SARS‐CoV‐2 coronavirus infection is characterised by a marked inflammatory state and viral pneumonitis. A striking clinical feature is severe hypoxaemia, often in the presence of near‐normal lung mechanics. Several hypotheses have been put forward to explain these findings, 1 , 2 including pulmonary microvascular thrombosis, dysregulated hypoxic pulmonary vasoconstriction and dysfunctional gas transport by red blood cells (RBCs). Derangement in convective O2 transport is an attractive hypothesis as this would explain why COVID‐19 hypoxaemia is often refractory to supplemental oxygen. A controversial in silico prediction postulated that the virus attacks haemoglobin (Hb), 3 and despite subsequent criticism, 4 a number of hypotheses have emerged linking Hb with COVID‐19, such as the association between thalassaemias or fetal Hb with disease severity. 5 , 6 , 7 Notwithstanding these opinions, studies in China have confirmed modestly lower Hb levels in severe COVID‐19 8 , 9 and greater heterogeneity in terms of RBC volume, quantified as RBC Distribution Width‐Standard Deviation (RDW‐SD). 10
In a recent letter to this Journal, Hb oxygen affinity was shown to be unaltered in a cohort of 14 patients infected with SARS‐CoV‐2. 11 However, steady‐state measurements of affinity cannot predict the kinetics of gas exchange by RBCs, which may become rate‐limiting in COVID‐19 due to impaired perfusion of the injured lung and inflammation‐triggered RBC deformations that expand intracellular diffusion path length. Moreover, measurements on whole blood report an ensemble population average, which cannot resolve the presence of small subpopulations of dysfunctional RBCs, if these emerge in COVID‐19. Indeed, given that RDW‐SD increases in COVID‐19, O2 handling must be interrogated with cellular resolution.
We recently designed single‐cell oxygen saturation imaging to assess O2 unloading kinetics and O2 storage capacity on a cell‐by‐cell basis. 12 We now applied this technique to study blood from COVID‐19 patients at the John Radcliffe Hospital, Oxford, UK. Ten SARS‐CoV‐2‐positive patients [9/10 confirmed by polymerase chain reaction (PCR) result, remaining patient diagnosed clinically] were recruited to this study through the Oxford GI Biobank (ethics 16/YH/0247). In half of the patients, blood was sampled within the first two weeks of diagnosis, and for the other half, sampling was in the subsequent fortnight. Three patients were asymptomatic healthcare workers, identified by voluntary PCR testing, and the remaining seven presented with COVID‐19 symptoms. None of the patients had a history of RBC disorders or haemoglobinopathy. For reference measurements, healthy donors were recruited. Venous blood samples were spun down (1200 RCF for 3 min at room temperature) to collect 10 µl of red cells, which were re‐suspended in 4 ml of HEPES‐buffered Tyrode solution containing 10 mmol/l ascorbate and then treated with UV‐C light (4000 µW/cm2 at a distance of 6 cm for 15 min, UVS‐18 EL Series UV lamp, Analytik‐Jena, Germany) to inactivate the virus. Ascorbate was included to protect Hb from oxidative damage by UV light. After re‐suspending in fresh Tyrode solution (containing no ascorbate), cells were loaded with CellTracker DeepRed and Green (ThermoFisher Scientific, Waltham, MA, USA) to produce HbO2‐sensitive fluorescence on a confocal imaging system. 12 Rapid solution switching between an oxygenated and deoxygenated microstream triggered O2 exchange, which was imaged at high temporal resolution for each RBC individually (Fig 1A). Repeating this several times yielded a measure of O2 unloading kinetics (time constant τ), normalised O2 binding capacity (κ) and cell radius in the horizontal plane. Data were analysed in terms of their frequency distribution (Fig 1B).
Fig 1.
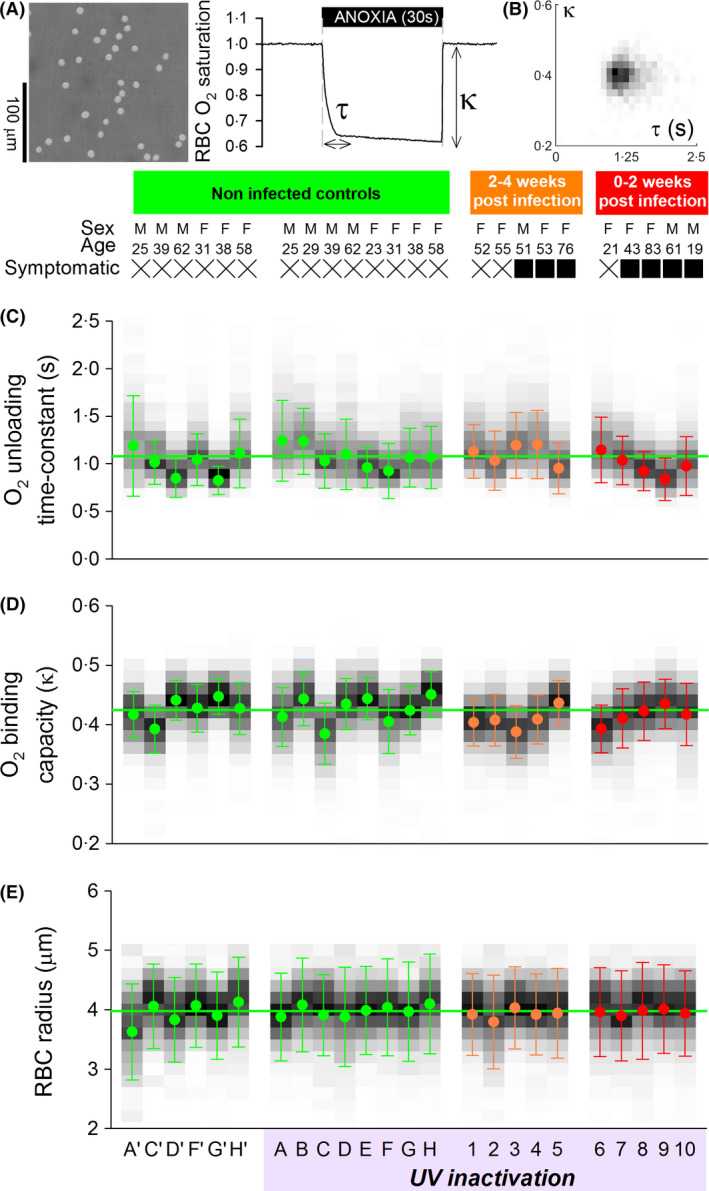
Single‐cell characterisation of O2 handling in red blood cells (RBCs) from patients with SARS‐CoV‐2 infection. (A) RBCs were loaded with CytoTracker Green and DeepRed to measure O2 saturation during rapid and transient deoxygenation (N2‐bubbled solution, with 1 mmol/l dithionite). Protocol quantifies O2 handling in terms of unloading time‐constant (τ) and binding capacity (κ) in fresh venous blood sample from COVID‐19 patients. (B) Cell‐by‐cell frequency distribution of τ and κ shows single population. Quantification of (C) τ, (D) κ and (E) radius in non‐infected controls (A–H), and patients (1–10) tested positive for SARS‐CoV‐2 at two time points relative to infection. Viral inactivation by UV light in the presence of 10 mmol/l ascorbate was performed; based on findings from control blood, this treatment had no effect on measured parameters. Greyscale shading illustrates histogram distribution of each parameter. Data presented as mean, and error bars denote width of distribution at half maximal height. >1000 cells per sample. [Colour figure can be viewed at wileyonlinelibrary.com]
RBC τ, κ and radius were no different in COVID‐19 patients, compared to controls (Fig 1C–E). There was also no evidence for a change in the distribution of these functional variables, arguing against the emergence of dysfunctional subpopulations over the course of infection. When expressed in terms of the fraction of cells that release >95% of stored oxygen in a given time (equivalent to capillary transit), there was no effect of SARS‐CoV‐2 infection on RBC O2 handling (Fig 2).
Fig 2.
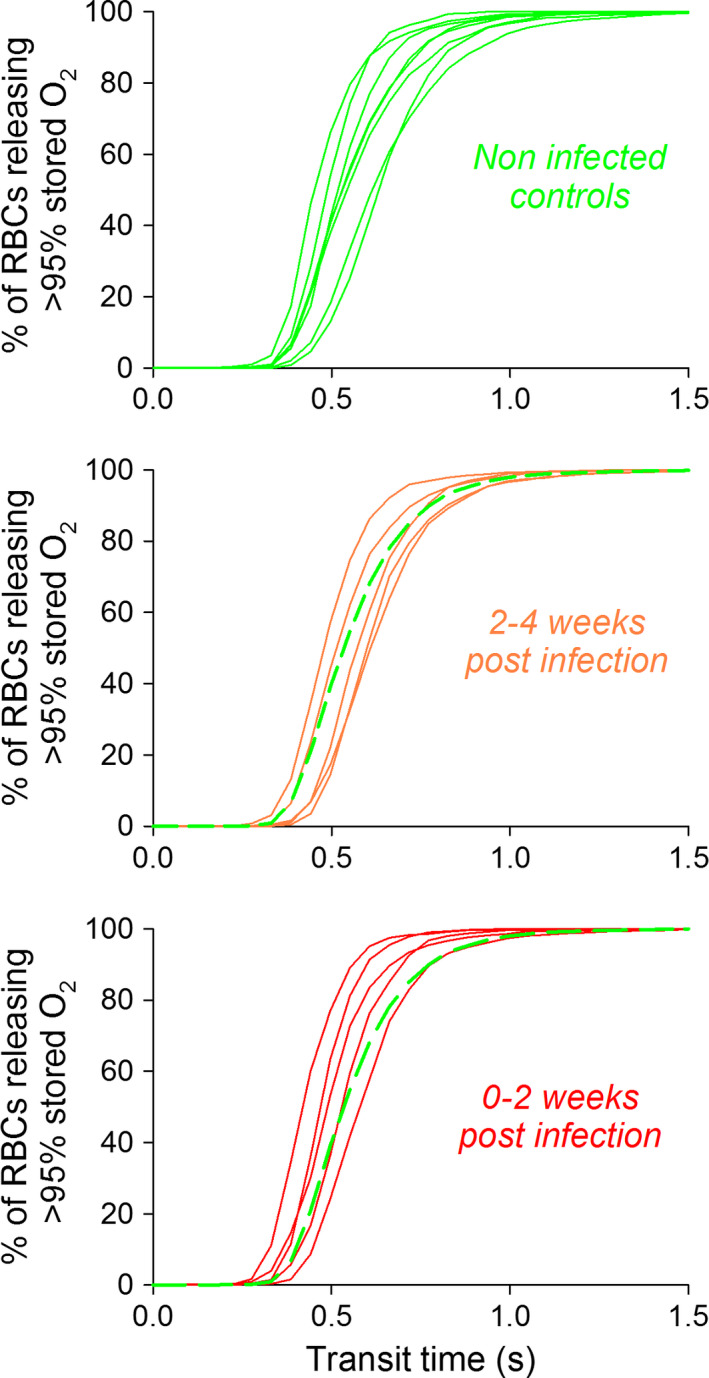
Fraction of red blood cells (RBCs) that deoxygenate by >95% during given transit time. SARS‐CoV‐2 infection does not affect O2 release kinetics. Analysis of histogram distributions shown in Fig 1 using equations derived previously in Richardson et al. 12 [Colour figure can be viewed at wileyonlinelibrary.com]
Our findings add new evidence that SARS‐CoV‐2 infection does not lead to dysfunctional convective transport. The cause of hypoxaemia in COVID‐19 patients is therefore unlikely to relate to impaired O2 handling by RBCs, either as a result of direct coronavirus infection or a consequence of the inflammatory state. Our findings argue against a mechanistic link between Hb variants and disease outcomes.
Funding information
Supported by NIHR Biomedical Research Centre, Oxford, the COVID‐19 Research Response Fund (#0009288), Oxford, European Research Council (SURVIVE #723997).
Author contributions
KCP, NR and PS performed the research. KD, SM and PS designed the research study. NR and PK contributed essential reagents or tools. KCP, KD and PS analysed the data. PS wrote the paper. The authors thank Dr Barbara Kronsteiner and Dr Emily Adland for coordinating blood sample supply.
References
- 1. Gattinoni L, Chiumello D, Rossi S. COVID‐19 pneumonia: ARDS or not? Crit Care. 2020a;24:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID‐19 does not lead to a "Typical" acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020b;201:1299–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu W, Li H. COVID‐19: attacks the 1‐beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. ChemRxiv. 2020. 10.26434/chemrxiv.11938173.v11938178. [DOI] [Google Scholar]
- 4. Read RJ. Flawed methods in “COVID‐19: Attacks the 1‐beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism”. ChemRxiv. 2020. 10.26434/chemrxiv.12120912.v12120912. [DOI] [Google Scholar]
- 5. Cavezzi A, Troiani E, Corrao S. COVID‐19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. 2020;10:1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lansiaux E, Pebay PP, Picard JL, Son‐Forget J. COVID‐19: beta‐thalassemia subjects immunised? Med Hypotheses. 2020;142:109827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sotoudeh E, Sotoudeh H. A hypothesis about the role of fetal hemoglobin in COVID‐19. Med Hypotheses. 2020;144:109994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bao J, Li C, Zhang K, Kang H, Chen W, Gu B. Comparative analysis of laboratory indexes of severe and non‐severe patients infected with COVID‐19. Clin Chim Acta. 2020;509:180–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu X, Zhang R, He G. Hematological findings in coronavirus disease 2019: indications of progression of disease. Ann Hematol. 2020;99:1421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang C, Deng R, Gou L, Fu Z, Zhang X, Shao F, et al. Preliminary study to identify severe from moderate cases of COVID‐19 using combined hematology parameters. Ann Transl Med. 2020;8:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daniel Y, Hunt BJ, Retter A, Henderson K, Wilson S, Sharpe CC, et al. Haemoglobin oxygen affinity in patients with severe COVID‐19 infection. Br J Haematol. 2020. 10.1111/bjh.16888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richardson SL, Hulikova A, Proven M, Hipkiss R, Akanni M, Roy NBA, et al. Single‐cell O2 exchange imaging shows that cytoplasmic diffusion is a dominant barrier to efficient gas transport in red blood cells. Proc Natl Acad Sci USA. 2020;117:10067–78. [DOI] [PMC free article] [PubMed] [Google Scholar]


