Abstract
To evaluate the efficacy of angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) vs calcium channel blockers (CCBs) on the progression of Corona Virus Disease 2019 (COVID‐19) patients with hypertension in Wuhan. This retrospective single‐center case series analyzed COVID‐19 patients with hypertension, treated with ACEIs/ARBs or CCBs at the Tongji Hospital of Wuhan City, China from 25th January to 15th March 2020. After propensity score matching analysis, 76 patients were selected into two groups. Univariate and multivariable analyses were conducted to determine factors related to improvement measures and outcome measures by Cox proportional hazard regression models. Among 157 patients with confirmed COVID‐19 combined hypertension, including 73 males and 84 females, a median age of 67.28 ± 9.11 vs 65.39 ± 10.85 years. A univariable analysis indicated that clinical classification, lymphocyte count, and interleukin‐2 receptor were associated with a lengthened negative time of nucleic acid, with a significant difference between two groups (P = .036). Furthermore, we found no obvious difference in nucleic acid conversion time between ACEIs/ARBs and CCBs groups (hazard ratio [HR]: 0.70; 95% confidence interval [CI]: [0.97, 3.38]; P = .18) in the multivariable analysis as well as chest computed tomography improved time (HR: 0.73; 95% CI [0.45, 1.2]; P = .87), and hospitalization time between ACEIs/ARBs and CCBs groups (HR: 1.06; 95% CI [0.44, 1.1]; P = .83). Our study provided additional evidence of no obvious difference in progress and prognosis between ACEIs/ACEIs and CCBs group, which may suggest ACEIs/ARBs may have scarcely influence on increasing the clinical severe situations of COVID‐19 patients with hypertension.
Keywords: ACEIs, ARBs, CCBs, COVID‐19, hypertension, RAS blockers
Highlights
-
1.
Antihypertensive drugs, either ACEIs/ARBs or CCBs had few effects on firstly negative nucleic acid time, firstly chest CT improved time and the hospitalization time of COVID‐19 by a cox Regression analysis.
-
2.
ACEIs/ARBs didn't increase the risk of extended course and poor prognosis of hypertension patients with COVID‐19.
Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- ACEIs/ARBs
angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers
- COVID‐19
Corona Virus Disease 2019
- RAS blockers
renin‐angiotensin system blockers
- RT‐PCR
real‐time reverse‐transcription‐polymerase chain reaction
- SARS
severe acute respiratory syndrome
- WHO
World Health Organization
1. INTRODUCTION
Corona Virus Disease 2019 (COVID‐19) is a new respiratory illness caused by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), first reported in Wuhan, China, in December 2019. The outbreak of COVID‐19 is currently continuously evolving globally, resulting in 1 133 758 confirmed cases including in healthcare workers, worldwide by 5 April 2020. 1 On 20 January 2020, the National Health Commission issued No. 1 announcement, which included COVID‐19 as an acute respiratory infectious disease into the class B infectious disease specified in the law of the people's Republic of China on the prevention and control of infectious diseases, and then managed it as class A infectious disease. The COVID‐19 epidemic situation was classified as “public health emergencies of international concern” on 31st January, the highest level in the World Health Organization (WHO) infectious disease emergency mechanism by WHO.
There was of particular interest to clinicians and investigators with a major interest in cardiovascular disease and mortality on infected patients, particularly in elderly people with comorbidities. subsequently, in an analysis of 45 000 confirmed cases in China, 2 , 3 the crude case fatality rate was 0.9% for patients without any documented comorbidities, whereas the case fatality rate was much higher for patients with cardiovascular disease (10.5%), diabetes (7.3%), or hypertension (6.3%).
Well, now the current study reports that hypertension may be associated with an increased risk of severe in hospitalized COVID‐19 patients. Previous experimental data 4 , 5 revealed angiotensin‐converting enzyme 2 (ACE2) receptors serve as binding sites for the anchoring spike (S) proteins on the exterior surfaces of beta coronaviruses. The beta coronavirus SARS‐CoV causes the severe acute respiratory syndrome (SARS). The phylogenetically related beta coronavirus, SARS‐Cov‐2, causes the novel coronavirus disease (nCoV‐2019) or COVID‐19. S proteins anchor both beta coronaviruses to ACE2 receptors in the lower respiratory tract of infected patients to gain entry into the lungs. Viral pneumonia and potentially fatal respiratory failure may result in susceptible persons after 10 to 14 days.
Currently, the hypertension patients are basically treated with blood pressure‐lowering drugs, mainly including renin‐angiotensin system (RAS) blockers‐angiotensin‐converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), calcium channel blockers (CCBs), β‐blockers, diuretics. In Southwest China, CCBs accounted for 58.6% of hypertension treatment, followed by ACEIs/ARBs accounted for 22.4%. 6 Since ACE2 was identified to the functional receptor of SARS‐CoV‐2. ACE2 level was increased following treatment with ACEIs and ARBs. The current research 7 , 8 revealed that intravenous infusions of ACEIs and ARBs in experimental animals increased the numbers of ACE2 receptors in the cardiopulmonary circulation. Patients taking ACEIs or ARBs chronically for cardiovascular diseases are assumed to have increased numbers of ACE2 receptors throughout their cardiopulmonary circulations as observed in experimental animal models.
Currently, a corollary concern needed to identify through real‐world clinical studies, whether these commonly used RAS blockers—ACEIs/ARBs may increase the severity of COVID‐19. The present study therefore retrospectively analyzed data from hospitalized COVID‐19 patients with hypertension in a single center in Wuhan, China, to compare the difference between ACEIs/ARBs and CCB groups, which may provide clinical evidence on the impact of ACEIs/ARBs on the clinical course of COVID‐19 infection.
2. METHODS
2.1. Study patients
We performed a retrospective study of COVID‐19 patients with hypertension who were recorded in Tongji Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China from 25th January to 15th March 2020. COVID‐19 was diagnosed via epidemiological history (Travel history or residence history in Wuhan and surrounding areas or other communities with case reports within 14 days before the onset of illness; history of exposure to COVID‐19 case infection within 14 days before onset of illness and so on), consistent with two clinical manifestations (fever and respiratory symptoms, normal or decreased white blood cells in early onset of illness), and microbiological evidence (laboratory test for the COVID‐19 from the respiratory specimens shows positive result by the real‐time reverse‐transcription‐polymerase chain reaction [RT‐PCR] assay) according to the Novel Coronavirus Pneumonia Diagnosis and Treatment Guideline (7th edition, in Chinese) published by the National Health Commission of China. Hypertension history and blood pressure medications Obtained through electronic medical record (EMR). Patients demographics as well as the use of antihypertensive drug, therapy protocol, imaging data, laboratory data, follow‐up records, and prognosis were collected from EMR. Routine tests, such as chest computed tomography (CT), serum biochemical indices, and complete blood counts were conducted during hospitalization.
From 25th January to 15th March 2020, 306 patients diagnosed with COVID‐19 combined hypertension using CCBs, ACEIs, or ARBs antihypertensive drugs. Patients with the following conditions were excluded from our study: (a) lung imaging was significantly improved before using antihypertensive; (b) before using antihypertensive, ucleic acid was negative for two consecutive times; (c) case use both CCBs and ACEIs/ARBs. This study was approved by the institutional ethics board of the Tongji Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology (No. TJ‐ⅠRB20200338).
According to the Novel Coronavirus Pneumonia Diagnosis and Treatment Guideline (7th edition, in Chinese), clinical classification of the COVID‐19 patients was classified into mild‐moderate, severe, and critically ill. Baseline clinical data were collected at admission, including blood routine, such as white blood cell count (3.5‐9.5) × 109/L, lymphocyte count (1.1‐3.2) × 109/L, coagulation function such as D‐dimer less than 0.5 mg/L, renal and liver function, such as alanine aminotransferase and aspartate aminotransferase less than 40 U/L, creatinine (59‐104 U/L), uric acid (202.3‐416.5 mmol/L), urea (3.6‐9.5 mmol/L), electrolytes such as potassium (3.5‐5.1 mmol/L), sodium (136‐145 mmol/L), C‐reactive protein (CRP) less than 5 mg/L, myocardial enzymes such as NT‐ProBNP less than 241 pg/mL, cytokine such as interleukin‐6 less than 7 mmol. These were collected routinely on admission. Radiologically, the area of affected lungs consistent with viral pneumonia in each patient's first chest CT after admission was measured and classified into one or two lungs. During hospitalization, lesion progression in chest CT was recorded. RT‐PCR assay was used to analyze the SARS‐CoV‐2 viral nucleic acid. The outcome data were collected prospectively by physicians who un‐knew the study group. The main improvement measures were the time from illness onset to negative nucleic acid for two time and chest CT gradually improved, and we selected firstly nucleic acid conversion time and chest CT improved. The secondary endpoints were worsened Chest CT during hospitalization and in‐hospital mortality. The outcome measures were the time from illness onset to discharge from hospital.
2.2. Statistical analysis
Continuous data accorded with normal distribution were expressed as mean ± standard deviation and compared by independent samples t test or expressed as median (25th‐75th percentile) and compared by the Wilcoxon rank‐sum test. Categorical variables were expressed as number (percentage) and compared by χ 2 test or Fisher's exact test. Among patients diagnosed with COVID‐19 combined hypertension using CCBs, ACEIs, or ARBs antihypertensive drugs, we sought to investigate the ACEIs or ARBs medication on progress and prognosis of COVID‐19 patients. The main improvement measures, such as the first nucleic acid conversion time, first chest CT improved time and outcome measures such as the hospitalization time was also calculated. Univariable and multivariable Cox regression models were used to describe the progression and prognosis of COVID‐19 patients between CCBs and ACEIs/ARBs. To account for sample size, we selected the variables with P < .05 which were significantly associated with outcome measures in the univariable analysis. One‐to‐one (1:1) propensity score matching (PSM) was conducted to construct a matched sample consisting of pairs of CCBs and ACEIs/ARBs subjects by optimal matching algorithm. Variables that were significantly different between the two groups were utilized to generate propensity scores. Specifically, we also conducted a stratified analysis with respect to outcome measures by age, gender, clinical classification, comorbidities, temperature, respiratory rate, diastolic blood pressure (BP), systolic BP, heart rate, white blood cell count, lymphocyte count, D‐dimer, interleukin‐6, interleukin‐2 receptor, alanine aminotransferase, aspartate aminotransferase, lung image, NT‐ProBNP, creatinine, urea, uric acid, potassium, sodium, and CRP, which was used to perform PSM. The data were imported and analyzed using R language (3.6.2 ed, 2019), implemented in RStudio (Version 1.2.5003 ed, 2015). 9 , 10 Package MatchIt, survival, and survminer were employed for PSM and survival analysis, separately, 11 , 12 , 13 with a two‐sided P value of less than .05 considered statistically significant.
3. RESULTS
3.1. Study the selected population
In total, 306 patients with a diagnosis of COVID‐19 combined hypertension using CCBs, ACEIs, or ARBs antihypertensive drugs between 25th January 2020 to 15th March 2020. The patient selection flow‐chart is displayed in Figure 1. All these patients were treated with one or multiple blood pressure‐lowering drugs, including RAS blockers—ACEIs/ARBs, calcium channel blockers, β‐Blockers, and diuretics. The ACEIs/ARBs group consisted of ACEIs or ARBs without CCBs, as well as CCBs group. Then, we selected 157 adult hypertension patients with COVID‐19 infections, including 73 males (46.50%) and 84 females (53.50%). The vast majority of patients were discharged from hospital. Unfortunately, there were five patients and one patient died from pneumonia in ACEIs/ARBs and CCBs groups, respectively. The in‐hospital mortality showed no significant difference between two groups (P = .191). After PSM, we selected 76 adult hypertension patients with COVID‐19 infections (Figures S1 and S2).
Figure 1.
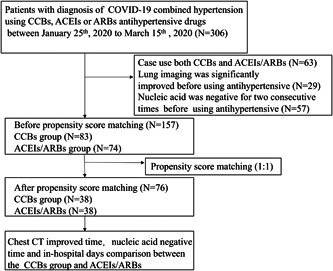
Flow‐chart for patient selection
3.2. Comparison of demographic and clinical characteristics
Before PSM, a total of 157 adult hypertension patients with COVID‐19 infections were involved in this study. Demographic features and clinical characteristics data are shown in Table 1. There is no obvious difference between ACEIs/ARBs and CCBs groups about 49 (59.0) vs 35 (47.3) female, a median age of 67.28 ± 9.11 vs 65.39 ± 10.85 years (P > .05). Compared with the respiratory rate 21.39 ± 7.22 or 19.96 ± 2.42, diastolic BP 83.23 ± 11.88 or 81.81 ± 13.47 mm Hg, systolic BP 138.66 ± 17.97 or 134.76 ± 18.53 mm Hg, Heart rate 89.01 ± 14.35 or 89.19 ± 17.62 bpm, respectively, we found that antihypertensive drugs in ACEIs/ARBs and CCBs groups exhibited a similarly antihypertensive efficacy. The underlying diseases, including diabetes mellitus and coronary disease, in ACEIs/ARBs and CCBs groups are listed in Table 2. There was no significant difference between two groups, as well as lymphocytes count (×109/L)1.26 ± 0.53 vs 1.29 ± 0.60, D‐dimer (mg/L) 1.43 ± 2.04 vs 1.38 ± 1.59, interleukin‐6 18.51 ± 32.41 vs 24.11 ± 52.72 mmol/L, interleukin‐2 receptor 32.69 ± 490.99 vs 752.82 ± 550.00 mmol/L, and CRP 29.57 ± 47.25 vs 24.25 ± 50.47 mg/L. The clinical severity was graded as mild‐moderate, severe, and critically ill. There is no difference in any of grade between ACEIs/ARBs and CCBs groups. It is worth noting that the significant differences between ACEIs/ARBs group and CCBs group on temperature 36.92°C ± 0.84°C vs 37.69°C ± 0.75℃, white blood cell count (×109/L) 5.92 ± 2.28 vs 7.54 ± 2.31, aspartate aminotransferase 22.82 ± 13.42 vs 29.95 ± 16.61 U/L. To further evaluate the detected differences between ACEIs/ARBs and CCBs groups, we performed a 1:1 matched case‐control analysis using the PSM method. PSM between the ACEIs/ARBs and CCBs groups was conducted by all variables (age, gender, clinical classification, comorbidities, temperature, respiratory rate, diastolic BP, systolic BP, heart rate, white blood cell count, lymphocytes count, D‐dimer, interleukin‐6, interleukin‐2 receptor, alanine aminotransferase, aspartate aminotransferase, lung image, NT‐ProBNP, creatinine, urea, uric acid, potassium, sodium, and CRP). After PSM, the ACEIs/ARBs and CCBs groups consisted of 38 patients, respectively. No statistical differences on demographic features and clinical characteristics data were observed between the two groups.
Table 1.
Comparison of demographic and clinical characteristics of patients with COVID‐19 between ACEIs/ARBs and CCBs groups
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Characteristics ID | CCBs group (n = 83) | ACEIs/ARBs group (n = 74) | P | CCBs group (n = 38) | ACEIs /ARBs group (n = 38) | P |
| Gender = female (%) | 49 (59.0) | 35 (47.3) | .19 | 22 (57.9) | 22 (57.9) | 1 |
| Age (mean ± SD) | 67.28 ± 9.11 | 65.39 ± 10.85 | .239 | 65.34 ± 9.65 | 68.16 ± 7.58 | .162 |
| Outcome = cure (%) | 82 (98.8) | 69 (93.2) | .163 | 38 (100.0) | 38 (100.0) | 1 |
| Clinical classification (%) | .191 | .818 | ||||
| Mild‐moderate (%) | 44 (53.0) | 38 (51.4) | ||||
| Severe (%) | 38 (45.8) | 31 (41.9) | 16 (42.1) | 18 (47.4) | ||
| Critically ill (%) | 1 (1.2) | 5 (6.8) | ||||
| Diabetes (%) | 22 (26.5) | 21 (28.4) | .934 | 9 (23.7) | 12 (31.6) | .608 |
| coronary disease (%) | 6 (7.2) | 10 (13.5) | .301 | 3 (7.9) | 5 (13.2) | .709 |
| Temperature (mean ± SD) | 36.92 ± 0.84 | 37.69 ± 0.75 | .012 | 36.85 ± 0.83 | 36.73 ± 0.74 | .505 |
| Respiratory rate (breaths per min) (mean ± SD) | 21.39 ± 7.22 | 19.96 ± 2.42 | .107 | 20.18 ± 2.99 | 20.11 ± 2.08 | .894 |
| diastolic BP (mean ± SD), mm Hg | 83.23 ± 11.88 | 81.81 ± 13.47 | .484 | 83.45 ± 11.72 | 82.66 ± 12.69 | .779 |
| systolic BP (mean ± SD), mm Hg | 138.66 ± 17.97 | 134.76 ± 18.53 | .182 | 140.53 ± 17.97 | 136.03 ± 18.58 | .287 |
| Heart rate (mean ± SD), bpm | 89.01 ± 14.35 | 89.19 ± 17.62 | .945 | 89.13 ± 13.71 | 88.82 ± 14.03 | .921 |
| White blood cell count (×109/L) (mean ± SD) | 5.92 ± 2.28 | 7.54 ± 2.31 | .019 | 6.53 ± 2.54 | 6.62 ± 2.42 | .887 |
| lymphocyte count (×109/L) (mean ± SD) | 1.26 ± 0.53 | 1.29 ± 0.60 | .757 | 1.38 ± 0.61 | 1.26 ± 0.55 | .38 |
| D‐dimer (mean ± SD), mg/L | 1.43 ± 2.04 | 1.38 ± 1.59 | .874 | 1.08 ± 1.55 | 1.49 ± 1.66 | .267 |
| Interleukin‐6 (mean ± SD), mmol/L | 18.51 ± 32.41 | 24.11 ± 52.72 | .419 | 14.97 ± 30.09 | 15.54 ± 27.05 | .932 |
| Interleukin‐2 receptor (mean ± SD), mmol/L | 732.69 ± 490.99 | 752.82 ± 550.00 | .809 | 631.87 ± 422.94 | 758.95 ± 393.16 | .179 |
| Alanine aminotransferase (mean ± SD), U/L | 26.04 ± 23.08 | 31.46 ± 21.82 | .134 | 26.03 ± 20.99 | 26.42 ± 16.25 | .927 |
| Aspartate aminotransferase (mean ± SD), U/L | 22.82 ± 13.42 | 29.95 ± 16.61 | .037 | 22.95 ± 13.44 | 22.92 ± 9.71 | .992 |
| lung image (%) | 75 (90.4) | 65 (87.8) | .802 | 32 (84.2) | 35 (92.1) | .478 |
| NT‐ProBNP (mean ± SD), pg/L | 1305.13 ± 7258.23 | 294.00 ± 385.08 | .233 | 164.18 ± 263.02 | 306.11 ± 442.72 | .094 |
| Creatinine (mean ± SD), U/L | 93.17 ± 116.46 | 80.32 ± 43.05 | .372 | 71.16 ± 17.44 | 76.32 ± 39.31 | .462 |
| Urea (mean ± SD), mmol/L | 5.69 ± 4.94 | 5.73 ± 3.45 | .957 | 4.79 ± 1.52 | 5.11 ± 1.96 | .431 |
| Uric acid (mean ± SD), mmol/L | 268.24 ± 82.61 | 285.67 ± 116.59 | .277 | 276.84 ± 83.11 | 264.35 ± 97.42 | .549 |
| Potassium (mean ± SD), mmol/L | 4.25 ± 0.56 | 4.22 ± 0.47 | .772 | 4.14 ± 0.44 | 4.24 ± 0.50 | .345 |
| Sodium (mean ± SD), mmol/L | 140.10 ± 2.90 | 139.45 ± 3.83 | .223 | 140.31 ± 2.83 | 140.32 ± 3.05 | .997 |
| C‐reactive protein (mean ± SD), mg/L | 29.57 ± 47.25 | 24.25 ± 50.47 | .496 | 13.45 ± 21.30 | 17.88 ± 25.46 | .413 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CCB, calcium channel blocker; PSM, propensity score matching; SD, standard deviation.
Table 2.
Univariate and multivariate Cox regression analysis for nucleic acid conversion time
| Characteristics | HR | P value | 95% CI | |
|---|---|---|---|---|
| ID | Lower | Upper | ||
| Univariate | ||||
| Gender | 0.94 | .78 | 0.59 | 1.5 |
| Age | 0.99 | .25 | 0.96 | 1 |
| Clinical classification | 0.19 | 1.20E‐08 | 0.11 | 0.34 |
| Diabetes | 1.6 | .092 | 0.93 | 2.6 |
| Coronary disease | 0.79 | .53 | 0.38 | 1.6 |
| Temperature | 1.1 | .68 | 0.78 | 1.5 |
| Respiratory rate (breaths per min) | 1 | .67 | 0.92 | 1.1 |
| Diastolic BP, mm Hg | 1 | .99 | 0.99 | 1 |
| Systolic BP, mm Hg | 1 | .8 | 0.99 | 1 |
| Heart rate, bpm | 1 | .65 | 0.98 | 1 |
| White blood cell count | 0.95 | .22 | 0.86 | 1 |
| Lymphpcyte count (×109/L) | 1.5 | .032 | 1 | 2.3 |
| D‐dimer, mg/L | 0.94 | .52 | 0.79 | 1.1 |
| Interleukin‐6, mmol/L | 1 | .27 | 0.99 | 1 |
| Interleukin‐2 receptor, mmol/L | 1 | .045 | 1 | 1 |
| Alanine aminotransferase, U/L | 1 | .47 | 0.99 | 1 |
| Aspartate aminotransferase, U/L | 1 | .69 | 0.98 | 1 |
| Lung image | 0.8 | .54 | 0.4 | 1.6 |
| NT‐ProBNP, pg/L | 1 | .3 | 1 | 1 |
| Creatinine, U/L | 1 | .72 | 0.99 | 1 |
| Urea, mmol/L | 0.95 | .43 | 0.84 | 1.1 |
| Uric acid, mmol/L | 1 | .6 | 1 | 1 |
| Potassium, mmol/L | 1.5 | .12 | 0.89 | 2.5 |
| Sodium, mmol/L | 0.97 | .38 | 0.89 | 1 |
| C‐reactive protein, mg/L | 0.99 | .087 | 0.98 | 1 |
| CCBs vs ACEI/ARBs groups | 0.6 | .036 | 0.38 | 0.97 |
| Multivariate | ||||
| Clinical classification | 0.201 | 2.36E‐07 | ||
| Lymphpcyte count (×109/L) | 1.363 | .107 | ||
| Interleukin‐2 receptor, mmol/L | 0.999 | .867 | ||
| CCBs vs ACEI/ARBs groups | 0.702 | .148 | ||
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CCB, calcium channel blocker; CI, confidence interval; HR, hazard ratio.
3.3. Comparison of nucleic acid conversion time between the ACEIs/ARBs and CCBs groups in all patients
We performed a stratified analysis according to different variables in 1:1 matched group. we used the Cox proportional hazards model to investigate the effect of baseline characteristics on nucleic acid conversion time. A univariable analysis indicated that clinical classification (P = 1.20E‐08), lymphocyte count (P = .032), and interleukin‐2 receptor (P = .045) were significantly associated with a lengthened negative time of nucleic acid (Table 2). In total, we found a significant difference in nucleic acid time between two groups in all univariable (P = .036) (Figure 2A). Furthermore, we included all variables mentioned earlier in the multivariable analysis. After adjustment for potential confounders, we found no obvious difference in nucleic acid conversion time between ACEIs/ARBs group and CCBs group (hazard ratio [HR]: 0.70; 95% confidence interval [CI]: [0.97, 3.38]; P = .18) (Table 2).
Figure 2.
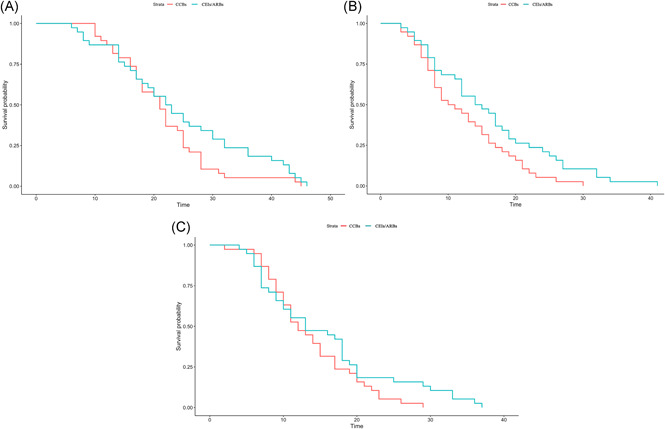
A, Cox regression analysis curve of nucleic acid conversion time of COVID‐19 patients with hypertension (after PSM). B, Cox regression analysis curve of chest CT improved time of COVID‐19 patients with hypertension (after PSM). C, Cox regression analysis curve of the hospitalization time of COVID‐19 patients with hypertension (after PSM). CT, computed tomography; PSM, propensity score matching
3.4. Comparison of chest CT improved time between the ACEIs/ARBs and CCBs groups in all patients
Furthermore, we used the Cox proportional hazards model to investigate the effect of baseline characteristics on chest CT improved time. Although a univariable analysis indicated that clinical classification (P = 1.70E‐08), lymphocyte count (P = .019), and uric acid (P = .024) were significantly associated with a lengthened negative time of chest CT improved (Table 3). However, we found no difference in chest CT improved time between two groups in all univariable (P = .19) (Figure 2B). subsequently, we included all variables mentioned earlier in the multivariable analysis. After adjustment for potential confounders, we also found no obvious difference in chest CT improved time between ACEIs/ARBs group and CCBs group (HR: 0.73; 95% CI: [0.45, 1.2]; P = .87) (Table 3).
Table 3.
Univariate and multivariate Cox regression analysis for chest CT improved time
| Characteristics | HR | P value | 95% CI | |
|---|---|---|---|---|
| ID | Lower | Upper | ||
| Univariate | ||||
| Gender | 0.91 | .7 | 0.57 | 1.5 |
| Age | 0.99 | .48 | 0.97 | 1 |
| Clinical classification | 0.21 | 1.70E‐08 | 0.12 | 0.34 |
| Diabetes | 1 | .96 | 0.6 | 2.6 |
| Coronary disease | 0.87 | .74 | 0.39 | 1.6 |
| Temperature | 1.3 | .11 | 0.95 | 1.5 |
| Respiratory rate (breaths per min) | 1 | .37 | 0.95 | 1.1 |
| Diastolic BP, mm Hg | 0.99 | .4 | 0.97 | 1 |
| Systolic BP, mm Hg | 1 | .83 | 0.99 | 1 |
| Heart rate, bpm | 0.99 | .48 | 0.98 | 1 |
| White blood cell count (×109/L) | 0.97 | .56 | 0.89 | 1 |
| lymphocyte count (×109/L) | 1.5 | .019 | 1.1 | 2.3 |
| D‐dimer, mg/L | 0.88 | .23 | 0.72 | 1.1 |
| Interleukin‐6, mmol/L | 0.99 | .24 | 0.99 | 1 |
| Interleukin‐2 receptor, mmol/L | 1 | .25 | 1 | 1 |
| Alanine aminotransferase, U/L | 1 | .089 | 1 | 1 |
| Aspartate aminotransferase, U/L | 1 | .36 | 0.99 | 1 |
| Lung image | 0.73 | .38 | 0.36 | 1.6 |
| NT‐ProBNP, pg/L | 1 | .37 | 1 | 1 |
| Creatinine, U/L | 1 | .86 | 0.99 | 1 |
| Urea, mmol/L | 0.94 | .3 | 0.82 | 1.1 |
| Uric acid, mmol/L | 1 | .024 | 1 | 1 |
| Potassium, mmol/L | 1.1 | .82 | 0.65 | 2.5 |
| Sodium, mmol/L | 0.98 | .62 | 0.9 | 1 |
| C‐reactive protein, mg/L | 0.99 | .26 | 0.99 | 1 |
| CCBs vs ACEI/ARBs groups | 0.73 | .19 | 0.45 | 0.97 |
| Multivariate | ||||
| Clinical classification | 0.211706 | 4.74E‐08 | ||
| Lymphpcyte count (×109/L) | 1.328439 | .0942 | ||
| Uric acid, mmol/L | 1.003377 | .0209 | ||
| CCBs vs ACEI/ARBs groups | 0.962869 | .8792 | ||
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CCB, calcium channel blocker; CI, confidence interval; CT, computed tomography; HR, hazard ratio.
3.5. Comparison of the hospitalization time between the ACEIs/ARBs and CCBs groups in all patients
To evaluate differences among antihypertensive drugs on the outcome measures of patients diagnosed with COVID‐19 combined hypertension, Cox proportional hazards model was performed to investigate these factors in the hospitalization time between ACEIs/ARBs and CCBs groups. As showed in Table 4, univariate Cox regression analysis identified six prognosis factors for hospitalization time: clinical classification (P < .001), lymphocyte count (P = .012) and interleukin‐2 receptor (P = .025), lung image (P = .037), uric acid (P = .045), potassium (P = .0086) (Figure 2C). Next, multivariate Cox regression analysis was performed to determine if these variables were independently related to hospitalization time. Multivariate Cox regression analysis revealed that no obvious difference in the hospitalization time between ACEIs/ARBs group and CCBs group (HR: 1.06; 95% CI: [0.44, 1.1]; P = .83) (Table 4).
Table 4.
Univariate and multivariate Cox regression analysis for the hospitalization time
| Characteristics | HR | P value | 95% CL | |
|---|---|---|---|---|
| ID | Lower | Upper | ||
| Univariate | ||||
| Gender | 0.83 | .44 | 0.52 | 1.3 |
| Age | 0.98 | .17 | 0.95 | 1 |
| Clinical classification | 0.13 | 3.40E‐11 | 0.072 | 0.24 |
| Diabetes | 1.4 | .16 | 0.86 | 2.4 |
| Coronary disease | 1.1 | .8 | 0.52 | 2.3 |
| Temperature | 1.2 | .3 | 0.87 | 1.6 |
| Respiratory rate (breaths per min) | 0.97 | .54 | 0.88 | 1.1 |
| Diastolic BP, mm Hg | 1 | .21 | 0.99 | 1 |
| Systolic BP, mm Hg | 1 | .29 | 0.99 | 1 |
| Heart rate, bpm | 1 | .91 | 0.98 | 1 |
| White blood cell count (×109/L) | 0.96 | .32 | 0.87 | 1 |
| Lymphpcyte count (×109/L) | 1.7 | .012 | 1.1 | 2.4 |
| D‐dimer, mg/L | 0.93 | .47 | 0.77 | 1.1 |
| Interleukin‐6, mmol/L | 1 | .64 | 0.99 | 1 |
| Interleukin‐2 receptor, mmol/L | 1 | .025 | 1 | 1 |
| Alanine aminotransferase, U/L | 1 | .33 | 0.99 | 1 |
| Aspartate aminotransferase, U/L | 1 | .84 | 0.98 | 1 |
| Lung image | 0.47 | .037 | 0.23 | 0.95 |
| NT‐ProBNP, pg/L | 1 | .86 | 1 | 1 |
| Creatinine, U/L | 1 | .68 | 0.99 | 1 |
| Urea, mmol/L | 0.92 | .19 | 0.81 | 1 |
| Uric acid, mmol/L | 1 | .045 | 1 | 1 |
| Potassium, mmol/L | 2 | .0086 | 1.2 | 3.3 |
| Sodium, mmol/L | 0.95 | .19 | 0.88 | 1 |
| C‐reactive protein, mg/L | 0.99 | .066 | 0.98 | 1 |
| CCBs vs ACEI/ARBs groups | 0.7 | .13 | 0.44 | 1.1 |
| Multivariate | ||||
| Clinical classification | 0.1114452 | 9.51E‐10 | ||
| Lymphpcyte count (×109/L) | 1.408974 | .1117 | ||
| Interleukin‐2 receptor | 0.9994966 | .1698 | ||
| Lung image | 0.5100719 | .1009 | ||
| Uric acid | 1.0030762 | .0347 | ||
| Potassium | 1.5275902 | .1536 | ||
| CCBs vs ACEI/ARBs groups | 1.0559883 | .8353 | ||
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CCB, calcium channel blocker; CI, confidence interval; HR, hazard ratio.
4. DISCUSSION
During the spread of the SARS‐CoV‐2, some reports of data still emerging and in need of full analysis indicated that certain groups of patients are at risk of COVID‐19, especially complicated with diabetes mellitus, hypertension, and coronary artery disease. 14 Currently, hypertension patients are basically treated with blood pressure‐lowering drugs, mainly including RAS blockers—ACEIs/ARBs, CCBs, β‐blockers, diuretics. However, it has been popularized in clinical treatment that RAS blockers might increase the risk of developing a severe and fatal SARS‐CoV‐2 infection. 15 Until now, the distinct effects of antihypertensives on prognosis remains unclear. The present study therefore retrospectively analyzed data from hospitalized COVID‐19 patients with hypertension in a single center in Wuhan, China, to compare the difference between ACEIs/ARBs groups and CCB groups, which may provide clinical evidence on the impact of ACEIs/ARBs on the clinical course of COVID‐19 infection.
Upon sufficient consideration of the relationship that ACE2 was the receptor that allowed coronavirus entry into cells, ACE2 overexpression facilitated the replication in cells that were otherwise resistant to the virus. In the RAAS, ACE2 catalyzes the conversion of angiotensin II to angiotensin 1 to 7, which acts as a vasodilator and exerts protective effects in the cardiovascular system. 5 In animal experiments, increased expression and activity of ACE2 in various organs including the heart were found in connection with ACEIs and ARBs administration. 16 In addition, more recent data 7 showing increased urinary secretion of ACE2 in hypertensive patients treated with the ARB olmesartan suggest that the upregulation of ACE2 may also occur in humans. These observations have been reiterated in the literature and on the web in recent days and the question arose whether RAAS inhibition may increase the risk of the deleterious outcome of COVID‐19 through upregulation of ACE2 and increase of viral load.
Nevertheless, we analyzed the clinical characteristics of 76 essential hypertension patients with COVID‐19 and then found a significant difference in nucleic acid time between two groups in all univariable (P = .036) in the univariable analysis. Specifically, compared with CCBs, we observed that clinical classification (P = 1.20E‐08), lymphocyte count (P = .032), and interleukin‐2 receptor (P = .045) were significantly associated with a lengthened negative time of nucleic acid of ACEI and ARB in univariable analysis. For further confirmation, we included all variables mentioned earlier in the multivariable analysis. After adjustment for potential confounders, we found no obvious difference in nucleic acid conversion time between ACEIs/ARBs and CCBs groups (HR: 0.70; 95% CI: [0.97, 3.38]; P = .18). Which indicated no clinical data providing a causal relationship between RAS blockers and SARS‐CoV2 associated increasing the entry and replication of viral.
RAS blockers such as ACEIs and ARBs were highly recommended medications for patients with cardiovascular diseases, such as refractory hypertension, coronary artery disease, heart failure, and postmyocardial infarction status. 17 Especially if elderly hypertensive patients suffered with cardiovascular diseases, diabetes, and renal insufficiency, with the highest level of evidence with regard to mortality reduction 18 Given that ACEIs/ARBs increase ACE2 expression and activity in the heart and kidneys in normotensive or hypertensive rats. ACE2 was also universally expressed in the heart, liver, kidney, blood vessels, and other tissues. Several studies 19 , 20 , 21 , 22 evidenced that ACEIs/ARBs prevent the progression of pulmonary complications in vulnerable populations, and reduce severe lung injury in certain viral pneumonias. Therefore, there is conflicting evidence that the use of ACEIs/ARBs has a positive or negative impact on COVID‐19. For this reason, we try to shed light on the relationship between the usage of ACEIs/ARBs and the lung injury of COVID‐19 combined hypertension in the real‐world study. We screened and analyzed the clinical data and found that no obvious difference in chest CT improved time between ACEIs/ARBs group and CCBs group (HR: 0.73; 95% CI: [0.45, 1.2]; P = .87) by the univariable and multivariable analysis.
Although the possible upregulation of ACE2 by RAAS inhibition and the theoretically associated risk of a higher susceptibility to infection, there is no direct evidence proving a causal relationship between ACE2 activity and SARS‐CoV2 associated mortality. 23 , 24 Consequently, we try to evaluate differences among antihypertensive drugs on the outcome measures of patients diagnosed with COVID‐19 combined hypertension. Until now, our current study manifested that no statistical differences in the in‐hospital mortality between ACEIs/ARBs and CCBs groups. We also evaluate the difference in ACEIs/ARBs on the outcome measures of patients diagnosed with COVID‐19 combined hypertension and found no obvious difference in the hospitalization time between ACEIs/ARBs and CCBs groups (HR: 1.06; 95% CI: [0.44, 1.1]; P = .83) by the univariable and multivariable analysis. ACEIs/ARBs did not increase the risk of extended course and poor prognosis of hypertension patients with COVID‐19. Notwithstanding there was conflicting evidence about the use of ACEIs/ARBs in the context of the pandemic COVID‐19 outbreak, our research suggested continuation of usual ACEIs/ARBs treatment for hypertension patients with COVID‐19.
4.1. Limitations
Our study has several limitations. First, only 76 patients with confirmed COVID‐19 were included, and a larger cohort study is needed to verify our conclusions. Second, as a retrospective study, some other specific information regarding cardiovascular complications such as echocardiography were not presented in the study because the data were incomplete owing to the limited conditions in the isolation ward and the urgency of containing the COVID‐19 epidemic. Third, we did not observe mild cases who were treated at home. Four, we did not observe who changed past usage of ACEIs/ARBs medication at home to CCBs in hospital.
4.2. Conclusion
Here, the current analysis shows that as compared with CCBs, ACEIs/ARBs did not increase the risk of extended course and poor prognosis of hypertension patients with COVID‐19. However, long‐term observation and prospective study design on the difference between the ACEIs/ARBs and non‐ACEIs/ARBs are needed.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
JL and DL conceived and designed the study. YL, SY, and XB collected clinical cases. KC performed statistical analysis and XL wrote the paper. JL, DL, and XL reviewed and edited the manuscript. All authors read and approved the manuscript.
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
We appreciate the healthcare workers fight in the front line of the outbreak of COVID‐19.
Liu X, Liu Y, Chen K, et al. Efficacy of ACEIs/ARBs vs CCBs on the progression of COVID‐19 patients with hypertension in Wuhan: A hospital‐based retrospective cohort study. J Med Virol. 2021;93:854–862. 10.1002/jmv.26315
Contributor Information
Juan Li, Email: 947281063@qq.com.
Dong Liu, Email: ld2069@outlook.com.
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this published article.
REFERENCES
- 1. Novel Coronavirus (2019‐nCoV) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed April 6, 2020.
- 2. Novel CPERE. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145‐151. [DOI] [PubMed] [Google Scholar]
- 3. Hanff TC, Harhay MO, Brown TS, Cohen JB, Mohareb AM. Is there an association between COVID‐19 mortality and the renin‐angiotensin system—a call for epidemiologic investigations. Clin Infect Dis. 2020;71:870‐874. 10.1093/cid/ciaa329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China: Life Sci. 2020;63(3):457‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation. 2005;111(20):2605‐2610. [DOI] [PubMed] [Google Scholar]
- 6. Zhang D, Song L, Li Y, et al. Application status of antihypertensive drugs in patients with hypertension in southwest China. Chin J Epidemiol. 2020;4:520‐525. [Google Scholar]
- 7. Furuhashi M, Moniwa N, Mita T, et al. Urinary angiotensin‐converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28(1):15‐21. [DOI] [PubMed] [Google Scholar]
- 8. Wang X, Ye Y, Gong H, et al. The effects of different angiotensin II type 1 receptor blockers on the regulation of the ACE‐AngII‐AT1 and ACE2‐Ang (1–7)‐Mas axes in pressure overload‐induced cardiac remodeling in male mice. J Mol Cell Cardiol. 2016;97:180‐190. [DOI] [PubMed] [Google Scholar]
- 9. Team RC. R : A language and environment for statistical computing. Published online 2013.
- 10. Team Rs. RStudio: integrated development for R. RStudio Inc Boston MA URL Httpwww Rstudio Com. 2015;42:14. [Google Scholar]
- 11. Stuart EA. Developing practical recommendations for the use of propensity scores: discussion of ‘A critical appraisal of propensity score matching in the medical literature between 1996 and 2003' by Peter Austin, Statistics in Medicine. Stat Med. 2008;27(12):2062‐2065. [DOI] [PubMed] [Google Scholar]
- 12. Drawing Survival Curves using “ggplot2.” https://rpkgs.datanovia.com/survminer/index.html. Accessed April 6, 2020.
- 13. Therneau T Therneau/Survival.; 2020. https://github.com/therneau/survival. Accessed April 6, 2020.
- 14. Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin‐converting enzyme inhibitors in hypertension: to use or not to use? J Am Coll Cardiol. 2018;71(13):1474‐1482. [DOI] [PubMed] [Google Scholar]
- 15. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuster GM, Pfister O, Burkard T, et al. SARS‐CoV2: should inhibitors of the renin‐angiotensin system be withdrawn in patients with COVID‐19? Eur Heart J. 2020;41(19):1801‐1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mendoza‐Torres E, Oyarzún A, Mondaca‐Ruff D, et al. ACE2 and vasoactive peptides: novel players in cardiovascular/renal remodeling and hypertension. Ther Adv Cardiovasc Dis. 2015;9(4):217‐237. [DOI] [PubMed] [Google Scholar]
- 18. Soto M, Bang SI, McCombs J, Rodgers KE. Renin angiotensin system‐modifying therapies are associated with improved pulmonary health. Clin Diabetes Endocrinol. 2017;3(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caldeira D, Alarcão J, Vaz‐Carneiro A, Costa J. Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: systematic review and meta‐analysis. BMJ. 2012;345:e4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pinheiro DS, Santos RS, Jardim PCBV, et al. The combination of ACE I/D and ACE2 G8790A polymorphisms revels susceptibility to hypertension: a genetic association study in Brazilian patients. PLoS One. 2019;14(8):e0221248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu C, Li Y, Guan T, et al. ACE2 polymorphisms associated with cardiovascular risk in Uygurs with type 2 diabetes mellitus. Cardiovasc Diabetol. 2018;17(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diaz JH. Hypothesis: angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID‐19. J Travel Med, 27(3):taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891‐975. [DOI] [PubMed] [Google Scholar]
- 24. Patel AB, Verma A. COVID‐19 and angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. 2020;323:1769‐1770. 10.1001/jama.2020.4812 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Data Availability Statement
All data generated or analysed during this study are included in this published article.


