Abstract
Solid organ transplant recipients (SOTr) with coronavirus disease 2019 (COVID-19) are expected to have poorer outcomes compared to nontransplant patients because of immunosuppression and comorbidities. The clinical characteristics of 47 SOTr (38 kidneys and 9 nonkidney organs) were compared to 100 consecutive hospitalized nontransplant controls. Twelve of 47 SOTr managed as outpatients were subsequently excluded from the outcome analyses to avoid potential selection bias. Chronic kidney disease (89% vs 57% P = .0007), diabetes (66% vs 33% P = .0007), and hypertension (94% vs 72% P = .006) were more common in the 35 hospitalized SOTr compared to controls. Diarrhea (54% vs 17%, P < .0001) was more frequent in SOTr. Primary composite outcome (escalation to intensive care unit, mechanical ventilation, or in-hospital all-cause mortality) was comparable between SOTr and controls (40% vs 48%, odds ratio [OR] 0.72 confidence interval [CI] [0.33-1.58] P = .42), despite more comorbidities in SOTr. Acute kidney injury requiring renal replacement therapy occurred in 20% of SOTr compared to 4% of controls (OR 6 CI [1.64-22] P = .007). Multivariate analysis demonstrated that increasing age and clinical severity were associated with mortality. Transplant status itself was not associated with mortality.
KEYWORDS: clinical research/practice, complication: infectious, infection and infectious agents - viral, infectious disease, kidney transplantation/nephrology, organ transplantation in general
Abbreviations: ACE2, angiotensin-converting enzyme 2; AKI, acute kidney injury; ALC, absolute lymphocyte counts; ARDS, acute respiratory distress syndrome; CKD, chronic kidney disease; CNIs, calcineurin inhibitors; COVID-19, coronavirus disease 2019; CPK, creatine phosphokinase; CRP, C-reactive protein; EMR, electronic medical record; GPU, general practice unit; HCQ, hydroxychloroquine; HFH, Henry Ford Hospital; HFHS, Henry Ford Health System; ICU, intensive care unit; LDH, lactate dehydrogenase; LOS, length of hospital stay; mTOR, mammalian target of rapamycin; NEWS, National Early Warning Score; qSOFA, quick sequential organ failure; RAAS, renin-angiotensin-aldosterone system; RT-PCR, reverse-transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOT, solid organ transplant
1. INTRODUCTION
In December 2019, a cluster of patients with acute respiratory illness of unknown origin was reported from the city of Wuhan, Hubei province, China. It was determined that the causative agent was a novel coronavirus—severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 , 2 As of May 15, 2020, the United States has over 1.4 million confirmed cases of coronavirus disease 2019 (COVID-19) and 85 000 deaths.3 This has created unprecedented challenges to healthcare systems across the United States particularly, in large urban areas such as in Detroit, Michigan, which has 50 079 confirmed cases and 4 825 deaths.4 Most patients will have mild illness, but older persons and those with comorbidities may develop severe disease necessitating hospitalization and escalation of care to the intensive care unit (ICU). The epidemiology, clinical characteristics, and outcomes of COVID-19 among solid organ transplant (SOT) recipients are undefined. Few early descriptive case reports and case series of SOT recipients with COVID-19 suggest poor outcomes; however, it is unknown if this is different from COVID-19 in the nontransplant population.5 , 6 We report a comparative cohort study of 47 SOT recipients and 100 nontransplant patients diagnosed with COVID-19. The clinical features, severity of disease, and outcomes of the hospitalized SOT recipients and non-SOT patients with COVID-19 were compared.
2. METHODS
2.1. Study setting
The study was performed at the Henry Ford Health System (HFHS) a quaternary-care academic institution that comprises 5 hospitals in southeast and south-central Michigan. The Henry Ford Transplant Institute housed in HFHS annually performs approximately 300 organ transplants including kidney, liver, heart, lung, pancreas, intestinal, and multiorgan transplants.
2.2. Study population
A confirmed case of COVID-19 was defined as a patient with a positive reverse transcription-polymerase chain reaction (RT-PCR) assay for SARS-CoV-2 in a nasopharyngeal sample tested by the Michigan Department of Health and Human Services or the HFHS centralized clinical microbiology laboratory.
Cases: SOT recipients with the confirmed diagnosis of COVID-19 from March 20, 2020 through April 18, 2020, were eligible for inclusion if they were 18 years of age or older. SOT recipients managed as outpatients were subsequently excluded from the outcome analyses to avoid potential selection bias.
Controls: A convenience sample of 100 consecutive nontransplant patients hospitalized with the confirmed diagnosis of COVID-19 from March 20, 2020 onwards were eligible for inclusion if they were 18 years of age or older.
The Henry Ford Hospital (HFH) COVID-19 severity scoring system was used to risk stratify patients on presentation to the hospital as mild, moderate, or severe COVID-19. Mild disease was defined as patients who had normal chest radiography and SpO2 of ≥94% without the need for supplemental oxygen. Moderate disease patients were those who had abnormal chest radiography, SpO2 of <94% and needing between 1 and 5 liters/min supplemental O2. Patients with severe disease were defined by abnormal chest radiography, SpO2 of <94% and requiring ≥6 liters/min of O2.
2.3. Study design
This was a retrospective cohort study examining epidemiologic, laboratory, and clinical characteristics for adverse outcomes comparing SOT recipients and nontransplant controls hospitalized for COVID-19. The study was approved by the institution’s institutional review board (#13739) with waiver of consent.
Both cases and controls received standard care, comprised of supplemental oxygen, high-flow nasal cannula support, mechanical ventilation, antibiotics, antiviral agents, immunomodulating medications, vasopressor support, and renal replacement therapy, as determined by the primary team.
2.3.1. Immunosuppression management
The management of immunosuppression was at the discretion of the transplant team. In general the approach was to decrease overall immunosuppression. Typically, antimetabolites were the first to either be withdrawn or dose reduced with consideration for modification of calcineurin inhibitors (CNIs).
2.3.2. Specific COVID-19 treatment (COVID-19 protocol)
HFHS deployed a COVID-19 team during the time of outbreak in an effort to standardize management. Laboratory tests ordered included baseline serum C-reactive protein (CRP), lactate dehydrogenase (LDH), ferritin, D-dimer, creatine phosphokinase (CPK), high-sensitivity troponin, and procalcitonin. Additionally, the protocol also defined the use of antiviral agents and adjuvant immunomodulation therapies in the treatment of patients with COVID-19.
COVID-19-positive SOT recipients and controls with moderate to severe disease as per our severity criteria along with QTc interval of <500 msec received hydroxychloroquine (HCQ) 400mg twice daily orally for the first 24 hours followed by 200mg twice a day for 4 days in patients as the antiviral agent. Adjuvant therapy included an early short course of corticosteroids (methylprednisolone 1mg/kg in 2 divided doses for 3-7 days).7
2.4. DATA collection
Data were abstracted from the electronic medical record (EMR) and recorded in a standardized electronic case report form. Data included patient demographics, clinical symptoms and signs, laboratory and radiologic results at the time of presentation. Patient data were censored on April 26, 2020.
2.5. Study definitions
The National Early Warning Score (NEWS) and quick sequential organ failure (qSOFA) were calculated to evaluate baseline illness severity based on vital signs obtained in the emergency department.8 , 9
2.6. Outcome measures
2.6.1. Primary end point
The primary composite end point was escalation to ICU from a general practice unit (GPU), progression to respiratory failure requiring mechanical ventilation, or in-hospital all-cause mortality. This composite end point has been utilized to measure outcomes in COVID-19 pneumonia previously.2 , 7
2.6.2. Secondary end points
Secondary end points included development and severity of acute respiratory distress syndrome (ARDS), acute kidney injury (AKI) requiring renal replacement therapy, median duration of ventilation, and length of hospital stay (LOS). ARDS was diagnosed and classified according to the Berlin Definition.10 AKI and chronic kidney disease (CKD) were diagnosed according to the Kidney Disease: Improving Global Outcomes definition.11
2.7. Statistical analysis
Continuous variables were reported as median and interquartile range (IQR) and compared using the Mann-Whitney test or t test, as appropriate. Categorical data were reported as number and percentage and compared using the chi-square test or Fisher’s exact t test as appropriate. No imputation was made for missing data points. The sample size was derived from all eligible consecutive hospitalized patients during the study period. A 2-sided α ≤0.05 was considered statistically significant. Bivariate and multivariable logistic regression analysis was planned a priori to test the association between the individual composite end point components and SOT status. Clinically observed risk factors for mortality were fit a priori into a multivariate logistic regression model examining key exposures and outcomes. Survival curves were modeled using Kaplan-Meier estimation censoring data at the end of follow-up. Statistical analysis was performed using SAS 9.4 (SAS Institute, Cary, NC).
3. RESULTS
We enrolled 47 SOT recipients (cases) and 100 consecutive hospitalized nontransplant COVID-19-positive patient (controls). All patients were followed for a median duration of 35 (IQR 20-36) days or until death.
The clinical characteristics of 47 SOT recipients (38 kidneys and 9 nonkidney organs) were compared to 100 controls. Twelve of 47 SOT recipients managed as outpatients were subsequently excluded from the outcome analyses to avoid potential selection bias.
3.1. Clinical characteristics
The demographics, presence of coexisting conditions, clinical symptoms, laboratory findings, and severity of illness on presentation are shown in Tables 1 and 2. We initially compared SOT recipients with COVID-19 that were hospitalized vs those who were managed as outpatients (Table 1). Overall 89% of all SOT recipients had undergone transplantation >1 year. Characteristics were comparable across all key demographics and coexisting conditions. Cough, fever, and shortness of breath were the most common presenting symptoms. Overall 55% of SOT recipients had diarrhea as a presenting symptom. Hospitalized SOT recipients were more likely to complain of shortness of breath compared to outpatients (68% vs 33%, P = .04). Hospitalized SOT recipients were more likely to have a lower median absolute lymphocyte counts (ALC) (0.5 vs 1.6 x 10-9 per liter, P = .02) and abnormal chest imaging (83% vs 25%, P = .0005). Overall, on presentation 28%, 66%, and 6% of SOT recipients were stratified as having mild, moderate, and severe disease, respectively, utilizing the HFH COVID-19 severity score. Hospitalized SOT recipients presented with a higher degree of illness severity, based on median qSOFA score (1 vs 0, P = .06), median NEWS score (6.5 vs 2, P = .0009), and HFH COVID-19 severity score.
TABLE 1.
Characteristics of hospitalized and nonhospitalized transplant recipients
| Characteristics | Total transplant recipients (n = 47) | Hospitalized transplant recipients (n = 35) | Nonhospitalized transplant recipients (n = 12) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Median age (IQR) - y | 61 (52-70) | 62 (48-71) | 59 (55-67) | .92 |
| Male sex – no. (%) | 32 (68.1) | 23 (65.7) | 9 (75) | .73 |
| Black race – no. (%) | 39 (83.0) | 30 (85.7) | 9 (75) | .40 |
| Median BMI (IQR) - kg/m2 | 27.4 (25.5-33) | 27.3 (24.9-33) | 27.5 (26.8-32.2) | .95 |
| Coexisting conditions – no. (%) | ||||
| Chronic obstructive pulmonary disease | 7 (14.9) | 6 (17.1) | 1 (8.3) | .66 |
| Chronic kidney disease | 42 (89.4) | 31 (88.6) | 11 (91.7) | 1.00 |
| Congestive heart failure | 11 (23.4) | 10 (28.6) | 1 (8.3) | .24 |
| Coronary artery disease | 6 (12.8) | 5 (14.29) | 1 (8.3) | 1.00 |
| Diabetes | 32 (68.1) | 23 (65.7) | 9 (75) | .73 |
| Hypertension | 44 (93.6) | 33 (94.3) | 11 (91.7) | 1.00 |
| Malignancy | 4 (8.5) | 4 (11.4) | 0 (0) | .55 |
| Smoking history | 11 (23.4) | 9 (25.7) | 2 (16.7) | .70 |
| Type of organ transplant (%)a | ||||
| Kidney | 38 (80.9) | 26 (74.3) | 12 (100) | .49 |
| Liver | 1 (2.1) | 0 (0) | 1 (8.3) | |
| Heart | 5 (10.6) | 5 (14.3) | 0 (0) | |
| Lung | 4 (8.5) | 4 (11.4) | 0(0) | |
| Pancreas | 1 (2.1) | 1 (2.9) | 0 (0) | |
| Exposure history | ||||
| Contact with person with COVID-19 – no (%) | 12 (25.5) | 10 (28.6) | 2 (16.7) | .70 |
| Symptoms | ||||
| Altered mentation – no. (%) | 4 (8.5) | 4 (11.4) | 0 (0) | .56 |
| Cough – no. (%) | 32 (68.1) | 22(62.9) | 10 (83.3) | .29 |
| Diarrhea – no. (%) | 26 (55.3) | 19 (54.3) | 7 (58.3) | .81 |
| Fatigue – no. (%) | 21 (44.7) | 16 (45.7) | 5 (41.7) | .81 |
| Fever – no. (%) | 29 (61.7) | 23 (65.7) | 6 (50) | .49 |
| Myalgia – no. (%) | 20 (42.6) | 17 (48.6) | 3 (25) | .15 |
| Shortness of breath – no. (%) | 28 (59.6) | 24 (68.6) | 4 (33.3) | .04 |
| Median duration from symptom onset to diagnosis (IQR) - d | 7 (3-10) | 7 (3-10) | 7 (3-10) | .76 |
| Laboratory test – median (IQR) | ||||
| White blood cell count (x10-9/L) | 5.6 (4.1 - 8.2) | 5.5 (4.1-8.1) | 6.2 (4.7-9.1) | .72 |
| Absolute lymphocyte count (x10-9/L) | 0.65 (0.40-0.80) | 0.50 (0.3-0.8) | 1.6 (0.8-2.0) | .02 |
| Hemoglobin (g/dL) | 11.9 (10.9-13.2 | 11.9 (10.7-13) | 13.5 (12.4-14.7) | .09 |
| Platelets (g/dL) | 174 (134-258) | 176 (133-261) | 166 (149-225) | .97 |
| C-reactive protein (mg/dL)b | 6.6 (2.2-13.5) | 7.5 (2.2-13.5) | 2.4 (2.4-2.4) | .74 |
| Imaging of the lungsc | ||||
| Abnormal - no. (%) | 32 (68.1) | 29 (82.8) | 3 (25) | .0005 |
| Severity of illness in emergency department | ||||
| Median qSOFA (IQR) | 1 (0-2) | 1 (0.5-2) | 0 (0-0) | .06 |
| Median NEWS (IQR) | 5 (3-8) | 6.5 (4-9) | 2 (0-4) | .0009 |
| Intubated at arrival – no. (%) | 1 (2.1) | 1 (2.9) | — | — |
| Direct admission to ICU – no. (%) | 5 (14.3) | 5 (14.3) | — | — |
| HFH COVID-19 Severity Score | ||||
| Mild – no. (%) | 13 (27.7) | 4 (11.4) | 9 (75) | .0001 |
| Moderate – no. (%) | 31 (65.9) | 28 (80) | 3 (25) | |
| Severe – no (%) | 3 (6.4) | 3 (8.6) | 0 (0) | |
| Changes in immunosuppression (%)d | 32 (69.5) | 28 (82.4) | 4 (33) | .006 |
| Reduction or cessation of antimetabolite | 27 (84) | 23(82) | 4 (100) | .08 |
| Reduction or cessation of CNI | 5 (15) | 5(17) | 0 (0 | — |
| Reduction or cessation of mTOR inhibitor | 1 (3) | 1 (3) | 0 (0) | — |
| Reduction or cessation of belatacept | 1 (3) | 1 (3) | 0 (0) | — |
Abbreviations: BMI, body mass index; CNI, calcineurin inhibitor; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; HFH, Henry Ford Hospital; ICU, intensive care unit; IQR, interquartile ratio; mTOR, mammalian target of rapamycin; NEWS, National Early Warning Score; q SOFA, quick sequential organ assessment.
Three SOT recipients had dual transplant.
Only 1 patient in the transplant nonhospitalized group had CRP level done.
Imaging of the lungs included chest X-ray or a chest computed tomography (CT) scan.
Total of 46 SOT recipients were on immunosuppressive therapy. One hospitalized SOT patient not receiving immunosuppression was excluded. Two patients had CNI reduced in addition to discontinuation of antimetabolite.
TABLE 2.
Characteristics of hospitalized transplant recipients and nontransplant controls
| Characteristics | Transplant recipients (n = 35) | Nontransplant controls (n = 100) | P value |
|---|---|---|---|
| Demographics | |||
| Median age (IQR) - y | 62 (48-71) | 60 (51-72) | .45 |
| Male sex - no. (%) | 23 (65.7) | 50 (50.0) | .11 |
| Black race - no. (%) | 30 (85.7) | 79 (79) | .39 |
| Median BMI (IQR) - kg/m2 | 27.2 (24.9-33) | 32.3 (28.0-37.9) | .02 |
| Coexisting conditions - no. (%) | |||
| Chronic kidney disease | 31 (88.6) | 57 (57) | .0007 |
| Chronic lung disease | 6 (17.1) | 13 (13) | .54 |
| Congestive heart failure | 10 (28.6) | 14 (14) | .05 |
| Coronary artery disease | 5 (14.3) | 12 (12) | .77 |
| Diabetes | 23 (65.7) | 33 (33) | .0007 |
| Hypertension | 33 (94.3) | 72 (72) | .006 |
| Malignancy | 4 (11.4) | 13 (13) | 1.00 |
| Smoking history | 9 (25.7) | 25 (25) | .94 |
| Exposure history | |||
| Contact with person with COVID-19 – no (%) | 10 (28.6) | 28 (28) | .95 |
| Symptoms | |||
| Altered mentation - no. (%) | 5 (14.3) | 8 (8) | .32 |
| Cough - no. (%) | 22 (62.9) | 74 (74) | .21 |
| Diarrhea - no. (%) | 19 (54.3) | 17 (17) | <.0001 |
| Fever - no. (%) | 23 (65.7) | 69 (69) | .72 |
| Myalgia - no. (%) | 17 (48.6) | 35 (35) | .16 |
| Shortness of breath - no. (%) | 24 (68.6) | 73 (73) | .62 |
| Median duration of symptoms (IQR) - d | 7 (3-10) | 5 (3-7) | .45 |
| Laboratory test median (IQR) | |||
| White blood cell count (x10-9/L) | 5.5 (4.1-8.1) | 6.05 (4.45-7.6) | .77 |
| Absolute lymphocyte count (x10-9/L) | 0.5 (0.3-0.8) | 0.8 (0.6-1) | .006 |
| Hemoglobin (g/dL) | 11.9 (10.7-13) | 13.3 (11.5-14.5) | .04 |
| Platelets (g/dL) | 176 (133-261) | 178 (150-229) | .77 |
| C-reactive protein (mg/dL) | 10.1 (5-15.8) | 7.5 (2.2-13.5) | .51 |
| D-dimer (ug/mL) | 1.02 (0.49-2.83) | 1.18 (0.75-2.13) | .70 |
| Ferritin, serum (ng/mL) | 617.5 (353-2314) | 586.5 (227-1130) | .73 |
| Lactate dehydrogenase serum (IU/L) | 288 (248-423) | 334 (245-443.5) | .16 |
| Lactic acid, plasma (mg/dL) | 1.5 (1.05-1.85) | 1.3 (1-1.8) | .75 |
| Procalcitonin (ug/L) | 0.23 (0.14-0.43) | 0.15 (0-0.44) | .50 |
| Imaging of lungs | |||
| Chest X-ray abnormal | 29 (85.3) | 79 (81.4) | .61 |
| Severity of illness in emergency department | |||
| Median qSOFA (IQR) | 1 (0.5-2) | 1 (0-1) | .02 |
| Median NEWS (IQR) | 6.5 (4-9) | 6.5 (4-9) | .75 |
| Intubated on admission - no. (%) | 1 (2.9) | 12 (12) | .18 |
| Direct admission to ICU - no. (%) | 5 (14.3) | 16 (16) | .81 |
| HFH COVID-19 Severity Score | |||
| Mild - no. (%) | 4 (11.4) | 13 (13) | .32 |
| Moderate - no. (%) | 28 (80) | 68 (68) | |
| Severe - no. (%) | 3 (8.6) | 19 (19) | |
| Treatment | |||
| Empiric antibiotic - no. (%) | 26 (74.3) | 72 (72) | .80 |
| Antiviral therapya | |||
| Hydroxychloroquine - no. (%) | 32 (91.4) | 79 (79) | .10 |
| Adjunctive therapy | |||
| Corticosteroid use - no. (%)b | 23 (65.7) | 65 (65) | .94 |
| Tocilizumab use - no. (%) | 3 (8.6) | 18 (18) | .19 |
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; HFH, Henry Ford Hospital; ICU, intensive care unit; IQR, interquartile ratio; NEWS, National Early Warning Score; q SOFA, quick sequential organ assessment.
At the time of the study, neither remdesivir nor convalescent plasma was administered to any patients.
Corticosteroids include methylprednisolone, prednisone, hydrocortisone, and dexamethasone.
Hospitalized SOT recipients and nontransplant patients with COVID-19 (Table 2) had similar demographics. SOT recipients had a higher proportion of chronic kidney disease (89% vs 57%, P = .0007), hypertension (94% vs 72%, P = .006), and diabetes mellitus (66% vs 33%, P = .0007) compared with nontransplant controls. SOT recipients were more likely to present with diarrhea (54% vs 17%, P ≤ 0.0001) and had lower median ALC (0.5 vs 0.8 x 10-9 per liter, P = .006) and lower median hemoglobin (11.9 vs 13.3 mg/dL, P = .04) compared to controls. SOT recipients presented with greater severity of illness as compared to controls (mean qSOFA 1.1 vs 0.7, P = .02). Using HFH COVID-19 severity score, the severity of illness among hospitalized transplant and nontransplant patients was comparable.
Additional subgroup analysis among kidney transplant recipients and recipients of other organs showed that demographics, clinical parameters, severity of illness at presentation, and outcomes were similar. (Table S1).
3.2. Management
Laboratory parameters including serum CRP, D-dimer, ferritin, LDH, procalcitonin, and abnormal chest radiography were comparable in hospitalized SOT recipients and nontransplant controls (Table 2).
Immunosuppression was reduced in 70% of all SOT recipients, more often in hospitalized patients than outpatients (Table 1). Withdrawal or dose reduction occurred in 84%, 15%, 3%, and 2% of patients receiving antimetabolite, CNIs, mammalian target of rapamycin (mTOR) inhibitors, and belatacept, respectively. Antiviral therapy with HCQ and adjunctive therapies with corticosteroids or tocilizumab was comparable (Table 2).
3.3. Outcomes
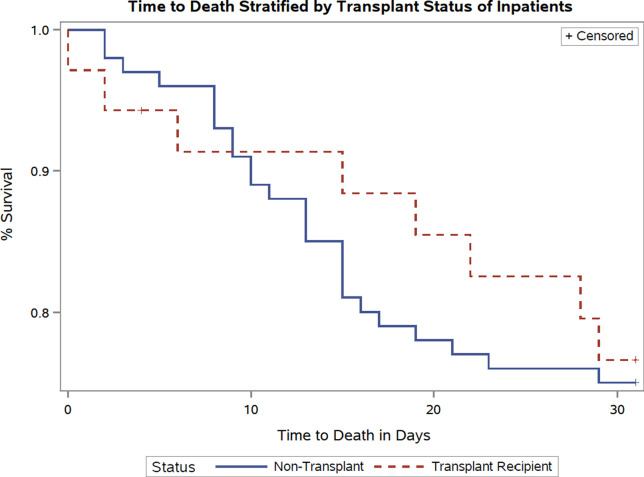
Overall mortality was 17% (8/47), in our transplant cohort, 23% (8/35) among those hospitalized and 58% (7/12) requiring mechanical ventilation. The primary composite outcome of ICU admission, mechanical ventilation, or death was comparable between hospitalized SOT recipients and nontransplant patients ( Table 3). Overall, mortality between these 2 groups was comparable (23% vs 25% odds ratio [OR] 0.88 confidence interval [CI] [0.36-2.21] P = .8). Beyond the first week of hospitalization, fewer deaths occurred in the transplant cohort compared to the controls ( Figure 1). Increasing HFH COVID-19 severity score was associated with greater risk of mortality, with no deaths among patients with mild disease (Figure S1).
TABLE 3.
Outcomes in hospitalized transplant recipients and nontransplant controls
| Transplant recipients (n = 35) | Nontransplant controls (n = 100) | Odds ratio (95% CI) | P value | |
|---|---|---|---|---|
| Primary composite outcome – no. (%) | 14 (40) | 48 (48) | 0.72 (0.33, 1.58) | .42 |
| Death – no. (%) | 8 (22.8) | 25 (25) | 0.88 (0.36, 2.21) | .80 |
| ICU admission – no. (%) | 13 (37.1) | 43 (43) | 0.78 (0.35, 1.73) | .55 |
| Mechanical ventilation – no. (%) | 12 (34.3) | 36 (36) | 0.93 (0.41, 2.08) | .86 |
| Median duration of mechanical ventilation (IQR) - d | 8 (3-18) | 8.5 (4-14) | .77 | |
| Failure to extubatea – no. (%) | 9 (75) | 17 (47.2) | 3.73 (0.87, 15.83) | .07 |
| ARDS – no. (%) | 12 (35.3) | 34 (34) | 1.06 (0.47, 2.39) | .89 |
| Mild | 0 | 3 (8.3) | ||
| Moderate | 4 (33) | 9 (25) | ||
| Severe | 8 (66) | 22 (61.1) | 1.05 (0.42, 2.64) | .92 |
| Shock – no. (%) | 8 (22.9) | 17 (17) | 0.69 (0.27, 1.78) | .44 |
| Acute kidney injury | 22 (46.8) | 43 (43) | 2.24 (1.02, 4.95) | .05 |
| Acute kidney injury requiring RRT – no. (%)b | 7 (20) | 4 (4) | 6.0 (1.64, 21.98) | .007 |
| Median hospital length of stay (IQR) - d | 4 (2-13) | 8 (5-14) | .22 | |
| Discharged from hospital – no. (%) | 24 (75) | 72 (72) | 1.04 (0.42, 2.61) | .93 |
| Remains hospitalized – no. (%) | 3 (8.6) | 3 (3) | 3.03 (0.58, 15.7) | .19 |
| Readmission within 30 d – no. (%) | 6 (19.4) | 9 (11.8) | 1.78 (0.57, 5.53) | .31 |
Abbreviations: ARDS, acute respiratory distress syndrome; CI, confidence interval; ICU, intensive care unit; IQR, interquartile ratio; RRT, renal replacement therapy.
Includes patients on mechanical ventilation that either died while ventilated or remained ventilated at the end of follow-up period.
Of the 7 transplant recipients (5 out 7 were kidneys): 2 died, 2 were discharged, and 3 remain hospitalized. Of the 4 controls: 1 died and 3 were discharged.
FIGURE 1.
Survival curves in hospitalized transplant recipients and nontransplant controls [Color figure can be viewed at wileyonlinelibrary.com]
Secondary outcome analysis showed both groups had similar median duration of mechanical ventilation, degree of ARDS, LOS, and readmissions. However, AKI (47% vs 43%, OR 2.24 CI [1.02-4.95] P = .05) and AKI requiring renal replacement therapy (20% vs 4%, OR 6.0 CI [1.64-21.98] P = .007) were more frequent in SOT recipients.
Univariate analysis of all hospitalized patients showed age >60 years, higher qSOFA, NEWS, HFH COVID-19 severity score, and ferritin >500 ng/mL were associated with mortality (Table S2). In all hospitalized patients and SOT recipients, ICU stay was significantly associated with death. Multivariate modeling was performed adjusting for age, coexisting conditions, transplant status, and HFH COVID-19 severity score. It showed that age >60 years, and HFH COVID-19 severity score were significantly associated with both the primary composite outcome and mortality ( Table 4). Transplant status itself was not associated with mortality in univariate (OR 0.9 CI [0.36-2.2] P = .8) or multivariate analysis (OR 1.11 CI [0.37-3.31] P = .85).
TABLE 4.
Multivariate analysis of all hospitalized patients - Odds of mortality and composite outcome as a function of clinically relevant risk factors
| Variable | Mortality | Composite outcome | ||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% Confidence interval | P value | Odds ratio | 95% Confidence interval | P value | |||
| Age > 60 y | 5.01 | 1.71 | 14.67 | .003 | 1.06 | 1.03 | 1.09 | .0007 |
| Coexisting conditions | ||||||||
| Diabetes | 1.48 | 0.55 | 3.93 | .44 | 4.07 | 1.52 | 10.89 | .005 |
| Chronic kidney disease | 0.84 | 0.29 | 2.49 | .76 | 1.32 | 0.47 | 3.65 | .60 |
| Severity of illness in the emergency department | ||||||||
| HFH COVID-19 Severity Score | 5.98 | 2.26 | 15.85 | .0003 | 18.35 | 4.19 | 80.36 | .0001 |
| Transplant status | 1.11 | 0.37 | 3.31 | .85 | 0.51 | 0.17 | 1.51 | .23 |
Abbreviations: COVID-19, coronavirus disease 2019; HFH, Henry Ford Hospital.
4. DISCUSSION
In our cohort study, mortality, need for ICU care, and mechanical ventilation support were comparable between COVID-19-positive hospitalized SOT recipients and nontransplant controls. Furthermore, our study demonstrates that transplant status by itself does not confer an increased risk for mortality. Additionally, we showed that our HFH COVID-19 severity score was strongly predictive of mortality.
In our transplant cohort, age >60 years and severity of COVID-19 on presentation strongly correlated with mortality, similar to what has been previously reported in the general population.12 , 13 Observational studies to date, as summarized in Table S3, report variable mortality in COVID-19-positive transplant recipients between 0% and 30%.5 , 6 , 14, 15, 16, 17, 18, 19, 20, 21 Our observed overall mortality rate of 17% in our transplant cohort is comparable to rates reported from urban centers. Lower mortality rates in other studies may be explained by smaller sample size, lesser severity of illness, limited follow-up, and population demographics.5 , 6 , 14, 15, 16, 17, 18, 19, 20, 21 In our hospitalized transplant cohort, those requiring ICU care and mechanical ventilation had a significantly higher mortality rate of 23% and 58%, respectively. This is comparable to recent reports from New York City.20
Of note, in our predominantly kidney transplant population, rates of AKI were high, 47%, comparable to rates of 25%-57% in other studies.14, 15, 16, 17 , 19 Of those who had AKI, about a third needed RRT, similar to previous reports.5 , 15 The high incidence of kidney injury in patients with COVID-19 may be multifactorial, including binding and direct renal injury caused by SARS-CoV-2 suggested by renal histopathological studies. Hua Su et al demonstrated virus nucleoprotein antigens in the renal tubules and clusters of coronavirus particles in the tubular epithelium and podocytes.22 Furthermore, the receptor of SARS-CoV-2, angiotensin-converting enzyme 2 (ACE2) found on renal epithelial cells, was found to be upregulated in patients with COVID-19. Modulators of the renin-angiotensin-aldosterone system (RAAS) including ACE2 and angiotensin receptor blockers may have a potential role in mitigating the risk for AKI.23 The high proportion of CKD, diarrhea, and CNIs use in our transplant cohort may also have contributed to AKI. It is notable, matched controls had similar rates of AKI as our SOT cohort.
Early reports have suggested poorer outcomes in SOT recipients with COVID-19 because of chronic immunosuppression and higher rates of comorbidities. In our study, a greater proportion of COVID-19-positive SOT recipients had coexisting conditions, particularly congestive heart failure, diabetes, CKD, and hypertension compared to nontransplant patients. Interestingly, despite this observation, mortality and other adverse outcomes did not occur more frequently among SOT recipients. The reasons for this are currently unknown but may be related to blunting of inflammatory cascades and cytokine release due to chronic immune suppression in transplant recipients. More research is needed to elucidate these theories further.
Similar to other studies, fever, cough, and fatigue were the most common presenting symptoms in our entire cohort. Interestingly, diarrhea was the presenting symptom in more than half of our transplant recipients. Previous studies estimate incidence of diarrhea to range from 14% to 50% in COVID-19-positive transplant patients (Table S3). Diarrhea may be a direct consequence of infection with SARS-CoV-2 that uses ACE2 for entry and serine protease TMPRSS2 for protein priming. Both ACE2 and TMPRSS2 are expressed in the epithelium of the small intestine.24 Diarrhea in our kidney transplant recipients may also be due to agents such as mycophenolate.
Hospitalized transplant patients were more likely to present with severe disease and COVID-19 pneumonia characterized by shortness of breath, hypoxia, and abnormal chest X-ray on presentation. Lymphopenia and anemia were more common among our hospitalized SOT recipients in comparison to nontransplant patients and may be the consequence of chronic immune suppression, and chronic disease.1 , 2 , 12 , 13 , 25, 26, 27, 28, 29
All of our hospitalized patients who qualified for treatment received HCQ as the antiviral agent. HCQ has been hypothesized to possess direct antiviral activity against SAR-COV-2 by increasing intracellular pH resulting in decreased phagolysosome fusion, impairing viral receptor glycosylation. In addition, it also has immune-modulating effect by inhibiting toll-like receptor signaling, and decreasing production of cytokines especially interleukin (IL)-1 and IL-6.30 Most of our transplant patients who had infectious complications during their hospitalization were managed in the usual fashion of reducing immune suppression in stepwise fashion and prompt antimicrobial therapy when needed. As per institutional COVID-19 treatment protocol, the majority of patients received corticosteroids early in their clinical course to mitigate the hyperinflammatory syndrome.7 Other immunomodulatory agents or other antiviral agents such as remdesivir may have a role in the management of COVID-19.31 , 32
This study has limitations. It is a single-center retrospective study with convenience sampling. Almost all SOT recipients were hospitalized for moderate or severe COVID-19 and so the spectrum of COVID-19 including mild disease is undefined. Most SOT recipients were remote (> 1year) from transplantation and were receiving maintenance immunosuppression. This is similar to other reports and consequently the impact of COVID-19 in the early posttransplant period remains undefined. Our cohort of hospitalized transplant patients was composed of mostly kidney transplant recipients, and when compared to nonkidney transplant recipients there was no difference in presenting symptoms, comorbidities, laboratory markers, severity of disease, and mortality. However, the lack of a large cohort of nonkidney transplant limits extrapolation of these findings. Even though our median follow-up was more than 28 days, long-term outcomes such as graft function in our SOT cohort need further study.
In conclusion, hospitalized SOT recipients with COVID-19 have comparable mortality to nontransplant patients, despite a greater proportion of coexisting conditions and immune suppression. Mortality in COVID-19-positive SOT recipients is driven by the severity of illness at presentation, independent of transplant status.
ACKNOWLEDGMENTS
We acknowledge the entire Henry Ford Transplant Institute team for their collaborative efforts. We also acknowledge Azza Elamin MD for her help with data extraction.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Funding information None.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
Supplementary Material
REFERENCES
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.W-jie G, Gandhi M, Arons MM, Gandhi RT. China Medical Treatment Expert Group. Clinical Characteristics of Coronavirus Disease 2019 in China: NEJM. N Engl J Med. https://www.nejm.org/doi/full/10.1056/NEJMoa2002032
- 3.Coronavirus Disease 2019 (COVID-19). Centers for disease control and prevention. https://www.cdc.gov/coronavirus/2019-nCoV/index.html. Accessed April 23, 2020.
- 4.Michigan Data. Coronavirus - Michigan Data. https://www.michigan.gov/coronavirus/0,9753,7-406-98163_98173--,00.html. Accessed May 11, 2020.
- 5.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and Kidney Transplantation. N Engl J Med. 2020;382(25):2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20(7):1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fadel R, Morrison A, Vahia A, et al. Early Short Course Corticosteroids in Hospitalized Patients with COVID-19. 2020. [preprint] 10.1101/2020.05.04.20074609. [DOI] [PMC free article] [PubMed]
- 8.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of Clinical Criteria for Sepsis. JAMA. 2016;315(8):762. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84(4):465–470. doi: 10.1016/j.resuscitation.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 10.The ARDS Definition Task Force* Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 11.Kellum JA, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney International Supplements. 2012;2(1):1–138. [Google Scholar]
- 12.Wang D, Hu BO, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020. [DOI] [PMC free article] [PubMed]
- 15.Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020. [DOI] [PMC free article] [PubMed]
- 16.Early Description of Coronavirus Disease in Kidney Transplant Recipients in New York. J Am Soc Nephrol. 2019;2020 doi: 10.1681/asn.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kates OS, Fisher CE, Stankiewicz-Karita HC, et al. Earliest cases of coronavirus disease 2019 (COVID-19) identified in solid organ transplant recipients in the United States. Am J Transplant. 2020;20(7):1885–1890. doi: 10.1111/ajt.15944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montagud-Marrahi E, Cofan F, Torregrosa JV, et al. Preliminary data on outcomes of SARS-CoV-2 infection in a Spanish single centre cohort of kidney recipients. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed]
- 19.Nair V, Jandovitz N, Hirsch JS, et al. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20(7):1819–1825. doi: 10.1111/ajt.15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu L, Gong N, Liu B, et al. Coronavirus Disease 2019 Pneumonia in Immunosuppressed Renal Transplant Recipients: A Summary of 10 Confirmed Cases in Wuhan, China [published online ahead of print, April 18, 2020] Eur Urol. 2020;S0302–2838(20) doi: 10.1016/j.eururo.2020.03.039. 30214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fishman JA, Grossi PA. Novel Coronavirus-19 (COVID-19) in the immunocompromised transplant recipient: #Flatteningthecurve. Am J Transplant. 2020;20(7):1765–1767. doi: 10.1111/ajt.15890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Amico F, Baumgart DC, Danese S, Peyrin-Biroulet L. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention and management. Clin Gastroenterol Hepatol. 2020;18(8):1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy [published online ahead of print, April 9, 2020] Lancet Gastroenterol Hepatol. 2020;S2468–1253(20):30116. doi: 10.1016/S2468-1253(20)30116-3. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latif F, Farr MA, Clerkin KJ, et al. Characteristics and Outcomes of Recipients of Heart Transplant With Coronavirus Disease 2019. JAMA Cardiology. 2020. [DOI] [PMC free article] [PubMed]
- 27.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2019;2020:m792. doi: 10.1136/bmj.m792. [DOI] [PubMed] [Google Scholar]
- 29.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against todays diseases. Lancet Infect Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCreary EK, Pogue JM. Oxford University Press; US: 2020. “Coronavirus disease 2019 treatment: a review of early and emerging options.” Open Forum Infectious Diseases. Vol. 7. No. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19). JAMA. 2020 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material