters, ↑ indicates elevated levels. GI = gastrointestinal; CRP = C‐reactive protein; ESR = erythrocyte sedimentation rate; LDH = lactate dehydrogenase; IL‐6 = interleukin‐6; PCR = polymerase chain reaction; COVID‐19 = coronavirus disease 2019.
In the RCPCH case definition, additional features include abdominal pain, confusion, conjunctivitis, cough, diarrhea, headache, lymphadenopathy, mucous membrane changes, neck swelling, rash, respiratory symptoms, sore throat, swollen hands and feet, syncope, and vomiting.
In the WHO case definition, cardiac involvement is defined as the presence of myocardial dysfunction, pericarditis, valvulitis, or coronary abnormalities (including findings on echocardiogram or elevated levels of troponin/N‐terminal pro–B‐type natriuretic peptide).
Evidence review
From May 22 to May 29, 2020, the work groups developed preliminary recommendation statements within their assigned topic, based on expert opinion and evidence reviewed from publications listed in PubMed, scientific briefings from the World Health Organization, health alerts from the Centers for Disease Control and Prevention (CDC), and guidance provided by the Royal College of Paediatrics and Child Health. Each work group generated an evidence report supporting the recommendations, which was shared with the entire task force.
Voting
Round 1
The task force voted virtually and anonymously using the RAND/University of California at Los Angeles (UCLA) Appropriateness Method (11). A 9‐point scale was used by panelists to rate the appropriateness of each of the statements. A score of 9 was considered to be the highest level of appropriateness, while a score of 1 indicated that the statement was entirely inappropriate. Prior to voting, median scores of 1–3 were defined as inappropriate, 4–6 as uncertain, and 7–9 as appropriate. Consensus was prespecified as high if all 16 votes coalesced within the same tertile, while low consensus was recognized when voting was dispersed widely along the 9‐point scale (with ≥5 votes in the 1–3 score range and ≥5 votes in the 7–9 score range). Moderate consensus encompassed all other scenarios. The votes of each task force member were counted equally and tallied. The results of the initial voting were distributed to the task force and reviewed during a 90‐minute webinar on June 4, 2020. Statements that were rated as uncertain (median score 4–6) and/or characterized by moderate or low consensus were addressed first. The panelists were then encouraged to discuss the remaining statements.
Round 2
Input from the initial voting and discussion was incorporated (by LAH and JJM) into the draft guidance statements, and the document was redistributed to the entire task force for a second round of voting. Voting in this phase was conducted in the same manner as described above, and results were reviewed at a third webinar on June 10, 2020. Guidance statements that earned a median score of 7–9 with moderate or high levels of consensus were approved by the panel.
Guidance approval
Following the final webinar, approved statements were refined and, in some instances, combined to reduce redundancy. A preliminary guidance document was generated, and the entire task force was given an opportunity to review and edit the document. Approval was obtained from each panelist on June 14, 2020 and by the ACR Board of Directors on June 17, 2020 (12). After further review, the authors decided to include measurement of C‐reactive protein (CRP) levels in the laboratory evaluation of hyperinflammation in severe COVID‐19 (Table 7), and the entire task force then voted again on the guidance statements and approved the modifications to this recommendation statement.
Table 2.
Diagnostic evaluation of MIS‐C*
| Guidance statement | Level of consensus |
|---|---|
| The vast majority of children with COVID‐19 present with mild symptoms and have excellent outcomes. MIS‐C remains a rare complication of SARS–CoV‐2 infections. | High |
| MIS‐C is temporally associated with SARS–CoV‐2 infections. Therefore, the prevalence of the virus in a given geographic location, which may change over time, should inform management decisions. | Moderate |
| A child “under investigation” for MIS‐C should also be evaluated for other possible infections and non–infection‐related conditions (e.g., malignancy) that may explain the clinical presentation. | High |
| Patients “under investigation” for MIS‐C may require additional diagnostic studies (not described in Figure 1), including, but not limited to, imaging of the chest, abdomen, and/or central nervous system and lumbar puncture. | High |
| Outpatient evaluation for MIS‐C may be appropriate for assessing well‐appearing children with stable vital signs and for ensuring that physical examinations provide close clinical follow‐up. | Moderate |
|
Patients “under investigation” for MIS‐C should be considered for admission to the hospital for further observation while the diagnostic evaluation is completed, especially if the patient displays any of the following symptoms:
|
Moderate to high |
| Patients presenting with shock, significant respiratory distress, neurologic changes (altered mental status, encephalopathy, focal neurologic deficits, meningismus, papilledema), dehydration, or features of KD should be admitted for further evaluation, regardless of MIS‐C status, in accordance with standard of care. | High |
|
Children admitted to the hospital with MIS‐C should be managed by a multidisciplinary team that includes pediatric rheumatologists, cardiologists, infectious disease specialists, and hematologists. Depending on the clinical manifestations, other subspecialties may need to be consulted as well; these include, but are not limited to, pediatric neurology, nephrology, hepatology, and gastroenterology. |
Moderate to high |
MIS‐C = multisystem inflammatory syndrome in children; COVID‐19 = coronavirus disease 2019; SARS–CoV‐2 = severe acute respiratory syndrome coronavirus 2; CRP = C‐reactive protein; EKG = electrocardiogram; BNP = B‐type natriuretic peptide; KD = Kawasaki disease.
RESULTS
In the first round of voting, the task force evaluated a total of 125 statements that addressed the management of MIS‐C and hyperinflammation in pediatric patients with COVID‐19. Of these, 112 statements met the criteria for approval, with a median score for appropriateness of 7–9 and with moderate or high consensus, while 13 statements were rated as uncertain (median score 4–6). After refining the statements based on the input from the initial phase, 128 guidance statements were approved in the second round of voting (see Supplementary Tables 1–4, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41454/abstract). These statements were organized into 40 final guidance statements as well as a flow diagram depicting the diagnostic pathway for MIS‐C (Figure 1), which were approved by the entire task force and the ACR Board of Directors (12). Topics covered in the guidance include the following: 1) diagnostic evaluation of MIS‐C (Table 2 and Figure 1); 2) comparing and contrasting features of MIS‐C and KD (Table 3); 3) cardiac management of MIS‐C (Table 4); 4) treatment of MIS‐C (Tables 5 and 6); and 5) hyperinflammation in COVID‐19 (Table 7).
Figure 1.
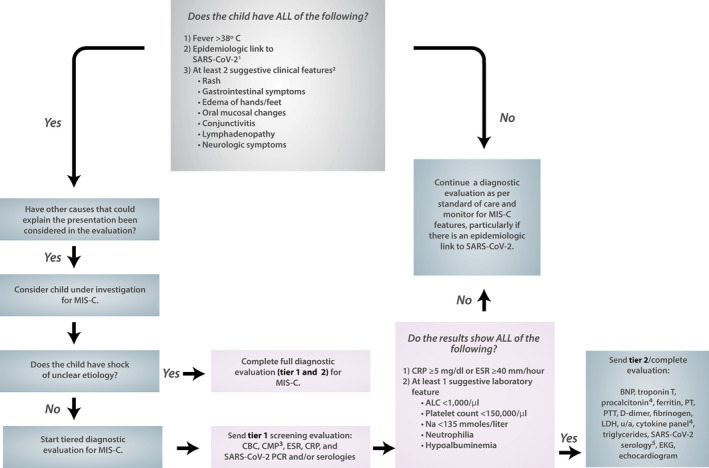
Diagnostic pathway for multisystem inflammatory syndrome in children (MIS‐C). Moderate‐to‐high consensus was reached by the task force in the development of this diagnostic pathway for MIS‐C associated with severe acute respiratory syndrome coronavirus 2 (SARS–CoV‐2). 1An epidemiologic link to SARS–CoV‐2 infection is defined as a child with any of the following criteria: positive for SARS–CoV‐2 by polymerase chain reaction (PCR), positive for SARS–CoV‐2 by serology, preceding illness resembling coronavirus disease 2019 (COVD‐19) or close contact with an individual with confirmed or suspected COVID‐19 in the past 4 weeks. 2Suggestive clinical features include rash (polymorphic, maculopapular, or petechial, but not vesicular), gastrointestinal symptoms (diarrhea, abdominal pain, or vomiting), oral mucosal changes (red and/or cracked lips, strawberry tongue, or erythema of the oropharyngeal mucosa), conjunctivitis (bilateral conjunctival infection without exudate), and neurologic symptoms (altered mental status, encephalopathy, focal neurologic deficits, meningismus, or papilledema). 3The complete metabolic panel (CMP) includes measurement of sodium, potassium, carbon dioxide, chloride, blood urea nitrogen, creatinine, glucose, calcium, albumin, total protein, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and bilirubin. 4Procalcitonin and cytokine panel results should be sent, if available. 5Serologic test results should be sent if not sent in tier 1 evaluation, and if possible, SARS–CoV‐2 IgG, IgM, and IgA test results should be sent. CRP = C‐reactive protein; ESR = erythrocyte sedimentation rate; ALC = absolute lymphocyte count; CBC = complete blood cell count; BNP = B‐type natriuretic peptide; PT = prothrombin time; PTT = partial thromboplastin time; LDH = lactate dehydrogenase; u/a = urinalysis; EKG = electrocardiogram.
Table 3.
Comparing and contrasting features of MIS‐C and KD*
| Guidance statement | Level of consensus |
|---|---|
| Patients with KD that is unrelated to SARS–CoV‐2 will continue to require evaluation, diagnosis, and treatment during the SARS–CoV‐2 pandemic. | High |
| MIS‐C and KD unrelated to SARS–CoV‐2 infections may share overlapping clinical features, including conjunctival infection, oropharyngeal findings (red and/or cracked lips, strawberry tongue), rash, swollen and/or erythematous hands and feet, and cervical lymphadenopathy. | Moderate to high |
|
Several epidemiologic, clinical, and laboratory features of MIS‐C may differ from KD unrelated to SARS–CoV‐2 in the following ways:
|
Moderate to high |
| It is unknown if the incidence of CAAs is different in MIS‐C compared to KD; however, MIS‐C patients without KD features can develop CAAs. | Moderate to high |
MIS‐C = multisystem inflammatory syndrome in children; KD = Kawasaki disease; SARS–CoV‐2 = severe acute respiratory syndrome coronavirus 2; GI = gastrointestinal; CRP = C‐reactive protein; CAAs = coronary artery aneurysms.
Table 4.
Cardiac management of MIS‐C*
| Guidance statement | Level of consensus |
|---|---|
| Patients with MIS‐C and abnormal BNP and/or troponin T levels at diagnosis should have these laboratory parameters trended over time until they normalize. | High |
| EKGs should be performed at a minimum of every 48 hours in MIS‐C patients who are hospitalized and during follow‐up visits. If conduction abnormalities are present, patients should be placed on continuous telemetry while in the hospital, and Holter monitors should be considered during follow‐up. | Moderate to high |
| Echocardiograms conducted at diagnosis and during clinical follow‐up should include evaluation of ventricular/valvar function, pericardial effusion, and coronary artery dimensions with measurements indexed to body surface area using z‐scores. | High |
| Echocardiograms should be repeated at a minimum of 7–14 days and 4–6 weeks after presentation. For those patients with cardiac abnormalities occurring in the acute phase of their illness, an echocardiogram 1 year after MIS‐C diagnosis could be considered. Patients with LV dysfunction and/or CAAs will require more frequent echocardiograms. | Moderate to high |
| Cardiac MRI may be indicated 2–6 months after MIS‐C diagnosis in patients who presented with significant transient LV dysfunction in the acute phase of illness (LV ejection fraction <50%) or persistent LV dysfunction. Cardiac MRI should focus on myocardial characterization, including functional assessment, T1/T2‐weighted imaging, T1 mapping and extracellular volume quantification, and late gadolinium enhancement. | High |
| Cardiac CT should be performed in patients with suspected presence of distal CAAs that are not well seen on echocardiogram. | Moderate |
MIS‐C = multisystem inflammatory syndrome in children; BNP = B‐type natriuretic peptide; EKG = electrocardiogram; LV = left ventricular; CAAs = coronary artery aneurysms; MRI = magnetic resonance imaging; CT = computed tomography.
Table 5.
Immunomodulatory treatment in MIS‐C*
| Guidance statement | Level of consensus |
|---|---|
| Patients “under investigation” for MIS‐C without life‐threatening manifestations should undergo diagnostic evaluation for MIS‐C, as well as other possible infections and non–infection‐related conditions, before immunomodulatory treatment is initiated. | Moderate |
| Patients “under investigation” for MIS‐C with life‐threatening manifestations may require immunomodulatory treatment for MIS‐C before the full diagnostic evaluation can be completed. | High |
| After evaluation by specialists with expertise in MIS‐C, some patients with mild symptoms may only require close monitoring without immunomodulatory treatment. The panel noted uncertainty around the empiric use of IVIG to prevent CAAs in this setting. | Moderate |
| A stepwise progression of immunomodulatory therapies should be used to treat MIS‐C with IVIG and/or glucocorticoids considered as first‐tier treatments. | Moderate to high |
| High‐dose IVIG (typically 1–2 gm/kg) may be considered for treatment of MIS‐C. Cardiac function and fluid status should be assessed in MIS‐C patients with shock before IVIG treatment is provided, and IVIG should be administered when cardiac function is restored. | Moderate to high |
| Low‐to‐moderate doses of glucocorticoids may be considered for treatment of MIS‐C. High‐dose IV pulse glucocorticoids may be considered to treat patients with life‐threatening complications, such as shock, and specifically, if a patient requires high‐dose or multiple inotropes and/or vasopressors. | Moderate to high |
| Anakinra (IV or SC) may be considered for treatment of MIS‐C refractory to IVIG and glucocorticoids or in patients with contraindications to these treatments. | Moderate to high |
| Serial laboratory testing and cardiac assessment should guide the immunomodulatory treatment response and tapering. Patients will often require a 2–3‐week taper of immunomodulatory medications. | High |
MIS‐C = multisystem inflammatory syndrome in children; IVIG = intravenous immunoglobulin; CAAs = coronary artery aneurysms; SC = subcutaneous.
Table 6.
Antiplatelet and anticoagulation therapy in MIS‐C*
| Guidance statement | Level of consensus |
|---|---|
| Low‐dose aspirin (3–5 mg/kg/day; maximum 81 mg/day) should be used in patients with MIS‐C and KD‐like features and/or thrombocytosis (platelet count ≥450,000/μl) and should be continued until the platelet count is normalized and normal coronary arteries are confirmed at ≥4 weeks after diagnosis. Treatment with aspirin should be avoided in patients with a platelet count of ≤80,000/μl. | Moderate |
| MIS‐C patients with CAAs and a maximal z‐score of 2.5–10.0 should be treated with low‐dose aspirin. Patients with a z‐score of ≥10.0 should be treated with low‐dose aspirin and therapeutic anticoagulation with enoxaparin (factor Xa level 0.5–1.0) or warfarin. | Moderate to high |
| Patients with MIS‐C and documented thrombosis or an EF of <35% should receive therapeutic anticoagulation with enoxaparin until at least 2 weeks after discharge from the hospital. | High |
| Indications for longer outpatient therapeutic enoxaparin dosing include the following: CAAs with a z‐score of >10.0 (indefinite treatment), documented thrombosis (treatment for ≥3 months pending thrombus resolution), or ongoing moderate‐to‐severe LV dysfunction. | High |
| For MIS‐C patients who do not meet the above criteria, the approach to antiplatelet and anticoagulation therapeutic management should be tailored to the patient’s risk for thrombosis. | High |
MIS‐C = multisystem inflammatory syndrome in children; KD = Kawasaki disease; CAAs = coronary artery aneurysms; EF = ejection fraction; LV = left ventricular.
Table 7.
Hyperinflammation in COVID‐19*
| Guidance statement | Level of consensus |
|---|---|
| Children with a complex medical history and those taking immunosuppressive medications, including moderate‐to‐high–dose glucocorticoids, may be at higher risk for severe outcomes in COVID‐19. | Moderate to high |
| Children and adults admitted to the hospital with COVID‐19 present with similar symptoms, including fever, upper respiratory tract symptoms, abdominal pain, and diarrhea. | Moderate |
| Children with severe respiratory symptoms due to COVID‐19 should be considered for immunomodulatory therapy if any of the following are present: ARDS, shock/cardiac dysfunction, substantially elevated LDH, d‐dimer, IL‐6, IL‐2R, CRP, and/or ferritin levels, and depressed lymphocyte count, albumin levels, and/or platelet count. | Moderate to high |
| Glucocorticoids may be considered for use as immunomodulatory therapy in patients with COVID‐19 and hyperinflammation (as outlined in the above statement). | Moderate |
| Anakinra treatment appears safe in severe infections and in children with hyperinflammatory syndromes. In children with COVID‐19 and hyperinflammation, anakinra (>4 mg/kg/day IV or SC) should be considered for immunomodulatory therapy. Initiation of anakinra before invasive mechanical ventilation may be beneficial. | High |
| Children with COVID‐19 treated with anakinra should be monitored for LFT abnormalities. | Moderate |
| Compared to standard care, tocilizumab may be effective in reducing mortality and ICU admission in patients with severe COVID‐19 pneumonia and signs of hyperinflammation; however, patients treated with tocilizumab may be at higher risk for bacterial and fungal infections. | Moderate |
| When tocilizumab is used to treat children with COVID‐19, weight‐based dosing should be employed (body weight <30 kg, 12 mg/kg IV; body weight ≥30 kg, 8 mg/kg IV, maximum 800 mg). Children treated with tocilizumab should be monitored for LFT abnormalities and elevated triglyceride levels. | Moderate to high |
| In the absence of randomized controlled trials or comparative effectiveness studies, if immunomodulation is to be used at all, the balance of risks and benefits suggests that anakinra be used as first‐line immunomodulatory treatment of children with COVID‐19 and hyperinflammation. There is insufficient evidence to support the use of other immunomodulatory agents, unless glucocorticoids, IL‐1–blocking therapies, and/or IL‐6–blocking therapies are contraindicated or have failed. | Moderate |
COVID‐19 = coronavirus disease 2019; ARDS = acute respiratory distress syndrome; LDH = lactate dehydrogenase; IL‐6 = interleukin‐6; IL‐2R = interleukin‐2 receptor; CRP = C‐reactive protein; IV = intravenous; SC = subcutaneous; LFT = liver function test; ICU = intensive care unit.
Our understanding of SARS–CoV‐2–related syndromes in the pediatric population continues to evolve. The recommendations provided by the task force reflect expert opinion and currently available evidence, which is of low quality and based on a limited number of case series and retrospective cohort studies. Thus, this guidance is meant to be a “living document” and will be modified as additional data become available. The recommendations provided in the guidance document do not replace the importance of clinical judgment tailored to the unique circumstances of an individual patient.
Diagnostic evaluation of MIS‐C
Maintaining a broad differential diagnosis
Multiple case definitions of MIS‐C have been proposed (4, 5, 6), some of which are broader than others (Table 1). Common clinical features of MIS‐C include fever, mucocutaneous findings (rash, conjunctivitis, edema of the hands/feet, red/cracked lips, and strawberry tongue), myocardial dysfunction, cardiac conduction abnormalities, shock, gastrointestinal symptoms, and lymphadenopathy (2, 7, 8, 9, 10, 13, 14, 15, 16, 17, 18). There are also increasing reports of neurologic involvement, manifesting as severe headache, altered mental status, cranial nerve palsies, or meningismus, in select patients (8, 9, 10, 13, 14). These findings are nonspecific and can also occur in other types of infections and in non–infection‐related conditions such as malignancy or inflammatory conditions. Therefore, it is imperative that a diagnostic evaluation for MIS‐C include investigation for other possible causes, as would be deemed appropriate by the treating provider. MIS‐C is temporally associated with SARS–CoV‐2 infections, and clusters of cases have been reported in geographic areas with dense COVID‐19 disease burden, typically being found to emerge within 2–6 weeks after the peak incidence of acute, infectious COVID‐19 (7, 13, 14, 17). Thus, the prevalence and chronology of SARS–CoV‐2 infection in a given location, which may change over time, should also inform the diagnostic evaluation.
The incidence of MIS‐C is unknown; however, it appears to be a rare complication of SARS–CoV‐2 infection, with some estimates indicating that MIS‐C occurs in 2 of 200,000 individuals <21 years old (19). The relative rarity of MIS‐C should also be considered in the diagnostic approach.
Tier 1 screening
Based on our review of the literature and diagnostic algorithms that are publicly available, the task force chose to cast a broad net with respect to the evaluation of patients with possible MIS‐C, while simultaneously balancing the need to reduce indiscriminate overtesting and to prevent unnecessary use of resources in the treatment of pediatric patients who have unrelated causes of fever (2, 7, 8, 10, 13, 14, 15, 16, 20, 21). To date, there are no clear data indicating the pretest positive predictive or negative predictive probabilities for each clinical symptom or laboratory value in diagnosing MIS‐C. It should be noted that due to the paucity of data, our recommendations reflect a multidisciplinary consensus that is likely to be revised as novel data become available.
Children with fever and epidemiologic link to SARS–CoV‐2 and whose clinical symptoms are suggestive of SARS–CoV‐2 infection should be considered “under investigation” for MIS‐C, while alternative diagnoses that could explain the patient’s clinical presentation are also explored (Figure 1). A tiered diagnostic approach is recommended in patients without life‐threatening manifestations; this includes performing an initial screening evaluation (tier 1), and thereafter proceeding to a complete diagnostic evaluation (tier 2) only in patients with laboratory results from the tier 1 screening that are concerning. Tier 1 consists of laboratory studies that are easily obtained at most clinical facilities (complete blood cell count with manual differential, complete metabolic panel, erythrocyte sedimentation rate [ESR], CRP measurement, and testing for SARS–CoV‐2 by polymerase chain reaction [PCR] or serology). The overwhelming majority of MIS‐C cases reported in the literature display elevations in inflammation markers, particularly the CRP level as values higher than 10 mg/dl or even 20 mg/dl are common (2, 7, 8, 9, 13, 14, 17). Thus, to enter the second stage of testing, children should have elevated ESR and/or CRP and at least 1 other suggestive laboratory feature: lymphopenia, neutrophilia, thrombocytopenia, hyponatremia, or hypoalbuminemia (2, 7, 8, 9, 13, 14, 17).
Tier 2 evaluation
Tier 2 encompasses more complex testing that typically requires additional time to complete. Reports in the literature and unpublished observations by members of the panel both note that some patients with MIS‐C can decompensate rapidly; however, the risk factors that predispose patients to such severe and progressive illness have not been identified (10, 13). Accordingly, children with abnormal vital signs, concerning physical examination findings, significantly elevated levels of inflammation markers, or signs of cardiac involvement will need to be admitted to the hospital for supportive care, during which time tier 2 testing should be completed.
The panel also noted that MIS‐C appears to be a continuum of disease that encompasses milder phenotypes, none of which have been fully described in the published literature. Some patients present with fever, rash, and systemic inflammation and no other organ damage. While these children require close monitoring, they do not always need to be hospitalized. Thus, in some cases, well‐appearing children with reassuring vital signs and physical examination findings may be suitable for outpatient diagnostic evaluations, as long as close clinical follow‐up can be ensured.
Prominent cardiac involvement has been reported in a proportion of MIS‐C patients in every retrospective cohort study published to date (2, 7, 8, 9, 13, 14, 17). These include left ventricular (LV) dysfunction, coronary artery dilation or coronary artery aneurysm (CAA), and electrical conduction abnormalities. Valvular dysfunction and pericardial effusion are less frequently described. Among the initial descriptions of MIS‐C, LV dysfunction was present in 20–55% of cases, and coronary artery dilation or CAA in ~20% (2, 7, 13). Although the early reports may overestimate the incidence of cardiac features—as they likely represent the most severe component of the MIS‐C spectrum—these numbers nonetheless highlight the significant risk of cardiac involvement in MIS‐C.
For these reasons, electrocardiogram (EKG) and echocardiogram are key testing components of the full diagnostic evaluation. The echocardiogram should include quantification of LV size and systolic function using end‐diastolic volume (and z‐score) and ejection fraction (EF) (22, 23). Detailed evaluation of all coronary artery segments and normalization of coronary artery measurements to body surface area using z‐scores is necessary (23, 24). Cardiac laboratory values at the time of diagnosis, specifically levels of troponin T and B‐type natriuretic peptide (BNP)/N‐terminal proBNP (NT‐proBNP), may help identify patients with cardiac sequelae resulting from MIS‐C (7, 8, 9, 13, 14, 17). In particular, highly elevated BNP/NT‐proBNP levels may be helpful in distinguishing between MIS‐C patients with and those without LV dysfunction; however, mild and transient elevations in these laboratory parameters are likely nonspecific and not necessarily indicative of cardiac involvement (14, 25, 26). BNP, in particular, is an acute‐phase reactant, and may therefore be elevated in inflammatory conditions without cardiac involvement (25).
Tier 2 testing should also include further assessment for systemic inflammation. In addition to the ESR and CRP level, MIS‐C patients typically demonstrate other markers of inflammation, including high d‐dimer levels, moderately elevated ferritin levels (often ranging from 500 to 2,000 ng/dl), profoundly increased procalcitonin levels in the absence of bacterial infection, and increased lactate dehydrogenase (LDH) levels (8, 9, 10, 13, 14, 17). Cytokine panels, when available, can assist in the diagnostic evaluation, since levels of interleukin‐6 (IL‐6), tumor necrosis factor (TNF), or IL‐10 are often increased; however, cytokine levels measured in this manner should not dictate treatment choices and are not required to determine treatment plans (8, 9, 13). In addition, serologic tests can be used. Serologic testing for SARS–CoV‐2 has yielded positive results in a greater proportion of MIS‐C patients (80–90%) as compared to the results of PCR testing in MIS‐C patients (20–40%), and therefore both serology and PCR should be used to evaluate the epidemiologic link to the infection (7, 8, 9, 13, 17).
Comparing and contrasting features of MIS‐C and KD
In an early sentinel report from Bergamo, the Italian epicenter of the COVID‐19 pandemic, KD and KD‐like illnesses were observed at a rate 30 times higher than that observed in the pre–pandemic era (7). Since this observation, the clinical symptoms of MIS‐C have frequently been compared to those of KD, given their similarity in profiles, including fevers, mucocutaneous features, and cardiac sequelae (2, 7, 8, 9, 10, 13, 14, 15, 16, 17, 22, 27). However, a closer examination of the literature shows that only about one‐quarter to one‐half of reported MIS‐C patients meet the full diagnostic criteria for KD (7, 8, 9, 13, 14).
Several epidemiologic, clinical, and laboratory features of MIS‐C that differ from KD unrelated to SARS–CoV‐2 are worthy of mention. First, whereas the incidence of KD is highest in Japan, MIS‐C appears to be frequent in patients of African descent and possibly those of Hispanic descent (2, 8, 9, 14, 28). It is unclear whether this racial/ethnic distribution of MIS‐C is due to genetic or biologic factors or whether it might be attributed to socioeconomic status and risk of SARS–CoV‐2 exposure.
Second, the age distribution of MIS‐C is broad, with reports of MIS‐C found in children ranging from age 3 months to age 17 years (2, 7, 8, 9, 10, 13, 14, 17). In contrast, the majority of children with KD are diagnosed before age 5 years (7, 14, 29, 30).
Third, as discussed above, the clinical presentation of LV dysfunction and shock that is characteristic of patients with MIS‐C is considerably less common in patients with KD, with fewer than 10% of KD patients presenting with KD shock syndrome (31). Close to one‐quarter of untreated KD patients develop CAAs (32). Coronary artery dilation or CAAs have been documented in up to 20% of MIS‐C patients, and at least 3 patients have developed giant CAAs (2, 7, 13, 14, 17). It is unknown if the incidence or progression of CAAs differ between MIS‐C and KD patients. Importantly, it is clear that MIS‐C patients without KD symptoms can develop CAAs, highlighting the need for cardiac evaluation in all patients with MIS‐C regardless of phenotypic features, and providing support for the treatment rationale discussed below (14).
Fourth, although gastrointestinal and neurologic symptoms are reported to occur in KD patients, the panel agreed that these findings are more frequently encountered in the MIS‐C patient population.
Finally, the laboratory parameters that have been found to differ between retrospective cohorts of MIS‐C patients and historical cohorts of KD patients include a lower platelet count, lower absolute lymphocyte count, and higher CRP levels in MIS‐C patients (7, 14).
Cardiac management of MIS‐C
Children with MIS‐C will need close clinical follow‐up with cardiology. Extrapolating data from KD, another condition that can be complicated by CAA, the panel recommended that repeat echocardiograms be obtained from all children with MIS‐C at a minimum of 7–14 days and then 4–6 weeks after the initial presentation (22). For those patients with cardiac involvement noted during the acute phase of illness, another echocardiogram at 1 year after MIS‐C diagnosis could be considered. Children with LV dysfunction and CAAs will require more frequent echocardiograms.
Although LV function improves rapidly in most MIS‐C patients, the long‐term complications of myocardial inflammation in this syndrome are not known, and may include myocardial fibrosis and scarring, features that have been seen in other forms of pediatric myocarditis (9, 13, 33). Cardiac magnetic resonance imaging at 2–6 months post–acute illness in those patients who had moderate‐to‐severe LV dysfunction will allow for evaluation of fibrosis and scarring. Electrical conduction abnormalities are increasingly noted in MIS‐C patients and may develop after the initial presentation; therefore, EKG should be performed at a minimum of every 48 hours in patients who are hospitalized and at each follow‐up visit (8, 9, 13, 14). If conduction abnormalities are present, the patient should be placed on telemetry while in the hospital and may need Holter monitoring at clinical follow‐up.
Treatment of MIS‐C
Immunomodulatory treatment in MIS‐C
Goals of treatment in the MIS‐C population are to stabilize patients with life‐threatening manifestations such as shock, and to prevent long‐term sequelae, which may include CAAs, myocardial fibrosis/scarring, and fixed cardiac conduction abnormalities. There is no available literature that directly compares therapeutic approaches in MIS‐C. Recommendations approved by the task force are derived from experience in managing MIS‐C and from higher quality data in other pediatric conditions with similar features. Initiation of treatment will often depend on the severity of the patient’s presentation. There was consensus among the panelists that patients under investigation for MIS‐C without life‐threatening manifestations should undergo a diagnostic evaluation for MIS‐C as well as for other possible infections and non–infection‐related conditions before immunomodulatory treatment is initiated. This is to prevent the use of therapies that could be potentially harmful in patients who do not have MIS‐C.
Further, a subgroup of patients with MIS‐C will develop progressive cardiac involvement rapidly; therefore, hospital admission and sequential monitoring of inflammation markers, BNP/NT‐proBNP levels, and troponin T levels without instituting treatment can sometimes inform the diagnostic evaluation (10, 13). Children with a life‐threatening presentation such as shock will clearly require supportive care and may benefit from early initiation of immunomodulatory treatment, sometimes before a full diagnostic evaluation can be completed. In such cases, ongoing diagnostic evaluation should be pursued with a multidisciplinary team, in parallel with treatment.
Finally, the current recommendations address the treatment of MIS‐C that is uncomplicated by macrophage activation syndrome (MAS). Importantly, there is a subgroup of patients with MIS‐C who may also develop overt MAS. The treatment of those patients may need to deviate from the recommendations presented herein (7).
A stepwise approach to immunomodulatory treatment in MIS‐C is recommended, with intravenous immunoglobulin (IVIG) and/or glucocorticoids considered as first‐tier agents. Both IVIG and glucocorticoids, either alone or in combination, are the most commonly used immunomodulatory medications reported to date in MIS‐C patients (2, 7, 8, 9, 10, 13, 14, 15, 17). There are insufficient data available to compare the efficacy of IVIG and glucocorticoids in MIS‐C or to determine whether these treatments should be provided individually or as dual therapy. Accordingly, the task force recommended that IVIG and glucocorticoids could be used alone or in combination to treat MIS‐C.
Evidence for the use of IVIG and glucocorticoids to treat MIS‐C is also based on the use of these treatments in patients with KD and fulminant myocarditis, both of which are conditions that resemble MIS‐C in some aspects. IVIG at a dose of 2 gm/kg prevents CAAs in KD, while the benefit of IVIG in myocarditis remains unclear; however, case reports of successful use of IVIG in coronavirus‐associated myocarditis have been published (22, 32, 34, 35, 36, 37, 38, 39, 40). Before IVIG is given, cardiac function and fluid status should be assessed. If these parameters are abnormal, the rate of IVIG infusion may be slowed or treatment delayed until cardiac function is restored.
Glucocorticoids reduce the rates of CAA development when used in KD patients at high risk of being resistant to IVIG treatment (41, 42). Verdoni and colleagues (7) reported a high rate of IVIG resistance in KD patients who presented during the COVID‐19 pandemic, as compared to that in a historical cohort of KD patients, which may suggest a role for glucocorticoids in MIS‐C. Panelists reported that low‐to‐moderate doses (1–2 mg/kg/day) of glucocorticoids were sufficient to treat many MIS‐C patients. Some children with shock requiring multiple inotropes and/or vasopressors have responded best to high doses of intravenous glucocorticoids. High‐dose intravenous glucocorticoids have been used safely in patients with KD and have been used successfully in small numbers of patients with MIS‐C and shock (10, 43, 44, 45). Adjunctive glucocorticoids have also been shown to shorten the duration of shock in patients with sepsis (46). Panelists agreed that MIS‐C patients who are treated with steroids, regardless of the dose, often require a 2–3‐week taper to avoid rebound inflammation.
Anakinra is a recommended treatment for MIS‐C patients who are refractory to IVIG and/or glucocorticoids. This recommendation is based on the relative safety of anakinra in pediatric patients with hyperinflammatory syndromes and active infection, the experience of panel members in using anakinra to treat MIS‐C patients, and the findings in a small number of MIS‐C patients reported in the literature (13, 14, 17, 47, 48, 49, 50). Similarly, anakinra has been used successfully in a small number of patients with IVIG‐resistant KD (51, 52, 53).
Treatment with immunomodulatory agents may not always be required in MIS‐C. Whittaker et al reported that 22% of MIS‐C patients recovered with supportive care (14). In close coordination with specialists who have expertise in MIS‐C, some patients with mild symptoms may require only close monitoring, without the use of IVIG and/or glucocorticoids. The panel noted uncertainty around the empiric use of IVIG in this setting to prevent CAAs.
Antiplatelet and anticoagulation therapy in MIS‐C
Published reports of patients with MIS‐C describe marked abnormalities in the coagulation cascade, including prominent elevations in d‐dimer and fibrinogen levels, a variable effect on the platelet count, and a high clot strength as determined by thromboelastography (2, 7, 8, 9, 13, 14). An increased risk of thrombosis is a concern in patients with MIS‐C, based on the data outlined above as well as the hypercoagulability noted in adults with COVID‐19 (54, 55, 56, 57). A recent report also described a small number of MIS‐C patients with deep vein thrombosis or pulmonary embolism, but the overall risk of thrombosis in this population is not known (58). Therefore, these recommendations are based on experience in analogous pediatric conditions, specifically KD and myocarditis, and the emerging data from adults with COVID‐19.
Antiplatelet agents such as aspirin are recommended in patients with KD, because of the presence of platelet activation, thrombocytosis, altered flow dynamics in the affected coronary arteries, and endothelial damage characteristic of this disease (22). Accordingly, low‐dose aspirin (3–5 mg/kg/day up to 81 mg once daily) is recommended in all MIS‐C patients with KD features, CAAs, and thrombocytosis. Anti‐acid treatments should be used to prevent gastrointestinal complications in MIS‐C patients who are taking steroids and aspirin. The risk of coronary artery thrombosis is directly related to the size of the CAA, with the probability of thrombosis occurring in the coronary arteries being exponentially increased with artery dimensions above a z‐score of 10.0 (22, 59, 60). Thus, anticoagulation with enoxaparin (factor Xa level 0.5–1.0) or warfarin in MIS‐C patients with a coronary artery z‐score of greater than 10.0 is advised. Patients with more‐than‐mild LV dysfunction are at risk for intracardiac thrombosis (61, 62). Given the lack of clarity about the exact risk of hypercoagulability in MIS‐C, the task force recommended considering anticoagulation therapy for MIS‐C patients who have moderate or severe LV dysfunction (EF <35%).
Hyperinflammation in children with COVID‐19
Severe COVID‐19 in children
The task force also addressed immunomodulatory treatment in children with severe COVID‐19, a condition that panelists (given the current information) deemed to be readily distinguishable from MIS‐C. The vast majority of children with COVID‐19 have mild symptoms in the acute, infectious phase of the disease, but a small minority of children become severely ill (63, 64, 65, 66, 67, 68). MIS‐C patients are often previously healthy and will present with symptoms of fever, inflammation, and multiorgan dysfunction that manifests late in the course of SARS–CoV‐2 infection (most are positive for SARS–CoV‐2 IgG antibodies). In contrast, children who develop severe COVID‐19 during their initial infection often have a complex medical history (64, 65, 66, 67). Shekerdemian and colleagues reported that 40% of patients admitted to the intensive care unit (ICU) for COVID‐19 had developmental delay or a genetic anomaly, or were dependent on technological support (e.g., tracheostomy) for survival (65). There is no definitive evidence suggesting that children with rheumatic diseases treated with immunosuppression are also at risk of developing poor outcomes from COVID‐19. Shekerdemian et al also observed that 23% of pediatric patients with COVID‐19 who were admitted to the ICU were either immunosuppressed or had cancer, but they did not specify whether any of these patients had a rheumatic condition (65). Extrapolating from adults with inflammatory bowel disease and rheumatic conditions, glucocorticoid use may be associated with worse outcomes in COVID‐19, while treatment with TNF inhibitors may actually be protective against severe COVID‐19 (69, 70). In addition, in cohorts of pediatric patients receiving immunosuppressive medications, an increased risk of severe COVID‐19 has not been identified (71, 72, 73).
Immunomodulatory treatment in children with hyperinflammation and COVID‐19
Data to guide the treatment of pediatric patients with severe illness during the early phase of SARS–CoV‐2 infection are limited. In adults, certain laboratory parameters associated with an exaggerated inflammatory response (hyperinflammation) portend worse outcomes in COVID‐19, including elevated levels of LDH, d‐dimer, IL‐6, IL‐2 receptor, CRP, and ferritin and a decreased lymphocyte count, albumin level, and platelet count (74, 75, 76, 77). In at least one case series of pediatric patients with COVID‐19, increased CRP levels, elevated procalcitonin levels, and decreased platelet counts were significantly more common in children requiring ICU admission as compared to those cared for on the floor; however, further studies are needed to identify laboratory parameters that would be predictive of poor outcomes in the pediatric population (78). These results suggest that patients with COVID‐19 and hyperinflammation have poor outcomes, and that the host immune response to SARS–CoV‐2 may contribute to disease severity. The panel agreed that children with severe COVID‐19, manifesting as acute respiratory distress syndrome (ARDS), shock, or signs of hyperinflammation as measured by the laboratory parameters discussed above, should be considered for immunomodulatory therapy in addition to supportive care and antiviral medications.
Glucocorticoids are a readily available and inexpensive option for immunomodulation; however, their use in adults with COVID‐19 is controversial. Prior experience with adjunctive glucocorticoid therapy in ARDS unrelated to COVID‐19 has been equivocal (79, 80, 81). Observational studies evaluating glucocorticoid treatment in other respiratory viral infections, such as influenza, suggest that it is associated with increased mortality; however, these studies are difficult to interpret, due to confounding by indication (82, 83). There are concerns that glucocorticoids given at high doses or early in the course of infection delay viral clearance (84, 85). Glucocorticoid use in critically ill patients is also associated with increased neuropathy and myopathy (86). In SARS–CoV‐2 infections, there is conflicting evidence about the impact of glucocorticoids on viral clearance (87, 88). A small number of cohort studies suggest a benefit in patients with severe COVID‐19 pneumonia who are treated with glucocorticoids (75, 89). Importantly, preliminary results from a large randomized controlled trial (the RECOVERY trial) indicated that low‐to‐moderate doses of dexamethasone significantly reduced mortality in COVID‐19 patients requiring mechanical ventilation (90, 91); however, those results were reported after the task force had already voted on this guidance. Based on these studies that suggest that patients with severe COVID‐19 pneumonia may benefit from immunomodulation with glucocorticoids, the task force achieved moderate consensus that glucocorticoid treatment could be considered in pediatric patients with severe COVID‐19 and signs of hyperinflammation.
Targeted neutralization of inflammatory cytokines is another approach that can be employed to reduce pathologic inflammation in COVID‐19. In contrast to glucocorticoids, the panel was able to achieve high consensus with regard to the statement that anakinra (recombinant human IL‐1 receptor antagonist) can be used to treat pediatric patients with COVID‐19 and hyperinflammation. Anakinra appears to be safe in patients with severe infections, based on the results of a randomized controlled trial in patients with sepsis in whom there was no difference in the frequency of adverse events in the anakinra group compared to the placebo group (49). Furthermore, a re‐analysis of the data from this trial in sepsis showed increased survival in patients treated with anakinra who also had excessive inflammation, manifested as hepatobiliary dysfunction and coagulopathy, which is commonly seen in COVID‐19 (92). IL‐1 blockade has also been used safely in children with inflammatory syndromes, including those with systemic juvenile idiopathic arthritis and those with MAS (47, 48, 50). In COVID‐19, observations from case series provide evidence of the safety and efficacy of anakinra in patients with elevated inflammation marker levels and moderate‐to‐severe disease; however, most of those studies do not have a comparison group (93, 94, 95, 96, 97). In one retrospective cohort of patients with COVID‐19–related moderate‐to‐severe ARDS, treatment with anakinra in addition to usual care significantly reduced mortality when compared to patients treated at the same center a week prior (98). The patients in this cohort received high‐dose anakinra (10 mg/kg/day) and were not yet mechanically ventilated, suggesting that treatment administered before intubation is beneficial.
Given the association between increased IL‐6 levels and negative outcomes in COVID‐19, IL‐6 neutralization with tocilizumab may be an appealing potential therapy (74, 75, 77). In some case series (reported without a comparison group), clinical improvement with tocilizumab treatment was demonstrated, while others have not shown any clinical improvement or have noted a high rate of bacterial and fungal infections after treatment with tocilizumab (99, 100, 101, 102). Cohort studies with comparison groups have demonstrated conflicting results with one study reporting safety and efficacy of tocilizumab while another found no improvement in clinical outcomes (103, 104). In a study by Capra and colleagues, treatment with tocilizumab showed some benefit in COVID‐19 patients who had not yet been mechanically ventilated (103).
The task force agreed that the features of severe COVID‐19 were sufficiently similar between the described adult cases and pediatric cases to cautiously extrapolate from adult studies. Overall, the consensus among panelists was that immunomodulatory treatment should be considered in pediatric patients with hyperinflammation and severe symptoms in the acute phase of illness. While the data are still too sparse to make definitive recommendations based on high‐quality evidence, the panel favored the use of anakinra in this setting.
DISCUSSION
There has been an evolution in our understanding of SARS–CoV‐2 infections in children. Initially, it was believed that COVID‐19 was almost entirely benign and of little consequence in the pediatric population. There has been a sudden reversal from this stance in the context of the emergence of MIS‐C cases. The goals of this ACR task force were to synthesize available data and expert opinion to provide a resource for clinicians on the frontlines caring for children with inflammatory syndromes due to recent or concurrent infections with SARS–CoV‐2.
Recognizing the need to address the unique challenges facing children with inflammatory conditions triggered by SARS–CoV‐2 infections, the ACR convened the task force to provide guidance in a short period of time. To accomplish this charge, a multidisciplinary panel was assembled that included clinicians from North America with expertise encompassing pediatric rheumatology, cardiology, infectious disease, and critical care. Well‐established methodology in the form of the RAND/UCLA Appropriateness Method was used to achieve consensus. There are limitations inherent in our approach. Given the need for expedited decision‐making, we were unable to provide guidance on all topics of interest. In particular, the task force focused its efforts on providing diagnostic and treatment recommendations for MIS‐C instead of developing a new case definition for this condition. This choice was made because several case definitions for MIS‐C exist, and the data needed to develop a sensitive and specific set of criteria are not yet available. The guidance provided in this document is targeted to clinicians with access to complex diagnostic tools and biologic treatments. Thus, some of the recommendations are not practical in less resource‐rich settings. The task force may consider providing additional recommendations for these settings in subsequent versions of this guidance. In addition, the work product of the task force is considered guidance instead of formal treatment guidelines that must adhere to the strict methodology endorsed by the ACR.
The guidance provided in this document is supported by reports from the scientific literature and recommendations from public health institutions. Yet, the available data remain restricted to low‐quality evidence that often must be extrapolated from the experience in adults. This approach is particularly problematic when confronting clinical questions regarding MIS‐C, which, to date, has been reported primarily in children. This unique manifestation of COVID‐19 in children and adolescents highlights the need to prioritize and fund rigorous research in the pediatric population. For now, our understanding of pediatric SARS–CoV‐2 infections is rudimentary and will continue to change as higher quality evidence becomes available. Thus, the recommendations contained in this document should be interpreted in the setting of this shifting landscape and will be modified prospectively as our understanding of COVID‐19 improves. For these reasons, this guidance does not replace the critical role of clinical judgment that is essential to address the unique needs of individual patients.
As the SARS–CoV‐2 pandemic continues to unfold, the ACR will support clinicians caring for children with COVID‐19 by enabling this task force to continue the work of reviewing evidence and providing expert opinion through revised versions of this guidance document. It is the ultimate goal of both the ACR and the task force panelists to disseminate knowledge quickly in an effort to improve outcomes for children with SARS–CoV‐2 infections.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Henderson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Henderson, Friedman, Gorelik, Lapidus, Behrens, Ferris, Seo, Turner, Mehta.
Acquisition of data
Henderson, Canna, Gorelik, Lapidus, Bassiri, Behrens, Ferris, Kernan, Schulert, Seo, Tremoulet, Yeung, Karp, Mehta.
Analysis and interpretation of data
Henderson, Canna, Friedman, Gorelik, Lapidus, Bassiri, Kernan, Schulert, Seo, Son, Tremoulet, Yeung, Mudano, Karp, Mehta.
Supporting information
Table S1‐S4
Supported by the American College of Rheumatology.
Dr. Henderson has received consulting fees from Sobi (less than $10,000) and research support from the Childhood Arthritis and Rheumatology Research Alliance. Dr. Bassiri owns stock or stock options in CSL Behring. Dr. Schulert has received consulting fees from Novartis and Sobi (less than $10,000 each). Drs. Son and Mehta have received salary support from the Childhood Arthritis and Rheumatology Research Alliance. Dr. Yeung has received consulting fees from Novartis and Eli Lilly (less than $10,000 each). No other disclosures relevant to this article were reported.
References
- 1. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time [letter]. Lancet Infect Dis 2020;20:533–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riphagen S, Gomez X, Gonzalez‐Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID‐19 pandemic [letter]. Lancet 2020;395:1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Federation of Pediatric Intensive and Critical Care Societies . Statement to the media following the 2 May Pediatric Intensive Care‐COVID‐19 International Collaborative conference call. May 2020. URL: http://www.wfpiccs.org/wp‐content/uploads/2020/05/Media‐statement‐Final.pdf.
- 4. World Health Organization . Multisystem inflammatory syndrome in children and adolescents with COVID‐19. May 2020. URL: https://www.who.int/publications/i/item/multisystem‐inflammatory‐syndrome‐in‐children‐and‐adolescents‐with‐COVID‐19.
- 5. Royal College of Paediatrics and Child Health . Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID‐19. June 2020. URL: https://www.rcpch.ac.uk/resources/guidance‐paediatric‐multisystem‐inflammatory‐syndrome‐temporally‐associated‐COVID‐19-pims.
- 6. Centers for Disease Control and Prevention . Emergency preparedness and response: health alert network. May 2020. URL: https://emergency.cdc.gov/han/2020/han00432.asp.
- 7. Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: an observational cohort study. Lancet 2020;395:1771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki‐like multisystem inflammatory syndrome in children during the COVID‐19 pandemic in Paris, France: prospective observational study. BMJ 2020;369:m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, et al. Multisystem inflammatory syndrome related to COVID‐19 in previously healthy children and adolescents in New York City [letter]. JAMA 2020;234:294–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chiotos K, Bassiri H, Behrens EM, Blatz AM, Chang J, Diorio C, et al. Multisystem inflammatory syndrome in children during the COVID‐19 pandemic: a case series. J Pediatric Infect Dis Soc 2020;9:393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brook R. US Agency for Health Care Policy and Research Office of the Forum for Quality and Effectiveness in Health Care clinical practice guideline development: methodology perspectives. In: McCormick K, Siegel R, editors. The RAND/UCLA Appropriateness Method. Rockville (MD): Agency for Healthcare Research and Quality; 1994: p. 59–70. [Google Scholar]
- 12. American College of Rheumatology . Clinical guidance for pediatric patients with multisystem inflammatory syndrome in children (MIS‐C) associated with SARS–CoV‐2 and hyperinflammation in COVID‐19. June 2020. URL: https://www.rheumatology.org/Portals/0/Files/ACR‐COVID‐19-Clinical‐Guidance‐Summary‐MIS‐C‐Hyperinflammation.pdf. [DOI] [PMC free article] [PubMed]
- 13. Belhadjer Z, Meot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS‐C) in the context of global SARS‐CoV‐2 pandemic. Circulation 2020;142:429–436. [DOI] [PubMed] [Google Scholar]
- 14. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2. JAMA 2020;324:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leon MP, Redzepi A, McGrath E, Abdel‐Haq N, Shawaqfeh A, Sethuraman U, et al. COVID‐19 associated pediatric multi‐system inflammatory syndrome. J Pediatric Infect Dis Soc 2020;9:407–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dallan C, Romano F, Siebert J, Politi S, Lacroix L, Sahyoun C. Septic shock presentation in adolescents with COVID‐19 [letter]. Lancet Child Adolesc Health 2020;4:e21–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Capone CA, Subramony A, Sweberg T, Schneider J, Shah S, Rubin L, et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory disease of childhood (MIS‐C) associated with SARS‐CoV‐2 infection. J Pediatr 2020;224:141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Licciardi F, Pruccoli G, Denina M, Parodi E, Taglietto M, Rosati S, et al. SARS‐CoV‐2-induced Kawasaki‐like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics 2020;146:e20201711. [DOI] [PubMed] [Google Scholar]
- 19. Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020;383:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hennon TR, Abdul‐Aziz R, Alibrahim OS, McGreevy MB, Prout AJ, Schaefer BA, et al. COVID‐19 associated multisystem inflammatory syndrome in children (MIS‐C) guidelines: a western New York approach. Prog Pediatr Cardiol 2020. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Children's Hospital of Philadelphia . Emergency Department, ICU, and inpatient clinical pathway for evaluation of possible multisystem inflammatory syndrome (MIS‐C). May 2020. URL: https://www.chop.edu/clinical‐pathway/multisystem‐inflammatory‐syndrome‐MIS‐c‐clinical‐pathway.
- 22. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long‐term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association [review]. Circulation 2017;135:e927–99. [DOI] [PubMed] [Google Scholar]
- 23. Colan SD. The why and how of Z scores [editorial]. J Am Soc Echocardiogr 2013;26:38–40. [DOI] [PubMed] [Google Scholar]
- 24. Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 2010;23:465–95. [DOI] [PubMed] [Google Scholar]
- 25. Yetkin O, Hacievliyagil SS, Gunen H. Assessment of B‐type natriuretic peptide in patients with pneumonia. Int J Clin Pract 2008;62:488–91. [DOI] [PubMed] [Google Scholar]
- 26. Melendez E, Whitney JE, Norton JS, Silverman M, Monuteaux MC, Bachur RG. A pilot study of the association of amino‐terminal pro‐B‐type natriuretic peptide and severity of illness in pediatric septic shock. Pediat Crit Care Med 2019;20:e55–60. [DOI] [PubMed] [Google Scholar]
- 27. Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics 1974;54:271–6. [PubMed] [Google Scholar]
- 28. Makino N, Nakamura Y, Yashiro M, Kosami K, Matsubara Y, Ae R, et al. Nationwide epidemiologic survey of Kawasaki disease in Japan, 2015–2016. Pediatr Int 2019;61:397–403. [DOI] [PubMed] [Google Scholar]
- 29. Holman RC, Curns AT, Belay ED, Steiner CA, Schonberger LB. Kawasaki syndrome hospitalizations in the United States, 1997 and 2000. Pediatrics 2003;112:495–501. [DOI] [PubMed] [Google Scholar]
- 30. Son MB, Gauvreau K, Ma L, Baker AL, Sundel RP, Fulton DR, et al. Treatment of Kawasaki disease: analysis of 27 US pediatric hospitals from 2001 to 2006. Pediatrics 2009;124:1–8. [DOI] [PubMed] [Google Scholar]
- 31. Kanegaye JT, Wilder MS, Molkara D, Frazer JR, Pancheri J, Tremoulet AH, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics 2009;123:e783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, et al. The treatment of Kawasaki syndrome with intravenous γ globulin. N Engl J Med 1986;315:341–7. [DOI] [PubMed] [Google Scholar]
- 33. Banka P, Robinson JD, Uppu SC, Harris MA, Hasbani K, Lai WW, et al. Cardiovascular magnetic resonance techniques and findings in children with myocarditis: a multicenter retrospective study. J Cardiovasc Magn Reson 2015;17:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Furusho K, Kamiya T, Nakano H, Kiyosawa N, Shinomiya K, Hayashidera T, et al. High‐dose intravenous γ globulin for Kawasaki disease. Lancet 1984;2:1055–8. [DOI] [PubMed] [Google Scholar]
- 35. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long‐term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics 2004;114:1708–33. [DOI] [PubMed] [Google Scholar]
- 36. Kociol RD, Cooper LT, Fang JC, Moslehi JJ, Pang PS, Sabe MA, et al. Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation 2020;141:e69–92. [DOI] [PubMed] [Google Scholar]
- 37. Dennert R, Velthuis S, Schalla S, Eurlings L, van Suylen RJ, van Paassen P, et al. Intravenous immunoglobulin therapy for patients with idiopathic cardiomyopathy and endomyocardial biopsy‐proven high PVB19 viral load. Antivir Ther 2010;15:193–201. [DOI] [PubMed] [Google Scholar]
- 38. Goland S, Czer LS, Siegel RJ, Tabak S, Jordan S, Luthringer D, et al. Intravenous immunoglobulin treatment for acute fulminant inflammatory cardiomyopathy: series of six patients and review of literature. Can J Cardiol 2008;24:571–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yen CY, Hung MC, Wong YC, Chang CY, Lai CC, Wu KG. Role of intravenous immunoglobulin therapy in the survival rate of pediatric patients with acute myocarditis: a systematic review and meta‐analysis. Sci Rep 2019;9:10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. E‐pub ahead of print. [DOI] [PMC free article] [PubMed]
- 41. Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, Arakawa H, et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open‐label, blinded‐endpoints trial. Lancet 2012;379:1613–20. [DOI] [PubMed] [Google Scholar]
- 42. Wardle AJ, Connolly GM, Seager MJ, Tulloh RM. Corticosteroids for the treatment of Kawasaki disease in children [review]. Cochrane Database Syst Rev 2017;1:CD011188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Newburger JW, Sleeper LA, McCrindle BW, Minich LL, Gersony W, Vetter VL, et al. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med 2007;356:663–75. [DOI] [PubMed] [Google Scholar]
- 44. Inoue Y, Okada Y, Shinohara M, Kobayashi T, Kobayashi T, Tomomasa T, et al. A multicenter prospective randomized trial of corticosteroids in primary therapy for Kawasaki disease: clinical course and coronary artery outcome. J Pediatr 2006;149:336–41. [DOI] [PubMed] [Google Scholar]
- 45. Ogata S, Ogihara Y, Honda T, Kon S, Akiyama K, Ishii M. Corticosteroid pulse combination therapy for refractory Kawasaki disease: a randomized trial. Pediatrics 2012;129:e17–23. [DOI] [PubMed] [Google Scholar]
- 46. Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med 2018;378:797–808. [DOI] [PubMed] [Google Scholar]
- 47. Quartier P, Allantaz F, Cimaz R, Pillet P, Messiaen C, Bardin C, et al. A multicentre, randomised, double‐blind, placebo‐controlled trial with the interleukin‐1 receptor antagonist anakinra in patients with systemic‐onset juvenile idiopathic arthritis (ANAJIS trial). Ann Rheum Dis 2011;70:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ter Haar NM, van Dijkhuizen EH, Swart JF, van Royen‐Kerkhof A, el Idrissi A, Leek AP, et al. Treatment to target using recombinant interleukin‐1 receptor antagonist as first‐line monotherapy in new‐onset systemic juvenile idiopathic arthritis: results from a five‐year follow‐up study. Arthritis Rheumatol 2019;71:1163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fisher Jr CJ, Dhainaut JF, Opal SM, Pribble JP, Balk RA, Slotman GJ, et al, for the Phase III rhIL‐1ra Sepsis Syndrome Study Group . Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome: results from a randomized, double‐blind, placebo‐controlled trial. JAMA 1994;271:1836–43. [PubMed] [Google Scholar]
- 50. Eloseily EM, Weiser P, Crayne CB, Haines H, Mannion ML, Stoll ML, et al. Benefit of anakinra in treating pediatric secondary hemophagocytic lymphohistiocytosis. Arthritis Rheumatol 2020;72:326–34. [DOI] [PubMed] [Google Scholar]
- 51. Kone‐Paut I, Cimaz R, Herberg J, Bates O, Carbasse A, Saulnier JP, et al. The use of interleukin 1 receptor antagonist (anakinra) in Kawasaki disease: a retrospective cases series. Autoimmun Rev 2018;17:768–74. [DOI] [PubMed] [Google Scholar]
- 52. Lind‐Holst M, Hartling UB, Christensen AE. High‐dose anakinra as treatment for macrophage activation syndrome caused by refractory Kawasaki disease in an infant. BMJ Case Rep 2019;12:e229708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guillaume MP, Reumaux H, Dubos F. Usefulness and safety of anakinra in refractory Kawasaki disease complicated by coronary artery aneurysm. Cardiol Young 2018;28:739–42. [DOI] [PubMed] [Google Scholar]
- 54. Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, et al. Pulmonary post‐mortem findings in a series of COVID‐19 cases from northern Italy: a two‐centre descriptive study. Lancet Infect Dis 2020;20:P1135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID‐19. J Thromb Haemost 2020;18:1559–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Klok FA, Kruip M, van der Meer NJ, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thrombosis Res 2020;191:145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhou B, She J, Wang Y, Ma X. Venous thrombosis and arteriosclerosis obliterans of lower extremities in a very severe patient with 2019 novel coronavirus disease: a case report. J Thromb Thrombolysis 2020;50:229–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MB, et al. Multisystem inflammatory syndrome in US children and adolescents. N Engl J Med 2020;50:229–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Friedman KG, Gauvreau K, Hamaoka‐Okamoto A, Tang A, Berry E, Tremoulet AH, et al. Coronary artery aneurysms in Kawasaki disease: risk factors for progressive disease and adverse cardiac events in the US population. J Am Heart Assoc 2016;5:e003289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tsuda E, Tsujii N, Hayama Y. Stenotic lesions and the maximum diameter of coronary artery aneurysms in Kawasaki disease. J Pediatr 2018;194:165–70.e2. [DOI] [PubMed] [Google Scholar]
- 61. Chen K, Williams S, Chan AK, Mondal TK. Thrombosis and embolism in pediatric cardiomyopathy. Blood Coagul Fibrinolysis 2013;24:221–30. [DOI] [PubMed] [Google Scholar]
- 62. Giglia TM, Massicotte MP, Tweddell JS, Barst RJ, Bauman M, Erickson CC, et al. Prevention and treatment of thrombosis in pediatric and congenital heart disease: a scientific statement from the American Heart Association. Circulation 2013;128:2622–703. [DOI] [PubMed] [Google Scholar]
- 63. Parri N, Lenge M, Buonsenso D, for the Coronavirus Infection in Pediatric Emergency Departments Research Group . Children with COVID‐19 in pediatric emergency departments in Italy [letter]. N Engl J Med 2020;383:187–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS‐CoV‐2 infection in children [letter]. N Engl J Med 2020;382:1663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID‐19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr 2020;174:868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tagarro A, Epalza C, Santos M, Sanz‐Santaeufemia FJ, Otheo E, Moraleda C, et al. Screening and severity of coronavirus disease 2019 (COVID‐19) in children in Madrid, Spain [letter]. JAMA Pediatr 2020. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. DeBiasi RL, Song X, Delaney M, Bell M, Smith K, Pershad J, et al. Severe COVID‐19 in children and young adults in the Washington, DC metropolitan region. J Pediatr 2020;223:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID‐19 among children in China. Pediatrics 2020;145:e20200702. [DOI] [PubMed] [Google Scholar]
- 69. Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous‐Hunt M, Lewis JD, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID‐19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology 2020;159:P481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gianfrancesco M, Hyrich KL, Al‐Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID‐19 in people with rheumatic disease: data from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Ann Rheum Dis 2020;79:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Marlais M, Wlodkowski T, Vivarelli M, Pape L, Tonshoff B, Schaefer F, et al. The severity of COVID‐19 in children on immunosuppressive medication [letter]. Lancet Child Adolesc Health 2020;4:e17–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Turner D, Huang Y, Martin‐de‐Carpi J, Aloi M, Focht G, Kang B, et al. Coronavirus disease 2019 and paediatric inflammatory bowel diseases: global experience and provisional guidance (March 2020) from the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2020;70:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Michelena X, Borrell H, Lopez‐Corbeto M, Lopez‐Lasanta M, Moreno E, Pascual‐Pastor M, et al. Incidence of COVID‐19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease‐modifying antirheumatic drugs. Semin Arthritis Rheum 2020;50:564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Invest 2020;130:2620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chao JY, Derespina KR, Herold BC, Goldman DL, Aldrich M, Weingarten J, et al. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 (COVID‐19) at a tertiary care medical center in New York City. J Pediatr 2020;223:P14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Villar J, Ferrando C, Martinez D, Ambros A, Munoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med 2020;8:267–76. [DOI] [PubMed] [Google Scholar]
- 80. Seam N, Meduri GU, Wang H, Nylen ES, Sun J, Schultz MJ, et al. Effects of methylprednisolone infusion on markers of inflammation, coagulation, and angiogenesis in early acute respiratory distress syndrome. Crit Care Med 2012;40:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yehya N, Servaes S, Thomas NJ, Nadkarni VM, Srinivasan V. Corticosteroid exposure in pediatric acute respiratory distress syndrome. Intensive Care Med 2015;41:1658–66. [DOI] [PubMed] [Google Scholar]
- 82. Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta‐analysis. Crit Care 2019;23:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lansbury LE, Rodrigo C, Leonardi‐Bee J, Nguyen‐van‐Tam J, Lim WS. Corticosteroids as adjunctive therapy in the treatment of influenza: an updated Cochrane systematic review and meta‐analysis. Crit Care Med 2020;48:e98–106. [DOI] [PubMed] [Google Scholar]
- 84. Cao B, Gao H, Zhou B, Deng X, Hu C, Deng C, et al. Adjuvant corticosteroid treatment in adults with influenza A (H7N9) viral pneumonia. Crit Care Med 2016;44:e318–28. [DOI] [PubMed] [Google Scholar]
- 85. Lee N, Chan KC, Hui DS, Ng EK, Wu A, Chiu RW, et al. Effects of early corticosteroid treatment on plasma SARS‐associated Coronavirus RNA concentrations in adult patients. J Clin Virol 2004;31:304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yang T, Li Z, Jiang L, Xi X. Corticosteroid use and intensive care unit‐acquired weakness: a systematic review and meta‐analysis. Crit Care 2018;22:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ling Y, Xu SB, Lin YX, Tian D, Zhu ZQ, Dai FH, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fang X, Mei Q, Yang T, Li L, Wang Y, Tong F, et al. Low‐dose corticosteroid therapy does not delay viral clearance in patients with COVID‐19. J Infect 2020;81:147–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fadel R, Morrison AR, Vahia A, Smith ZR, Chaudhry Z, Bhargava P, et al. Early short course corticosteroids in hospitalized patients with COVID‐19. Clin Infect Dis 2020. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. University of Oxford . News and events. Low‐cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID‐19. June 2020. URL: http://www.ox.ac.uk/news/2020-06-16-low‐cost‐dexamethasone‐reduces‐death‐one‐third‐hospitalised‐patients‐severe.
- 91. Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, et al. Dexamethasone in hospitalized patients with COVID‐19: preliminary report. N Engl J Med 2020. E‐pub ahead of print. [Google Scholar]
- 92. Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, et al. Interleukin‐1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med 2016;44:275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Franzetti M, Pozzetti U, Carugati M, Pandolfo A, Molteni C, Faccioli P, et al. Interleukin‐1 receptor antagonist anakinra in association with remdesivir in severe coronavirus disease 2019: a case report. Int J Infect Dis 2020;97:215–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Filocamo G, Mangioni D, Tagliabue P, Aliberti S, Costantino G, Minoia F, et al. Use of anakinra in severe COVID‐19: a case report. Int J Infect Dis 2020;96:607–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Dimopoulos G, de Mast Q, Markou N, Theodorakopoulou M, Komnos A, Mouktaroudi M, et al. Favorable anakinra responses in severe COVID‐19 patients with secondary hemophagocytic lymphohistiocytosis. Cell Host Microbe 2020;28:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Aouba A, Baldolli A, Geffray L, Verdon R, Bergot E, Martin‐Silva N, et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID‐19 pneumonia: case series [letter]. Ann Rheum Dis 2020;79:1381–2. [DOI] [PubMed] [Google Scholar]
- 97. Pontali E, Volpi S, Antonucci G, Castellaneta M, Buzzi D, Tricerri F, et al. Safety and efficacy of early high‐dose IV anakinra in severe COVID‐19 lung disease [letter]. J Allergy Clin Immunol 2020;136:213–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cavalli G, de Luca G, Campochiaro C, Della‐Torre E, Ripa M, Canetti D, et al. Interleukin‐1 blockade with high‐dose anakinra in patients with COVID‐19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol 2020;2:e325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: a single center experience. J Med Virol 2020;92:814–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020;117:10970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Morena V, Milazzo L, Oreni L, Bestetti G, Fossali T, Bassoli C, et al. Off‐label use of tocilizumab for the treatment of SARS‐CoV‐2 pneumonia in Milan, Italy. Eur J Intern Med 2020;76:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, et al. Tocilizumab for the treatment of severe COVID‐19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy [review]. Autoimmun Rev 2020;19:102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Capra R, de Rossi N, Mattioli F, Romanelli G, Scarpazza C, Sormani MP, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID‐19 related pneumonia. Eur J Intern Med 2020;76:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Colaneri M, Bogliolo L, Valsecchi P, Sacchi P, Zuccaro V, Brandolino F, et al. Tocilizumab for treatment of severe COVID‐19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms 2020;8:695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S4


