Abstract
Aim
To assess the effects of oral care on prolonged viral shedding in coronavirus disease 2019 (COVID‐19) patients.
Methods and results
We evaluated the clinical course of eight COVID‐19 patients, including their duration of viral shedding, by PCR testing of nasopharyngeal swabs. The average time from the onset of symptoms until the virus was no longer detectable was 31.6 ± 11.8 days (mean ± SD; range 17‐53). Thus, it took 15.1 ± 14.7 (1‐40) days from the time of clinical recovery for the virus to become undetectable. In two patients who had mental retardation and psychiatric disorders, the viral shedding period continued for 44 days or 53 days. These two patients did not voluntarily brush their teeth. When they were instructed on the importance of oral care, including tooth brushing and gargling, their tests for the coronavirus became negative.
Conclusion
Most of the patients with COVID‐19 had a viral shedding period of 30 days or less. In cases of prolonged viral shedding (≥44 days), noninfectious viral nucleic acid may have accumulated in uncleaned oral cavities and continued to be detected. We propose that tooth brushing and gargling remove such viral nucleic acid and improve the accuracy of PCR testing.
Keywords: coronavirus disease 2019 (COVID‐19), mental retardation, oral care, prolonged viral shedding, psychiatric disorders, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), tooth brushing
1. INTRODUCTION
The novel coronavirus disease 2019 (COVID‐19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). There are no data on the duration of viral shedding after clinical recovery from COVID‐19. Median duration of viral shedding was reported to be 20.0 days, 1 but prolonged SARS‐CoV‐2 RNA shedding was reported to be common regardless of symptomatic relief among cases in Wuhan, China. 2 According to the World Health Organization's guidelines on clinical management, the Japanese Ministry of Health, Labor, and Welfare has set criteria for hospital discharge of COVID‐19 patients as follows: (a) clinically recovered to asymptomatic and (b) two negative PCR tests from respiratory specimens at 24‐hour intervals. We could have correct PCR test results with proper discharge in most cases. However, these criteria contain two possibilities for error. One is persistent infections, with false negative PCR test results allowing patients to be discharged, and the other is prolonged hospitalizations due to false positive test results. 3
In the present study, we hypothesized that inappropriate oral care may influence the prolonged duration of viral shedding after clinical recovery in patients with COVID‐19 and that this results in positive PCR tests, which impact the hospitalizations of patients. We first evaluated the clinical course of a series of COVID‐19 patients, including their duration of viral shedding after clinical recovery. Then, we assessed the status of oral hygiene and the role it may play in detected viral shedding.
2. PATIENTS AND METHODS
We evaluated eight COVID‐19 patients who were admitted to the Department of Neurology, Tokyo Metropolitan Neurological Hospital, Japan, between April 30 and May 14, 2020 and followed these patients until their discharge from the hospital. All patients were admitted to the hospital from Tokyo Metropolitan Tama Medical Center, which is a designated medical facility for infectious diseases and is affiliated with the hospital. The patients had passed the acute phase of COVID‐19, but they were transferred to the hospital because they could not be discharged due to persistent positive PCR tests for the coronavirus, and they had underlying diseases. None of the patients had been given antiviral or anti‐cytokine therapeutics. As symptomatic treatments for COVID‐19, acetaminophen and dextromethorphan hydrobromide hydrate had been used.
Clinical recovery was determined as returning to normal body temperature and termination of supplemental oxygen inhalation. PCR tests of COVID‐19 were performed with samples from nasopharyngeal swabs. SARS‐CoV‐2 nucleic acid detection by real‐time reverse transcription‐PCR was outsourced to a clinical laboratory testing company (BML, Inc., Tokyo, Japan). According to the criteria for hospital discharge of COVID‐19 patients, if two PCR tests on consecutive days were negative after clinical recovery, patients would be discharged. For these cases, the first negative day was defined as the day the virus was no longer detectable. In cases where the first test was negative but the second test was positive, the initial tests were judged to be false negatives and were included in the viral shedding period.
3. RESULTS
Demographics of the eight COVID‐19 patients are presented in Table 1. Age was 60 ± 14 (mean ± SD, range 31‐81) years. All of the patients had some underlying diseases, which included mental retardation and psychiatric disorders, hypertension, dyslipidemia, cerebral hemorrhage, bronchial asthma, and cancer. Of the initial symptoms, selected from a multiple choice list, 100% of the patients had fever, 75% had a cough, and 50% had diarrhea. Pneumonia was detected by chest X‐rays in six (75%) of the patients, and three (37.5%) of the patients required 2‐5 L of supplemental oxygen inhalation for 8‐12 days. None of the patients needed a ventilator or extracorporeal membrane oxygenation. As shown in Table 2, from the date of onset of the initial symptoms, fever lasted 14.0 ± 8.2 days, clinical recovery was observed at 16.5 ± 6.5 days, and the virus was no longer detectable by PCR at 31.6 ± 11.8 days (range 17‐53). Thus, it took an average of 15.1 ± 14.7 days (range 1‐40) from the day of clinical recovery to the day the virus was undetectable. As shown in the Figure 1, for patients 1 and 2, the viral shedding period continued for more than 44 days and 53 days, respectively, which in each case was more than 33 days after clinical recovery. For patients 3 through 8, once the COVID‐19 symptoms disappeared, a PCR‐negative status was confirmed within 18 days, and the patients were discharged.
TABLE 1.
Demographics of the patients
| Number of patients (n) | 8 |
| Age in years (mean ± SD [range]) | 60 ± 14 (31‐81) |
| Male:female | 2:6 |
| Underlying diseases (n) | |
| Mental retardation and/or psychiatric disorders | 3 |
| Hypertension | 3 |
| Dyslipidemia | 3 |
| Cerebral hemorrhage | 2 |
| Bronchial asthma | 2 |
| Cancer | 1 |
| Voluntarily brush their teeth (n [%]) | 6 (75) |
| Initial symptoms from multiple choice (n [%]) | |
| Fever | 8 (100) |
| Cough | 6 (75) |
| Diarrhea | 4 (50) |
| Pneumonia detected in chest X‐rays (n [%]) | 6 (75) |
| Required supplemental oxygen inhalation (n [%]) | 3 (37.5) |
TABLE 2.
Clinical course and viral shedding period
| Duration of fever | 14.0 ± 8.2 days a |
| Duration from onset of initial symptoms to clinical recovery | 16.5 ± 6.5 days a |
| Viral shedding period | 31.6 ± 11.8 (17‐53) days b |
| Viral shedding period after clinical recovery | 15.1 ± 14.7 (1‐40) days b |
Mean ± SD.
Mean ± SD (range).
FIGURE 1.
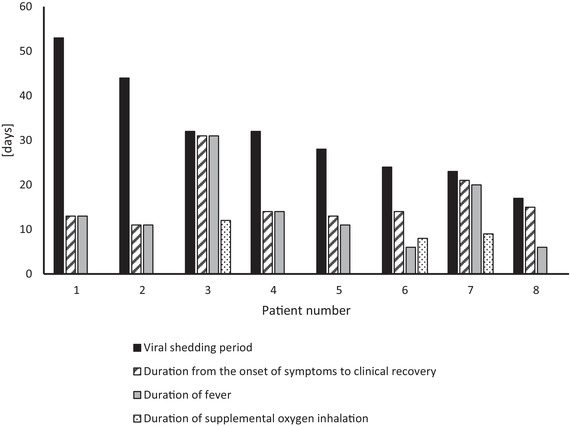
Viral shedding period and clinical course of each patient. The viral shedding period was 31.6 ± 11.8 days (mean ± SD, range 17‐53). In patients 1 and 2, who had mental retardation and psychiatric disorders, the viral shedding period continued for 44 and 53 days, respectively, and for more than 33 days after clinical recovery. In patients 3 through 8, once the COVID‐19 symptoms resolved, consecutive negative PCR test results were confirmed within 18 days
We considered various reasons why the PCR tests of patients 1 and 2 continued to be positive for such an extended period. We found that these two patients had mental retardation and/or psychiatric disorders and did not voluntarily brush their teeth during hospitalized life isolated in private rooms. Patients 3 through 8 were able to keep their bodies clean and groomed and voluntarily brushed their teeth on a regular basis. Therefore, we instructed patients 1 and 2 on the importance of oral care, including tooth brushing and gargling. Subsequently, their PCR tests became negative in 4‐9 days, and they were able to leave the hospital. Their clinical courses are as follows.
Patient 1 was a 31‐year‐old woman with schizophrenia. After having a meal in the downtown area, she developed COVID‐19 with a fever, cough, and diarrhea, and she was admitted to the medical center 1 week after the onset of the symptoms. She had no pneumonia and did not need supplemental oxygen. The fever disappeared in 12 days, and the patient was diagnosed as clinically recovered. She was given a PCR test for the coronavirus 24 hours later that gave a positive result. After that, repeated PCR tests were performed, but all gave positive results, so patient 1 could not leave the medical center and was transferred to our hospital. Patient 1 had schizophrenia and was unable to voluntarily keep herself clean during isolated hospitalized life. She brushed her teeth for the first time on the 18th day of hospitalization, but after that, she did not brush her teeth at all. Her virus shedding period reached 46 days, with consistently positive PCR test results. We speculated that her inappropriate oral care might have caused the persistence of PCR test positivity. In collaboration with the nurse, we repeatedly encouraged patient 1 to brush her teeth and gargle. Two days after the start of this instruction, on the 49th day after the patient's onset of symptoms, the patient's PCR test result was negative for the first time. On day 51, her PCR test result was positive again, but she had two negative test results on days 53 and 56. After 9 days of intensive tooth brushing with only water, patient 1 was discharged from the hospital with two consecutive negative PCR test results.
Patient 2, a 61‐year‐old woman, was the mother of patient 1. Patient 2 had dissociative disorder and mild mental retardation as underlying diseases. Two days after patient 1 developed a fever, patient 2 developed COVID‐19 with a fever and cough, and she was admitted to the medical center on the ninth day. She had no pneumonia and did not need supplemental oxygen inhalation. The fever disappeared in 11 days, at which time the patient was diagnosed as clinically recovered. She was given a PCR test for the coronavirus 24 hours later, which yielded a positive result. Subsequently, repeated PCR tests were performed. The PCR test result was negative once on the 26th day and once on the 37th day, but two consecutive negative results were not obtained, and the virus shedding duration reached 43 days. At that time, we found that patient 2 rarely brushed her teeth. Since then, we repeatedly instructed her to brush her teeth. With 4 days of intensive tooth brushing with only water, patient 2 had two consecutive negative PCR test results on days 44 and 47, so she was discharged.
4. DISCUSSION
Although we cannot make a statistical argument due to the small number of patients examined, this study has two important findings. Six of the patients with mild to moderate COVID‐19 had a viral shedding period of 30 days or less, but two of the patients had significantly longer shedding periods.
A previous virologic analysis of nine cases of COVID‐19 showed that pharyngeal virus shedding was very high during the first week of symptoms, with a peak on day 4, and the shedding of viral RNA from sputum outlasted the end of symptoms. 4 In a report of 191 patients from Wuhan, China, the median duration of viral shedding was 20.0 days in survivors, and the longest observed duration of viral shedding was 37 days. 1 However, another report from Wuhan showed that prolonged SARS‐CoV‐2 RNA shedding was not a rare phenomenon regardless of symptomatic relief. 2 In that study, 36 of 378 patients diagnosed with COVID‐19 continued to shed viral RNA for longer than 30 days. The median duration of viral RNA shedding was 53.5 days in these 36 patients, and the longest duration of viral RNA shedding was 83 days. A limited number of papers have pointed out that factors affecting prolonged SARS‐CoV‐2 RNA shedding are age older than 45 years, chest tightness, the highest temperature at admission, time from symptom onset to admission, and length of hospital stay. 5 , 6 However, in our case, patient 1 was 31 years old, and in this study there was no significant difference between this patient and the others in the body temperature and the time from onset to hospitalization. Therefore, these factors could not explain the reason for prolonged virus shedding period for patient 1.
Oral care, including tooth brushing and gargling, may a factor in the duration of viral shedding, especially in patients with mental retardation and psychiatric disorders who cannot brush their teeth voluntarily during isolated hospitalized lives. In addition to sputum and oropharyngeal secretions, saliva is reported as a reliable sample for the detection of SARS‐CoV‐2. 7 The SARS‐CoV‐2 viral load was found to be relatively higher in the saliva than that in the oropharynx during the early stage of COVID‐19. 8 However, the viral load in the saliva decreased in day 9 of the illness. Moreover, infectious virus was not isolated from stool samples in spite of high concentrations of virus RNA, although it was readily isolated from samples derived from the throat or lung. 4 These findings indicate that infectious virus is present in saliva and the digestive tract only in the early stages of COVID‐19. Even if a high concentration of viral RNA is detected in the prolonged viral shedding cases, it is highly likely that an infectious virus is not isolated. Considering this phenomenon, noninfectious viral nucleic acid may accumulate in an uncleaned nasopharynx and oral cavity and may continue to be detected by PCR. Concentrations of 1‐1.5% hydrogen peroxide solution or 1‐0.2% povidone‐iodine seem to be effective mouthwashes to reduce the viral load in the oral cavity of COVID‐19 patients. 9 Chlorhexidine mouthwash was also reported to be effective in reducing the SARS‐CoV‐2 viral load in the saliva for a short‐term period. 8 Brushing the teeth with toothpaste for 2 minutes twice a day is recommended for those individuals who are at risk of contracting COVID‐19, including the elderly, particularly those in nursing and retirement homes, where hygiene practices often depend on caregivers. 10 In addition, we propose not only a medicated mouthwash and toothpaste, but also brushing the teeth and gargling, even if only with water, to physically remove accumulated viral nucleic acid.
During the recent COVID‐19 pandemic, it was necessary to isolate infected patients, even those with mental retardation and psychiatric disorders. These patients were unable to keep their bodies clean and groomed or voluntarily brush their teeth on a regular basis. Two of our patients suffered from particularly inappropriate oral care regimens, where they barely brush their teeth for more than 43 days. We speculated that this poor oral care led to the prolonged viral shedding and extension of the patients’ hospital stays. When patients who cannot brush their teeth voluntarily must be isolated due to an infectious disease, including COVID‐19, clinicians should be conscious to confirm that the patients have appropriate oral care. Proper oral hygiene may decrease the observed viral shedding period and prevent unnecessarily long hospital stays.
5. CONCLUSIONS
In this study, six of the eight patients with mild to moderate COVID‐19 had a viral shedding period of 30 days or less, but two patients had significantly longer shedding periods. In such prolonged viral shedding cases, noninfectious viral nucleic acid may accumulate in an uncleaned oral cavity and may continue to be detected by PCR. We propose tooth brushing and gargling to remove accumulated noninfectious viral nucleic acid, leading to consistently negative PCR test results and thus avoiding unnecessarily long hospital stays.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ETHICAL STATEMENT
This study was performed in accordance with the Declaration of Helsinki and was approved by the ethical committee of Tokyo Metropolitan Neurological Hospital, Tokyo, Japan (TS‐R02‐020; June 5, 2020).
ACKNOWLEDGMENTS
We thank Dr. Tetsuya Kashiyama, vice‐president of Tokyo Metropolitan Tama Medical Center, for successful collaboration in the practice of COVID‐19. We thank all the nurses on the eighth floor ward for their dedication to COVID‐19 patients.
Warabi Y, Tobisawa S, Kawazoe T, et al. Effects of oral care on prolonged viral shedding in coronavirus disease 2019 (COVID‐19). Spec Care Dentist. 2020;40:470–474. 10.1111/scd.12498
REFERENCES
- 1. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li N, Wang X, Lv T. Prolonged SARS‐CoV‐2 RNA shedding: not a rare phenomenon [published online ahead of print April 29, 2020.]. J Med Virol. 2020. 10.1002/jmv.25952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoang VT, Dao TL, Gautret P. Recurrence of positive SARS‐CoV‐2 in patients recovered from COVID‐19 [published online ahead of print May 25, 2020]. J Med Virol. 2020. 10.1002/jmv.26056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581:465‐469. [DOI] [PubMed] [Google Scholar]
- 5. Hu X, Xing Y, Jia J, et al. Factors associated with negative conversion of viral RNA in patients hospitalized with COVID‐19. Sci Total Environ. 2020;728:138812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qi L, Yang Y, Jiang D, et al. Factors associated with the duration of viral shedding in adults with COVID‐19 outside of Wuhan, China: a retrospective cohort study. Int J Infect Dis. 2020;96:531‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Azzi L, Carcano G, Gianfagna F, et al. Saliva is a reliable tool to detect SARS‐CoV‐2. J Infect. 2020;81:e45‐e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoon JG, Yoon J, Song JY, et al. Clinical significance of a high SARS‐CoV‐2 viral load in the saliva. J Korean Med Sci. 2020;35:e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruiz Roca JA. What is the most effective mouthwash in patients infected with COVID‐19 to minimize possible transmission by saliva? Update. J Dent Maxillofacial Res. 2020;3:1‐5. [Google Scholar]
- 10. Addy M. Toothbrushing against coronavirus. Br Dent J. 2020;228:487. [DOI] [PubMed] [Google Scholar]


