Abstract
Several lines of evidence support a link between the essential element zinc and the coronavirus disease 2019 (COVID‐19). An important fact is that zinc is present in proteins of humans and of viruses. Some zinc sites in viral enzymes may serve as drug targets and may liberate zinc ions, thus leading to changes in intracellular concentration of zinc ions, while increased intracellular zinc may induce biological effects in both the host and the virus. Drugs such as chloroquine may contribute to increased intracellular zinc. Moreover, clinical trials on the use of zinc alone or in addition to other drugs in the prophylaxis/treatment of COVID‐19 are ongoing. Thereby, we aim to discuss the rationale for targeting zinc metalloenzymes as a new strategy for the treatment of COVID‐19.
Linked Articles
This article is part of a themed issue on The Pharmacology of COVID‐19. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.21/issuetoc
Keywords: COVID‐19, metalloenzyme, SARS‐CoV‐2, zinc, zinc ejecting drug, zinc finger
Abbreviations
- COVID‐19
coronavirus disease 2019
- MERS
Middle East respiratory syndrome
- MERS‐CoV
Middle East respiratory syndrome coronavirus
- Mpro
main protease (3CLpro, 3‐chymotrypsin‐like protease)
- PAC‐1
procaspase‐activating compound 1
- PLpro
papain‐like protease
- RdRp
RNA‐dependent RNA polymerase
- SARS‐CoV
severe acute respiratory syndrome coronavirus
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SARS
severe acute respiratory syndrome
- TPEN
N,N,N′,N′‐tetrakis(2‐pyridylmethyl)etylenediamine
- TSQ
p‐toluenesulfonamido‐quinoline
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) has emerged in December 2019 in the city of Wuhan, China and since then has spread worldwide. It is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) (Zhou et al., 2020). Until now, no drugs designed specifically against SARS‐CoV‐2 proteins have been developed. Novel drugs are urgently needed in view of the fact that the treatment with drugs, which are being repurposed for COVID‐19, such as chloroquine/hydroxychloroquine, are not safe in patients with cardiovascular co‐morbidities, constituting a large group of patients dying from this disease (Kalil, 2020).
Ananda Prasad (2012) recognized the nutritional essentiality of zinc in humans and the consequences of zinc deficiency in 1963 in Iran. Later, they observed recurrent opportunistic infections in patients with acrodermatitis enteropathica, in whom zinc deficiency is due to malabsorption of zinc caused by a mutation in ZIP4, an intestinal zinc transporter. Dysfunction of the immune system in acrodermatitis enteropathica patients has been corrected with zinc supplementation, thus demonstrating that zinc is essential for the function of the immune system (Shankar & Prasad, 1998). Potential benefits of zinc administration in COVID‐19 in terms of improved immunity, which may be foreseen in populations at risk for COVID‐19 and zinc deficiency, such as the elderly, have recently been discussed by Derwand and Scholz (2020), Rahman and Idid (2020) and Skalny et al. (2020).
Due to anti‐inflammatory properties, zinc has been suggested to limit the cytokine storm (Skalny et al., 2020), which might occur in patients with severe COVID‐19 (Mehta et al., 2020). A cytokine storm, also termed macrophage activation syndrome or secondary haemophagocytic lymphohistocytosis, is a potentially fatal systemic hyperinflammation associated with hypercytokinaemia and multiple organ failure (McGonagle, Sharif, O'Regan, & Bridgewood, 2020; Sun et al., 2020). Noteworthy, a combination of zinc, hydroxychloroquine and azithromycin has been proposed as an early treatment of COVID‐19 in the outpatient setting, which would prevent disease progression and hospitalization (Derwand & Scholz, 2020; Risch, 2020).
In the human body, zinc is the second most abundant metal. It plays catalytic, structural and signalling function. The biochemistry of zinc began in 1939 with the observation that the enzyme carbonic anhydrase contains zinc. Moreover, zinc was shown to be indispensable for its enzymatic activity (Lindskog, 1997; Maret, 2013). Since then, this element has been found in hundreds of other enzymes, which are called zinc metalloenzymes (Haraguchi, 2017; Maret, 2013). ACE and ACE2 belong to zinc metalloenzymes (Turner, Hiscox, & Hooper, 2004). Furthermore, zinc fingers, relatively small protein domains consisting of cysteines or cysteines and histidines bound to zinc ions have been discovered. It is predicted that 10% of the human genome encodes zinc fingers (Krishna, Majumdar, & Grishin, 2003; Maret, 2013).
Zinc plays an important role not only in proteins and enzymes of humans and other life forms but also in viruses. For example, RNA‐dependent DNA polymerase from the avian myeloblastosis virus was demonstrated to be a zinc‐dependent enzyme in 1974 (Poiesz, Seal, & Loeb, 1974). Zinc fingers are present in many viral proteins (Lei, Kusov, & Hilgenfeld, 2018; Ma et al., 2015; Ma‐Lauer et al., 2016; Tijms, van Dinten, Gorbalenya, & Snijder, 2001). Versatile functions of zinc fingers are being increasingly uncovered (Fu & Blackshear, 2017; Jen & Wang, 2016; Laity, Lee, & Wright, 2001). As structural motifs, they are gaining attention as drug targets—the disruption of zinc fingers in viral proteins, which causes destabilization of proteins, has been proposed as a therapeutic approach to treat viral diseases (Abbehausen, 2019; Garcia & Damonte, 2007).
Our aim is to discuss the possibility of targeting zinc as a therapeutic strategy for COVID‐19. We start with background information on potential drug targets for SARS‐CoV‐2 and classes of compounds that can be collectively termed as zinc targeting drugs. We will attempt to analyse the data on the effects of agents targeting zinc fingers in viral metalloenzymes. These agents cause the removal of zinc from the proteins, which in turn destabilises these proteins leading to an increase in the intracellular concentration of zinc ions plus other agents that induce changes in intracellular levels of zinc (zinc ionophores), with information on the consequences of altered level of intracellular zinc, with will focus particularly on SARS‐CoV‐2 and related pathogens. Furthermore, we will provide examples of compounds targeting zinc that have entered clinical trials in order to demonstrate that investigating zinc drugs may lead to success in the clinic. Finally, we summarize current clinical trials on the use of zinc in the treatment and prophylaxis of COVID‐19.
2. DRUG TARGETS FOR SARS‐CoV‐2
The human pathogen responsible for the outbreak of COVID‐19 has a positive‐sense RNA genome and has been placed within the Coronaviridae family (Coronaviridae Study Group of the International Committee on Taxonomy of Viruses, 2020). Because of the relatedness to severe acute respiratory syndrome coronavirus (SARS‐CoV) (Zhou et al., 2020), which causes severe acute respiratory syndrome (SARS), the virus responsible for the 2019 outbreak has been designated as SARS‐CoV‐2 (Coronaviridae Study Group of the International Committee on Taxonomy of Viruses, 2020). SARS‐CoV‐2, SARS‐CoV and Middle East respiratory syndrome (MERS) coronavirus (MERS‐CoV) are the three coronaviruses behind the major epidemics of the last two decades.
SARS‐CoV (Turner et al., 2004) and SARS‐CoV‐2 (Shang et al., 2020) use the host zinc metalloenzyme, ACE2, as an entry point to cells. Inside cells, the RNA of coronaviruses is translated into two large polyproteins, pp1a and pp1ab. These polyproteins are cleaved by the main protease (Mpro, or 3‐chymotrypsin‐like protease, 3CLpro) and the papain‐like protease (PLpro) into non‐structural proteins. Non‐structural protein 12 contains the RNA‐dependent RNA polymerase (RdRp) domain. Non‐structural proteins assemble into the replicase–transcriptase complex and are responsible for replication and transcription (de Wit, van Doremalen, Falzarano, & Munster, 2016; Fehr & Perlman, 2015). As indispensable enzymes in virus replication, the two SARS‐CoV‐2 proteases, Mpro and PLpro, and RdRp are attractive therapeutic target for future drugs against SARS‐CoV‐2.
The 3D structure of the Mpro of SARS‐CoV‐2 has been deposited into the Protein Data Bank database under entry 6LU7. The comparison of Mpros deposited in the Protein Data Ban has demonstrated that the substrate‐binding pocket of Mpros is highly conserved among coronaviruses, thus suggesting that inhibitors targeting this site should have broad activity against coronaviruses (Jin et al., 2020). Furthermore, other drug targets such as PLpro or RdRp are conserved between SARS‐CoV‐2 and SARS‐CoV (Sargsyan et al., 2020; Wu et al., 2020). Thus, although no specific drugs have been discovered during SARS or MERS epidemics (de Wit et al., 2016), the outcomes of studies on drug leads for SARS‐CoV may give some insights into the possible treatments for SARS‐CoV‐2.
For that reason, data on the relationship between zinc and Mpro, PLpro or RdRp of SARS‐CoV‐2, SARS‐CoV or MERS‐CoV will be discussed in detail in subsequent sections of this review, as they are potentially important for drug discovery.
3. DRUGS TARGETING ZINC
Ionophores are molecules forming complexes with ions and facilitate ion transport across lipid bilayers. There are ionophores promoting transport of cations (cationophores) and anion (anionophores), but the latter are less common (Alfonso & Quesada, 2013). Cationic ionophores may transfer proton, alkali, alkaline earth or transition metal ions and may display selectivity for some of them (Alfonso & Quesada, 2013; Freedman, 2011; Riddell, 2002). Because of the similarities between these targets it is unlikely that an ionophore will bind one ion at the exclusion of others (Helsel & Franz, 2015). However, ionophores may be selective in terms of, for example kinetics, as they may transport one ion faster than the others (Helsel & Franz, 2015; Riddell, 2002).
Because the plasma membrane is non‐permeable to ions, ionophores comprise a lipophilic exterior that facilitates transport across the membrane and a hydrophilic interior, where an ion is bound (Kaushik, Yakisich, Kumar, Azad, & Iyer, 2018). When the pH of the extracellular space is higher than the pKa of the ionophore, the compound binds a metal ion. A complex is formed, which diffuses across the plasma membrane. When the pH in the intracellular space is lower than the ionophore's pKa, the compound releases the ion. As a result, the intracellular concentration of the ion rises. Thus, some compounds may release ions in the cytosol. Because there are differences in pH between organelles, some compounds may release ions, for example in acidic organelles, such as lysosomes (Riddell, 2002).
With a view of potential clinical administration, compounds with low or moderate metal affinity shall be tested as ionophores. Such compounds shall bind metals in regions of high concentration and transport metals to regions where the concentration is lower, thus restoring equilibrium (Bush, 2008; Ding & Lind, 2009).
In contrast, the use of chelators may be associated with removal of metal ions from regions where they are essential, which may lead to unwanted effects. Chelators also bind ions, forming complexes, but the functional effect is opposite to ionophores. Traditionally, chelating agents have been used to remove toxic substances (Helsel & Franz, 2015). Such chelates should be water soluble, thus easily excreted in the urine. For some compounds (e.g. diethyldithiocarbamate, a metabolite of disulfiram, a drug that has been long used in alcoholism (Kranzler & Soyka, 2018) has both actions, in that it is an ionophore (Kim et al., 2000) and a chelator (Jones et al., 1980).
The examples of zinc ionophores are given in Table 1. In these studies cell cultures were used. The cells were exposed to metals and the test compounds. Cell membrane‐permeable fluorescent probes, which detect intracellular zinc ions, such as p‐toluenesulfonamido‐quinoline (TSQ), mag‐fura‐ or FluoZin‐3 were used (Andersson et al., 2009; Kim, Kim, Moon, et al., 1999; Kim et al., 2000; Kim, Kim, Xu, et al., 1999; Reeder et al., 2011; Wiggins et al., 2015; Xue et al., 2014). In some of the studies, these probes were combined with probes staining for lysosomes, such as LysoTracker or dextran‐Alexa 647 (Wiggins et al., 2015; Xue et al., 2014). In others, inductively coupled plasma mass spectrometry was used to monitor changes in intracellular concentration of zinc ions (Adlard et al., 2008; White et al., 2006).
TABLE 1.
Examples of zinc ionophores
| Mechanism of action | Method | References | |
|---|---|---|---|
| Disulfiram | Ionophore: zinc | Cell culture, FluoZin‐3, and dextran‐Alexa 647 | (Wiggins et al., 2015) |
| Dithiocarbamates (e.g., DEDTC and pyrrolidine dithiocarbamate) | Ionophore: zinc and copper | Cell culture, mag‐fura‐2, and TSQ | (Kim et al., 2000; Kim, Kim, Xu, Hsu, & Ahn, 1999) |
| Pyrithione | Ionophore: zinc and copper | Cell culture, mag‐fura‐2, and FluoZin‐3 | (Andersson, Gentry, Moss, & Bevan, 2009; Kim, Kim, Moon, et al., 1999; Reeder et al., 2011) |
| Clioquinol | Ionophore: zinc and copper | Cell culture and ICPMS | (White et al., 2006) |
| PBT2 | Ionophore: zinc and copper | Cell culture and ICPMS | (Adlard et al., 2008) |
| Chloroquine | Ionophore: zinc | Cell culture, FluoZin‐3, and LysoTracker | (Xue et al., 2014) |
Abbreviations: DEDTC, diethyldithiocarbamate; ICPMS, inductively coupled plasma mass spectrometry; TSQ, p‐toluenesulfonamido‐quinoline.
Furthermore, it was demonstrated that cysteine4 or cysteine3histidine zinc fingers in which zinc‐bound cysteine has no hydrogen bonds are reactive and can liberate zinc ions, which causes protein unfolding and increases intracellular zinc. A search algorithm based on physical properties has been employed in order to search for such zinc fingers, which have been termed “labile zinc fingers” (Lee, Wang, Duh, Yuan, & Lim, 2013). The following terms:‐ “zinc finger targeting agents” and “zinc ejecting agents” or “zinc ejectors” ae used in the literature (Lee et al., 2013; Supuran, Innocenti, Mastrolorenzo, & Scozzafava, 2004). For example, disulfiram has been demonstrated to act as zinc ionophore (Wiggins et al., 2015) and as an agent ejecting zinc from zinc fingers (Lin et al., 2018; Sargsyan et al., 2020). In order to examine the latter feature, the purified recombinant proteins predicted to contain labile zinc fingers were mixed with disulfiram in the presence of FluoZin‐3 probe and an increase in fluorescence was observed (Sargsyan et al., 2020).
4. DRUGS TARGETING ZINC AND MERS‐CoV, SARS‐CoV AND SARS‐CoV‐2
Several lines of evidence suggest a link between zinc and COVID‐19, including the observation that chloroquine, a drug being repurposed for COVID‐19 (Gautret et al., 2020), is a known as a zinc ionophore (Xue et al., 2014). Studies on zinc ionophores and zinc finger targeting agents as well as zinc in relation to SARS‐CoV‐2, SARS‐CoV or MERS‐CoV will be therefore discussed in detail.
4.1. Chloroquine is a zinc ionophore
The dramatic outbreak of COVID‐19 worldwide prompted to search for possible treatment options from already available drugs (Harrison, 2020). Chloroquine, an old antimalarial drug (Blount, 1967), was demonstrated to block virus infection at low micromolar concentration in Vero E6 cells infected with SARS‐CoV‐2 (Wang et al., 2020), thus suggesting the possible use of chloroquine in patients with COVID‐19. Moreover, its derivative, hydroxychloroquine, was found to inhibit SARS‐CoV‐2 infection in vitro (Liu et al., 2020). Furthermore, hydroxychloroquine was shown to be more potent than chloroquine at inhibiting SARS‐CoV‐2 (Yao et al., 2020).
Mode of chloroquine action has been extensively reviewed (Slater, 1993). In addition, several mechanisms have been recently proposed with regard to the use of chloroquine for COVID‐19 (Devaux, Rolain, Colson, & Raoult, 2020; Shittu & Afolami, 2020; Skalny et al., 2020), including its activity as zinc ionophore (Xue et al., 2014). It was found that administration of zinc and chloroquine to the human ovarian carcinoma cell line, A2780, produced an increase in the fluorescence of FluoZin‐3 probe, which was reversed by the application of N,N,N′,N′‐tetrakis(2‐pyridylmethyl)etylenediamine (TPEN), a cell membrane‐permeable zinc chelator. Moreover, chloroquine did not induce zinc uptake to the cell in the presence of Ca‐EDTA, a cell membrane‐impermeable metal chelator, showing that chloroquine produces an increase in intracellular concentration of zinc ions by transporting zinc from outside the cell and not by mobilizing zinc from intracellularly localized zinc proteins. Furthermore, the fluorescent signals of FluoZin‐3, indicating zinc ions, co‐localized with signals of LysoTracker, a cell membrane‐permeable probe selective for acidic organelles. These observations suggest that chloroquine is zinc ionophore, which transports zinc to lysosomes (Xue et al., 2014).
An important finding from this study is that treatment of cells with zinc chloride alone or with chloroquine alone produced less pronounced increase in intracellular zinc ions, compared with the effects induced by administration of zinc chloride and chloroquine (Xue et al., 2014), which suggest that combined treatment with ionophore and zinc is necessary in order to substantially increase the level of zinc inside a cell.
4.2. Disulfiram inhibits MERS‐CoV, SARS‐CoV, SARS‐CoV‐2 PLpro and SARS‐CoV‐2 Mpro
Similar issues to the above, which have been examined in relation to chloroquine (Xue et al., 2014), have been addressed with regard to disulfiram (Wiggins et al., 2015). It was shown with the aid of FluoZin‐3 probe that disulfiram increases intracellular zinc in MCF‐7 and BT474 breast cancer cells. The increase in FluoZin‐3 fluorescence in cells treated with disulfiram depended on extracellular zinc, thus supporting the hypothesis that disulfiram acts as zinc ionophore. Moreover, the fluorescence of FluoZin‐3 was not observed following disulfiram treatment under low‐zinc and low‐copper conditions. Under such conditions, zinc, but not copper, was able to restore the fluorescence, demonstrating that the increase in fluorescence after administration of disulfiram was due to selective interaction with zinc. Finally, it was shown that disulfiram sequesters intracellular zinc in lysosomes (Wiggins et al., 2015).
Disulfiram produced a dose‐dependent inhibitory effect of both SARS‐CoV and MERS‐CoV PLpro with IC50 in the micromolar range, as it was measured by the deubiquitination assay (Lin et al., 2018), since PLpro has deubiquitinating activity in vitro (Barretto et al., 2005). Disulfiram was found to be a non‐competitive and competitive (or mixed) inhibitor of MERS‐CoV and SARS‐CoV PLpro, respectively. Furthermore, in the above‐mentioned study, the protein and FluoZin‐3 probe were mixed in the presence or absence of disulfiram. An increase in the fluorescence signal was observed following incubation of both MERS‐CoV and SARS‐CoV PLpro with disulfiram, compared with the signal induced by MERS‐CoV and SARS‐CoV PLpro without disulfiram, thus showing increased concentration of zinc ions. This observation suggests that disulfiram destabilizes these enzymes by releasing zinc from them (Lin et al., 2018). It was also demonstrated that mutation of the zinc‐coordinating cysteine caused a significant loss of enzymatic activity of SARS‐CoV PLpro. This observation demonstrates that zinc‐binding ability is essential for SARS‐CoV PLpro enzymatic function (Barretto et al., 2005).
Recently, Sargsyan et al. (2020) found labile zinc fingers, thus likely to be targeted and disrupted, in three SARS‐CoV‐2 proteins, that is, PLpro, Nsp10 and Nsp13. In this study, the protease activity was determined using a fluorogenic substrate Dabcyl–FTLKGGAPTKVTE–Edans–NH2. Disulfiram and organoselenium compound, ebselen, inhibited SARS‐CoV‐2 PLpro with an IC50 in the micromolar range. Moreover, incubation of SARS‐CoV‐2 PLpro with ebselen and disulfiram was associated with increased concentration of zinc ions measured with the aid of FluoZin‐3 probe (Sargsyan et al., 2020).
Furthermore, disulfiram and ebselen are among inhibitors of another crucial SARS‐CoV‐2 enzyme, that is Mpro, with an IC50 in the micromolar range (Jin et al., 2020). The effects of disulfiram on SARS‐CoV‐2, SARS‐CoV and MERS‐CoV enzymes are summarized in Table 2. In addition, disulfiram and ebselen decreased the number of SARS‐CoV‐2 viral RNA copies (as it was determined by qRT‐PCR analysis) in SARS‐CoV‐2‐infected Vero E6 cells (Jin et al., 2020).
TABLE 2.
The effects of disulfiram on MERS‐CoV, SARS‐CoV and SARS‐CoV‐2 enzymes
| Compound | Mechanism | Reference |
|---|---|---|
| Disulfiram | MERS‐CoV PLpro inhibitor (μM) | (Lin et al., 2018) |
| SARS‐CoV PLpro inhibitor (μM) | (Lin et al., 2018) | |
| SARS‐CoV‐2 PLpro inhibitor (μM) | (Sargsyan et al., 2020) | |
| SARS‐CoV‐2 Mpro inhibitor (μM) | (Jin et al., 2020) |
Abbreviations: MERS‐CoV, Middle East respiratory syndrome‐related coronavirus; Mpro, main protease; PLpro, papain‐like protease; SARS‐CoV, severe acute respiratory syndrome‐related coronavirus; SARS‐CoV‐2, severe acute respiratory syndrome‐related coronavirus 2.
4.3. Intracellular zinc inhibits RdRp of SARS‐CoV
Te Velthuis et al. (2010) employed several in vitro approaches to study the effects of zinc on SARS‐CoV. First, they examined the effects of combination of zinc acetate and pyrithione, another zinc ionophore (Andersson et al., 2009; Kim, Kim, Moon, et al., 1999), on the replication of a recombinant SARS‐CoV in Vero E6 cells. The recombinant SARS‐CoV was generated by deletion of open reading frame 7a/7b (ORF 7a/7b) and insertion of the GFP, resulting in SARS‐CoV‐GFP, which replicates to similar titres as wild‐type viruses in Vero E6 cells (Sims, Burkett, Yount, & Pickles, 2008). Pyrithione inhibited the reporter gene expression of SARS‐CoV‐GFP. This effect was enhanced by the addition of zinc acetate. Approximately 98% reduction of the GFP signal for SARS‐CoV‐GFP was observed at concentrations that did not induce cytotoxicity, that is, 2‐μM pyrithione and 2‐μM zinc acetate. Furthermore, zinc acetate alone also reduced virus replication but to a lesser extent than the combination of zinc and its ionophore (te Velthuis et al., 2010).
Moreover, te Velthuis et al. (2010) used two more approaches that allowed to study the direct effects of zinc ions on replicase–transcriptase complex and RdRp, thus eliminating the need to transport zinc across the plasma membrane with the aid of ionophore. They tested the effects of zinc in an in vitro system of active replicase–transcriptase complexs isolated from infected cells (van Hemert et al., 2008). In this system, zinc acetate dose‐dependently decreased the amount of synthesized RNA. The inhibition of replicase–transcriptase complex by zinc was reversed by the addition of zinc chelator, Mg‐EDTA. In addition, they used an in vitro recombinant RdRp assay. Zinc inhibited the initiation and elongation phase in this assay (te Velthuis et al., 2010).
4.4. The proposed mechanism of action of drugs targeting zinc on SARS‐CoV‐2
Based on the above‐mentioned studies, a chain of events can be hypothesized, which may happen after administration of zinc ionophore (and zinc) and/or a zinc finger targeting drug, which causes ejection of zinc from zinc fingers in viral metalloenzymes.
Zinc ionophore and/or zinc finger targeting agent may enter a cell, as does SARS‐CoV‐2. An agent targeting zinc fingers may bind labile zinc fingers in essential viral enzymes such as PLpro. It may cause ejection of zinc from the enzyme, which will destabilize the enzyme, thus increasing intracellular concentration of zinc ions, as has been demonstrated for SARS‐CoV and MERS‐CoV by Lin et al. (2018) and for SARS‐CoV‐2 by Sargsyan et al. (2020). Moreover, zinc ionophores such as chloroquine may contribute to increased intracellular concentration of zinc ions (Xue et al., 2014). Additionally, compounds such as ebselen may contribute to increased intracellular zinc by releasing zinc from metallothioneins (Jacob, Maret, & Vallee, 1998), a family of cysteine‐rich, low MW, metal‐binding proteins (Thirumoorthy et al., 2011). Furthermore, zinc ions may inhibit RdRp, as shown for SARS‐CoV by te Velthuis et al. (2010) (Figure 1).
FIGURE 1.
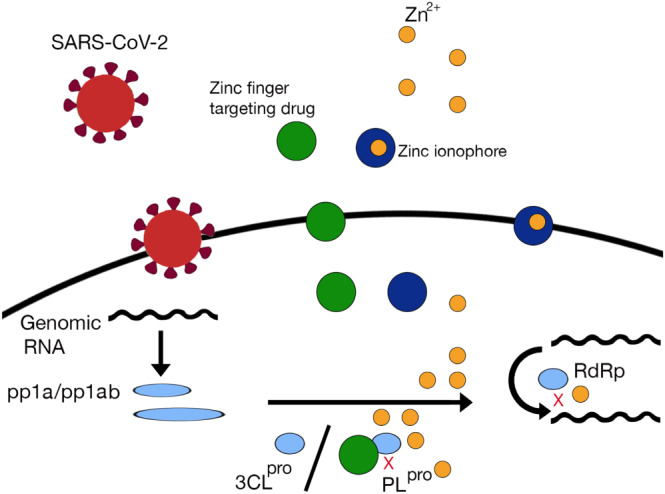
The possible mechanism of action of drugs targeting zinc metalloenzymes in coronavirus disease 2019. A drug targeting zinc fingers in zinc metalloenzymes would bind zinc in papain‐like protease (PLpro) (or another essential enzyme of severe acute respiratory syndrome coronavirus 2 [SARS‐CoV‐2]). Such drug would remove zinc from the enzyme, thus destabilizing the enzyme, and produce an increase in intracellular zinc concentration. Zinc administered together with its ionophore would contribute to increased intracellular zinc. Intracellular zinc would inhibit RNA‐dependent RNA polymerase (RdRp) of the virus
In addition to presumed inhibition of RdRp, intracellular zinc may initiate a cascade of events in the host. Intracellular zinc acts as a second messenger and modulates a variety of signalling pathways. All immune cells are affected by intracellular zinc signalling, which has been comprehensively reviewed by Maywald, Wessels, and Rink (2017). Thus, treatment strategies based on targeting zinc in viral enzymes, leading to increased intracellular zinc or other approaches also leading to increases intracellular zinc, will have potential consequences for many functions of the immune system (Read, Obeid, Ahlenstiel, & Ahlenstiel, 2019; Skalny et al., 2020).
It has also been suggested that tetracyclines may exert beneficial effects in COVID‐19 based on their ability to chelate zinc in MMPs (Sodhi & Etminan, 2020). MMPs are another group of zinc metalloenzymes. They are endopeptidases, which are involved in the degradation of proteins in the extracellular matrix (Cui, Hu, & Khalil, 2017). However, recent evidence demonstrates that MMPs are multitasking proteins working in both the extracellular and intracellular compartments. Most of MMP substrates are non‐extracellular matrix proteins and include chemokines, cytokines, cell surface receptors and proteins involved in immune signalling (Chopra, Overall, & Dufour, 2019).
It has been demonstrated in vitro that the neurotropic strain JHM.SD of the murine coronavirus mouse hepatitis virus uses an unidentified batimastat‐sensitive metalloprotease for both viral entry and virus‐mediated cell–cell fusion. Batimastat is a potent, broad spectrum MMP inhibitor. Thus, this study suggests the importance of MMPs for JHM.SD infection (Phillips, Gallagher, & Weiss, 2017). Moreover, coronavirus HCoV‐229E infection of primary monocytes was associated with increased production of MMP‐9 (Desforges, Miletti, Gagnon, & Talbot, 2007).
Tetracyclines are well‐known MMPs inhibitors (Boelen et al., 2019; Castro, Kandasamy, Youssef, & Schulz, 2011), but the direct relationship between MMPs and SARS‐CoV‐2 has yet to be examined.
5. CLINICAL POTENTIAL OF DRUGS TARGETING ZINC
Targeting metal homeostasis with the aid of chelators or ionophores has been suggested as a therapeutic strategy for a variety of diseases, for example, cancer (Ding & Lind, 2009; Vaden et al., 2019), diseases of the CNS (Doboszewska et al., 2017; Doboszewska et al., 2019; Weekley & He, 2017) and infectious diseases, such as malaria (Bharti, Singal, Raza, Ghosh, & Nag, 2019). An important fact is that there are ongoing clinical trials in which metal‐binding compounds are being tested because of their influence on metal homeostasis. For example, activation of procaspase‐3 by procaspase‐activating compound 1 (PAC‐1) was shown to be dependent on the chelation of zinc (Sarkar et al., 2016). There are several ongoing clinical trials on the use of PAC‐1 in cancer patients (ClinicalTrials.gov identifiers: NCT03927248, NCT03332355 and NCT02355535).
In relation to cancer, there are ongoing clinical trials on the use of disulfiram (NCT04265274, NCT03950830, NCT03714555, NCT03363659, NCT03323346, NCT03151772, NCT02715609 and NCT02671890).
Clioquinol was a registered drug worldwide until its use was associated with the occurrence of subacute myelo‐optic neuropathy, a condition primarily endemic to Japan. Today, in view of new information that may explain this phenomenon, clioquinol serves as a drug lead to treat cancer (Perez, Sklar, & Chigaev, 2019).
PBT2 is a next‐generation derivative of clioquinol, which is characterized by higher solubility and increased blood–brain barrier permeability. These features, together with its activity as a zinc–copper ionophore, make it a possible disease‐modifying drug for Alzheimer's disease (Adlard et al., 2008). According to the metal hypothesis of Alzheimer's disease, in the brain there is a failure in endogenous regulatory mechanisms, which leads to an unbalance of two metals, zinc and copper, resulting in their toxic excess in some compartments and deficit in others (Sensi, Granzotto, Siotto, & Squitti, 2018). Moreover, deposition of amyloid‐β has long been regarded as the leading substance which may be responsible for the development of Alzheimer's disease (Hardy & Higgins, 1992). The proposed mechanism of action of PBT2 is related to its ability to react with zinc and copper ions in oligomerized and precipitate forms of amyloid‐β, thus promoting the soluble form of amyloid‐β. PBT2 transports also ions captured from the amyloid‐β oligomers into the nearby cells (Adlard et al., 2008).
PBT2 was well tolerated and significantly improved executive function in two tests: category fluency and trails B as well as lowering CSF levels of amyloid‐β in patients with Alzheimer's disease in a Phase II, double‐blind, randomized, placebo‐controlled trial (Lannfelt et al., 2008). In addition, a Phase II, double‐blind, randomized, placebo‐controlled trial of patients with Huntington's disease revealed that PBT2 was generally safe and well tolerated, although it was concluded that the therapeutic potential on cognition needs to be confirmed in larger studies. The suicidal ideation was higher in patients with Huntington's disease taking PBT2, which urges careful observation of suicidality in future studies with this compound (Huntington Study Group Reach2HD Investigators, 2015). Nevertheless, these clinical results show that novel drugs targeting metal ions can be successfully developed.
In regard to zinc fingers, zinc finger nuclease technology is a tool in the field of genome editing, which is being increasingly developed and has entered clinical trials (Lee et al., 2020; Mullard, 2017; Paschon et al., 2019; Tebas et al., 2014). Zinc finger nucleases are enzymes that selectively bind, cleave and enable the repair of DNA. Zinc finger nuclease drugs are currently in clinical trials for mucopolysaccharidosis (e.g., ClinicalTrials.gov identifier: NCT02702115) and HIV infection (e.g. NCT04201782). Azodicarbonamide was the first compound targeting zinc fingers (Rice et al., 1997), which was in clinical trials for the treatment of HIV (Goebel et al., 2001). A few compounds that replace zinc in zinc fingers by another metal ion have also entered clinical trials (Abbehausen, 2019). In addition, a registered anticancer drug cisplatin (Dasari & Tchounwou, 2014) was found to interact with zinc fingers and to eject zinc (Castiglione Morelli, Ostuni, Cristinziano, Tesauro, & Bavoso, 2013).
6. CLINICAL TRIALS ON COVID‐19 RELATED TO ZINC
Clinical trials on the use of zinc in COVID‐19 are associated with repurposing of chloroquine/hydroxychloroquine. The outcomes of clinical trials with chloroquine in a variety of acute or chronic viral diseases have recently been discussed (Touret & de Lamballerie, 2020). Generally, in randomized trials it was found not to be effective in humans in the prevention or the treatment of acute viral diseases (Touret & de Lamballerie, 2020). In relation to COVID‐19, hydroxychloroquine was demonstrated to be effective in a small, open‐label, non‐randomized clinical trial (Gautret et al., 2020). Currently, there is no evidence coming from randomized controlled trials supporting the use of chloroquine/hydroxychloroquine in patients with COVID‐19. Therefore, so far, no such registration has been made by the Food and Drug Administration (Mahase, 2020). Clinical trials using these medications have been registered, including the SOLIDARITY study, a large‐scale, multicentre, randomized clinical trial to evaluate the safety and efficacy of treatments for patients diagnosed with COVID‐19.
In some of the registered clinical trials on hydroxychloroquine repurposing, zinc will be administered as an adjuvant treatment to hydroxychloroquine therapy, in both the prophylaxis and treatment of COVID‐19. Studies NCT04377646 (COVID‐Milit) and NCT04384458 will examine the effects of combined treatment with hydroxychloroquine and zinc in healthcare professionals providing care for patients with COVID‐19, as a prophylactic strategy. Two studies will assess the impact of combined treatment with hydroxychloroquine, zinc, vitamin C and vitamin D in the prophylaxis of COVID‐19 in healthcare professionals (NCT04326725 and NCT04335084).
Several clinical trials will explore the combination of hydroxychloroquine, azithromycin and zinc in the treatment of patients with the diagnosis of COVID‐19. Awaiting the outcomes of the clinical trials, a combination of zinc, hydroxychloroquine and azithromycin has been proposed as an early treatment of COVID‐19 in the outpatient setting. Early outpatient treatment can prevent disease progression and hospitalization (Derwand & Scholz, 2020; Risch, 2020). Table 3 contains data on clinical trials in regard to zinc and COVID‐19 and information whether they are scheduled in the inpatient or outpatient setting.
TABLE 3.
Clinical studies on zinc in COVID‐19 as of June 15, 2020
| Type of the study | Purpose of the study | Treatment/intervention | Participants/diagnosis | ClinicalTrials.gov identifier/acronym |
|---|---|---|---|---|
| Interventional | Prevention |
Hydroxychloroquine Zinc |
Military healthcare professionals |
COVID‐Milit |
|
Hydroxychloroquine Zinc |
Healthcare professionals | NCT04384458 | ||
|
Hydroxychloroquine Zinc Vitamin C Vitamin D |
Healthcare professionals |
HELPCOVID‐19 |
||
| Treatment |
Zinc Vitamin C |
Adult outpatients COVID‐19 |
COVIDAtoZ |
|
|
Zinc Vitamin D |
Institutionalized elderly patients COVID‐19 |
ZnD3CoVici |
||
|
Hydroxychloroquine Azithromycin Zinc Vitamin C Vitamin D |
Adult patients COVID‐19 |
HAZDpaC |
||
|
Hydroxychloroquine Azithromycin Zinc Vitamin D Vitamin B12 Vitamin C |
Adult inpatients and outpatients COVID‐19 |
ALLIANCE |
||
|
Hydroxychloroquine Azithromycin Zinc Favipiravir |
Adult inpatients COVID‐19 |
PIONEER |
||
|
Hydroxychloroquine Azithromycin Zinc Doxycycline |
30 years and older outpatients COVID‐19 |
NCT04370782 | ||
|
Nitazoxanide Ribavirin Ivermectin Zinc |
12 years and older inpatients COVID‐19 inpatients |
NCT04392427 | ||
| Immunonutrition |
Adult inpatients COVID‐19 |
NCT04323228 | ||
| Observational | Prevention |
Hydroxychloroquine Zinc Vitamin C Vitamin D |
Healthcare professionals | NCT04326725 |
| Other |
Hydroxychloroquine Azithromycin Zinc Lopinavir Ritonavir |
Diabetes, COVID‐19 |
COVIDIAB‐13 |
Abbreviation: COVID‐19, coronavirus disease 2019.
The study NCT04334512 (HAZDpaC) will examine the efficacy of quintuple therapy compromising hydroxychloroquine, azithromycin, zinc, vitamin D and vitamin C in the treatment of adult patients with the diagnosis of COVID‐19. The international ALLIANCE study (NCT04395768) will investigate the treatment with hydroxychloroquine, azithromycin, zinc, vitamin D and vitamin B12 with or without vitamin C. The study NCT04392427 will assess the effects of the combination of nitazoxanide, ribavirin, ivermectin and zinc in children or adults. The study NCT04373733 will compare treatment with hydroxychloroquine, azithromycin and zinc versus favipiravir.
Moreover, the study NCT04370782 will examine the effects of hydroxychloroquine and zinc in combination with either azithromycin or doxycycline in COVID‐19 patients. With regard to doxycycline, the study NCT04371952 (DYNAMIC Study [DoxycYcliNe AMbulatoIre COVID‐19]) is aimed to compare a treatment with doxycycline versus a placebo. Chelation of zinc in MMPs of the host by tetracyclins is a rationale for this study.
Furthermore, two studies have been registered in order to assess the effects of combination of zinc and vitamin D or zinc and vitamin C in the treatment in patients with COVID‐19: institutionalized elderly patients or in adult outpatients (NCT04351490, ZnD3CoVici; NCT04342728, COVIDAtoZ). Noteworthy is the fact that the study NCT04342728 (COVIDAtoZ) includes a group of patients who will receive only zinc gluconate (without vitamins). Inclusion of this group will allow to draw conclusions regarding the role of zinc in the treatment of COVID‐19.
Finally, the study NCT04323228 aims at assessing immunonutrition in patients with COVID‐19. Immunonutrition is a concept of nutrition that has an impact on the immune system. This strategy is often used in critical illnesses (Calder, 2003). For example, a meta‐analysis of 61 randomized controlled trials on immunonutrition in cancer patients has shown that immunonutrition was associated with reduced risk of post‐operative infectious complications, including reduced risk for respiratory tract infection (Yu et al., 2019), compared with standard nutrition. In the study on immunonutrition in COVID‐19, patients with confirmed SARS‐Cov‐2 infection, who do not require intensive care unit admission, will receive oral nutrition supplement (ONS) enriched in eicosapentaenoic acid (EPA), γ‐linolenic acid (GLA), vitamin A, vitamin C, vitamin E, selenium and 5.7‐mg zinc (Oxepa, Abbott Nutrition, Abbott Laboratories) or isocaloric–isonitrogenous product (prepared by the same manufacturer). The ONS or control product will be administered in the morning.
In addition, the study NCT04407572 is an observational study aimed at measuring serum zinc, vitamin D and vitamin B12 levels in pregnant women with COVID‐19. Another observational study (NCT04412746) will assess the prevalence of diabetes among hospitalized patients with COVID‐19 receiving hydroxychloroquine, azithromycin and zinc or lopinavir/ritonavir.
7. CONSIDERATIONS FOR FUTURE DEVELOPMENT OF DRUGS TARGETING ZINC
Many lines of evidence suggest the relationship between zinc and COVID‐19 and support the hypothesis that targeting zinc may lead to the development of new drugs for COVID‐19. Increasing intracellular zinc is among mechanisms of action of chloroquine, a drug being repurposed for COVID‐19 (Xue et al., 2014). It is plausible that the therapeutic mechanisms induced by chloroquine in patients with COVID‐19 at least in part result from its impact on zinc levels. Disulfiram (Sargsyan et al., 2020) and tetracyclines (Sodhi & Etminan, 2020) are among already known drugs that have been proposed to combat COVID‐19 based on their effects on zinc. Disulfiram increases intracellular zinc similarly to chloroquine (Sargsyan et al., 2020; Wiggins et al., 2015).
Although the above‐mentioned agents apparently have many mechanisms of action, the entrance of other metal‐binding compounds into clinical studies raises the possibility that investigating zinc‐binding drugs may lead to the development of pharmacotherapy. An example of such successful metal‐binding drug is PBT2, which was safe and well tolerated in clinical trials (Huntington Study Group Reach2HD Investigators, 2015).
The principles of a potential strategy of combating SARS‐CoV‐2 with the aid of zinc targeting agent would be similar to the mechanism of action of PBT2 in Alzheimer's disease. In the case of Alzheimer's disease, amyloid‐β is enriched in zinc, which is taken away and redistributed by PBT2. With regard to COVID‐19, a novel drug would target labile zinc fingers in SARS‐CoV‐2 proteins, thus destroying the proteins and producing an increase in intracellular concentration of zinc ions.
The design of therapeutic agents selectively binding a labile zinc finger motif in viral protein is theoretically feasible (Huang et al., 1998) and would be a solution to overcome the problem of binding of such agent to host's proteins (Garcia & Damonte, 2007). The time is ripe for the design, synthesis and evaluation of new zinc‐binding drugs, which may be helpful during this and future pandemics.
As intracellular zinc signalling is critically involved in antiviral immunity (Read et al., 2019), increased intracellular zinc following administration of zinc and/or zinc ionophore and/or a labile zinc finger targeting drug will affect function of the immune system. A question arises what level of intracellular zinc will be beneficial and detrimental, since also zinc excess may produce changes in immune cell number and function (Maywald et al., 2017). On the other hand, a question is whether disruption of zinc fingers in viral proteins with subsequent ejection of zinc ions will produce a rise in zinc ions, which will be sufficient to inhibit RdRp or this effect has to be enhanced by administration of zinc and/or its ionophore.
Currently, there is no evidence that administration of zinc will be beneficial with regard to COVID‐19, in terms of prophylaxis or treatment. The ongoing clinical trials will hopefully answer this question in the near future. The clinical trials on COVID‐19 in which zinc will be administered in addition to chloroquine will shed a light on the involvement of ionophoric activity of chloroquine towards zinc at the level of clinical pharmacology.
7.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Doboszewska U, Wlaź P, Nowak G, Młyniec K. Targeting zinc metalloenzymes in coronavirus disease 2019. Br J Pharmacol. 2020;177:4887–4898. 10.1111/bph.15199
REFERENCES
- Abbehausen, C. (2019). Zinc finger domains as therapeutic targets for metal‐based compounds—An update. Metallomics, 11, 15–28. 10.1039/C8MT00262B [DOI] [PubMed] [Google Scholar]
- Adlard, P. A. , Cherny, R. A. , Finkelstein, D. I. , Gautier, E. , Robb, E. , Cortes, M. , … Bush, A. I. (2008). Rapid restoration of cognition in Alzheimer's transgenic mice with 8‐hydroxy quinoline analogs is associated with decreased interstitial Aβ. Neuron, 59, 43–55. 10.1016/j.neuron.2008.06.018 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso, I. , & Quesada, R. (2013). Biological activity of synthetic ionophores: Ion transporters as prospective drugs? Chemical Science, 4, 3009–3019. 10.1039/c3sc50882j [DOI] [Google Scholar]
- Andersson, D. A. , Gentry, C. , Moss, S. , & Bevan, S. (2009). Clioquinol and pyrithione activate TRPA1 by increasing intracellular Zn2+ . Proceedings of the National Academy of Sciences of the United States of America, 106, 8374–8379. 10.1073/pnas.0812675106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretto, N. , Jukneliene, D. , Ratia, K. , Chen, Z. , Mesecar, A. D. , & Baker, S. C. (2005). The papain‐like protease of severe acute respiratory syndrome coronavirus has deubiquitinating activity. Journal of Virology, 79, 15189–15198. 10.1128/JVI.79.24.15189-15198.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti, H. , Singal, A. , Raza, M. , Ghosh, P. C. , & Nag, A. (2019). Ionophores as potent anti‐malarials: A miracle in the making. Current Topics in Medicinal Chemistry, 18, 2029–2041. 10.2174/1568026619666181129125950 [DOI] [PubMed] [Google Scholar]
- Blount, R. E. (1967). Chloroquine‐resistant falciparum malaria. JAMA, 200, 886 10.1001/jama.1967.03120230138027 [DOI] [PubMed] [Google Scholar]
- Boelen, G. J. , Boute, L. , d'Hoop, J. , EzEldeen, M. , Lambrichts, I. , & Opdenakker, G. (2019). Matrix metalloproteinases and inhibitors in dentistry. Clinical Oral Investigations, 23, 2823–2835. 10.1007/s00784-019-02915-y [DOI] [PubMed] [Google Scholar]
- Bush, A. I. (2008). Drug development based on the metals hypothesis of Alzheimer's disease. Journal of Alzheimer's Disease, 15, 223–240. 10.3233/JAD-2008-15208 [DOI] [PubMed] [Google Scholar]
- Calder, P. C. (2003). Immunonutrition. BMJ, 327, 117–118. 10.1136/bmj.327.7407.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglione Morelli, M. A. , Ostuni, A. , Cristinziano, P. L. , Tesauro, D. , & Bavoso, A. (2013). Interaction of cisplatin with a CCHC zinc finger motif. Journal of Peptide Science, 19, 227–232. 10.1002/psc.2490 [DOI] [PubMed] [Google Scholar]
- Castro, M. M. , Kandasamy, A. D. , Youssef, N. , & Schulz, R. (2011). Matrix metalloproteinase inhibitor properties of tetracyclines: Therapeutic potential in cardiovascular diseases. Pharmacological Research, 64, 551–560. 10.1016/j.phrs.2011.05.005 [DOI] [PubMed] [Google Scholar]
- Chopra, S. , Overall, C. M. , & Dufour, A. (2019). Matrix metalloproteinases in the CNS: Interferons get nervous. Cellular and Molecular Life Sciences, 76, 3083–3095. 10.1007/s00018-019-03171-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses . (2020). The species Severe acute respiratory syndrome‐related coronavirus: Classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nature Microbiology, 5, 536–544. 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, N. , Hu, M. , & Khalil, R. A. (2017). Biochemical and biological attributes of matrix metalloproteinases. Progress in Molecular Biology and Translational Science, 147, 1–73. 10.1016/bs.pmbts.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari, S. , & Tchounwou, P. B. (2014). Cisplatin in cancer therapy: Molecular mechanisms of action. European Journal of Pharmacology, 740, 364–378. 10.1016/j.ejphar.2014.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derwand, R. , & Scholz, M. (2020). Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today's battle against COVID‐19? Medical Hypotheses, 142, 109815 10.1016/j.mehy.2020.109815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges, M. , Miletti, T. C. , Gagnon, M. , & Talbot, P. J. (2007). Activation of human monocytes after infection by human coronavirus 229E. Virus Research, 130, 228–240. 10.1016/j.virusres.2007.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux, C. A. , Rolain, J. M. , Colson, P. , & Raoult, D. (2020). New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID‐19? International Journal of Antimicrobial Agents, 55(5), 105938 10.1016/j.ijantimicag.2020.105938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit, E. , van Doremalen, N. , Falzarano, D. , & Munster, V. J. (2016). SARS and MERS: Recent insights into emerging coronaviruses. Nature Reviews. Microbiology, 14, 523–534. 10.1038/nrmicro.2016.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, W. Q. , & Lind, S. E. (2009). Metal ionophores—An emerging class of anticancer drugs. IUBMB Life, 61, 1013–1018. 10.1002/iub.253 [DOI] [PubMed] [Google Scholar]
- Doboszewska, U. , Mlyniec, K. , Wlaz, A. , Poleszak, E. , Nowak, G. , & Wlaz, P. (2019). Zinc signaling and epilepsy. Pharmacology & Therapeutics, 193, 156–177. 10.1016/j.pharmthera.2018.08.013 [DOI] [PubMed] [Google Scholar]
- Doboszewska, U. , Wlaz, P. , Nowak, G. , Radziwon‐Zaleska, M. , Cui, R. , & Mlyniec, K. (2017). Zinc in the monoaminergic theory of depression: Its relationship to neural plasticity. Neural Plasticity, 2017, 3682752 10.1155/2017/3682752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr, A. R. , & Perlman, S. (2015). Coronaviruses: An overview of their replication and pathogenesis. Methods in Molecular Biology, 1282, 1–23. 10.1007/978-1-4939-2438-7_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman, J. C. (2011). Ionophores in planar lipid bilayers In Speralakis N. (Ed.), Cell physiology source book (pp. 61–66). Amsterdam: Academic Press. [Google Scholar]
- Fu, M. , & Blackshear, P. J. (2017). RNA‐binding proteins in immune regulation: A focus on CCCH zinc finger proteins. Nature Reviews. Immunology, 17, 130–143. 10.1038/nri.2016.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, C. C. , & Damonte, E. B. (2007). Zn finger containing proteins as targets for the control of viral infections. Infectious Disorders Drug Targets, 7, 204–212. 10.2174/187152607782110004 [DOI] [PubMed] [Google Scholar]
- Gautret, P. , Lagier, J. C. , Parola, P. , Hoang, V. T. , Meddeb, L. , Mailhe, M. , … Raoult, D. (2020). Hydroxychloroquine and azithromycin as a treatment of COVID‐19: Results of an open‐label non‐randomized clinical trial. International Journal of Antimicrobial Agents, 105949 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel, F. D. , Hemmer, R. , Schmit, J. C. , Bogner, J. R. , de Clercq, E. , Witvrouw, M. , … Tassignon, J. P. (2001). Phase I/II dose escalation and randomized withdrawal study with add‐on azodicarbonamide in patients failing on current antiretroviral therapy. Aids, 15, 33–45. 10.1097/00002030-200101050-00007 [DOI] [PubMed] [Google Scholar]
- Haraguchi, H. (2017). Metallomics: The history over the last decade and a future outlook. Metallomics, 9, 1001–1013. 10.1039/C7MT00023E [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy, J. A. , & Higgins, G. A. (1992). Alzheimer's disease: The amyloid cascade hypothesis. Science, 256, 184–185. 10.1126/science.1566067 [DOI] [PubMed] [Google Scholar]
- Harrison, C. (2020). Coronavirus puts drug repurposing on the fast track. Nature Biotechnology, 38, 379–381. 10.1038/d41587-020-00003-1 [DOI] [PubMed] [Google Scholar]
- Helsel, M. E. , & Franz, K. J. (2015). Pharmacological activity of metal binding agents that alter copper bioavailability. Dalton Transactions, 44, 8760–8770. 10.1039/C5DT00634A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M. , Maynard, A. , Turpin, J. A. , Graham, L. , Janini, G. M. , Covell, D. G. , & Rice, W. G. (1998). Anti‐HIV agents that selectively target retroviral nucleocapsid protein zinc fingers without affecting cellular zinc finger proteins. Journal of Medicinal Chemistry, 41, 1371–1381. 10.1021/jm9708543 [DOI] [PubMed] [Google Scholar]
- Huntington Study Group Reach2HD Investigators . (2015). Safety, tolerability, and efficacy of PBT2 in Huntington's disease: A phase 2, randomised, double‐blind, placebo‐controlled trial. Lancet Neurology, 14, 39–47. [DOI] [PubMed] [Google Scholar]
- Jacob, C. , Maret, W. , & Vallee, B. L. (1998). Ebselen, a selenium‐containing redox drug, releases zinc from metallothionein. Biochemical and Biophysical Research Communications, 248, 569–573. 10.1006/bbrc.1998.9026 [DOI] [PubMed] [Google Scholar]
- Jen, J. , & Wang, Y. C. (2016). Zinc finger proteins in cancer progression. Journal of Biomedical Science, 23, 53 10.1186/s12929-016-0269-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, Z. , Du, X. , Xu, Y. , Deng, Y. , Liu, M. , Zhao, Y. , … Yang, H. (2020). Structure of Mpro from COVID‐19 virus and discovery of its inhibitors. Nature, 582, 289–293. 10.1038/s41586-020-2223-y [DOI] [PubMed] [Google Scholar]
- Jones, M. M. , Burka, L. T. , Hunter, M. E. , Basinger, M. , Campo, G. , & Weaver, A. D. (1980). Dithiocarbamate chelating agents for toxic heavy metals. Journal of Inorganic and Nuclear Chemistry, 42, 775–778. 10.1016/0022-1902(80)80230-2 [DOI] [Google Scholar]
- Kalil, A. C. (2020). Treating COVID‐19‐off‐label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA, 323, 1897 10.1001/jama.2020.4742 [DOI] [PubMed] [Google Scholar]
- Kaushik, V. , Yakisich, J. S. , Kumar, A. , Azad, N. , & Iyer, A. K. V. (2018). Ionophores: Potential use as anticancer drugs and chemosensitizers. Cancers (Basel), 10, 360 10.3390/cancers10100360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, C. H. , Kim, J. H. , Moon, S. J. , Chung, K. C. , Hsu, C. Y. , Seo, J. T. , & Ahn, Y. S. (1999). Pyrithione, a zinc ionophore, inhibits NF‐κB activation. Biochemical and Biophysical Research Communications, 259, 505–509. 10.1006/bbrc.1999.0814 [DOI] [PubMed] [Google Scholar]
- Kim, C. H. , Kim, J. H. , Moon, S. J. , Hsu, C. Y. , Seo, J. T. , & Ahn, Y. S. (2000). Biphasic effects of dithiocarbamates on the activity of nuclear factor‐κB. European Journal of Pharmacology, 392, 133–136. 10.1016/S0014-2999(00)00109-6 [DOI] [PubMed] [Google Scholar]
- Kim, C. H. , Kim, J. H. , Xu, J. , Hsu, C. Y. , & Ahn, Y. S. (1999). Pyrrolidine dithiocarbamate induces bovine cerebral endothelial cell death by increasing the intracellular zinc level. Journal of Neurochemistry, 72, 1586–1592. 10.1046/j.1471-4159.1999.721586.x [DOI] [PubMed] [Google Scholar]
- Kranzler, H. R. , & Soyka, M. (2018). Diagnosis and pharmacotherapy of alcohol use disorder: A review. JAMA, 320, 815–824. 10.1001/jama.2018.11406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna, S. S. , Majumdar, I. , & Grishin, N. V. (2003). Structural classification of zinc fingers: Survey and summary. Nucleic Acids Research, 31, 532–550. 10.1093/nar/gkg161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laity, J. H. , Lee, B. M. , & Wright, P. E. (2001). Zinc finger proteins: New insights into structural and functional diversity. Current Opinion in Structural Biology, 11, 39–46. 10.1016/S0959-440X(00)00167-6 [DOI] [PubMed] [Google Scholar]
- Lannfelt, L. , Blennow, K. , Zetterberg, H. , Batsman, S. , Ames, D. , Harrison, J. , … Ritchie, C. W. (2008). Safety, efficacy, and biomarker findings of PBT2 in targeting Aβ as a modifying therapy for Alzheimer's disease: A phase IIa, double‐blind, randomised, placebo‐controlled trial. Lancet Neurology, 7, 779–786. 10.1016/S1474-4422(08)70167-4 [DOI] [PubMed] [Google Scholar]
- Lee, J. , Bayarsaikhan, D. , Bayarsaikhan, G. , Kim, J. S. , Schwarzbach, E. , & Lee, B. (2020). Recent advances in genome editing of stem cells for drug discovery and therapeutic application. Pharmacology & Therapeutics, 209, 107501 10.1016/j.pharmthera.2020.107501 [DOI] [PubMed] [Google Scholar]
- Lee, Y. M. , Wang, Y. T. , Duh, Y. , Yuan, H. S. , & Lim, C. (2013). Identification of labile Zn sites in drug‐target proteins. Journal of the American Chemical Society, 135, 14028–14031. 10.1021/ja406300c [DOI] [PubMed] [Google Scholar]
- Lei, J. , Kusov, Y. , & Hilgenfeld, R. (2018). Nsp3 of coronaviruses: Structures and functions of a large multi‐domain protein. Antiviral Research, 149, 58–74. 10.1016/j.antiviral.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, M. H. , Moses, D. C. , Hsieh, C. H. , Cheng, S. C. , Chen, Y. H. , Sun, C. Y. , & Chou, C. Y. (2018). Disulfiram can inhibit MERS and SARS coronavirus papain‐like proteases via different modes. Antiviral Research, 150, 155–163. 10.1016/j.antiviral.2017.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindskog, S. (1997). Structure and mechanism of carbonic anhydrase. Pharmacology & Therapeutics, 74, 1–20. 10.1016/S0163-7258(96)00198-2 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Cao, R. , Xu, M. , Wang, X. , Zhang, H. , Hu, H. , … Wang, M. (2020). Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS‐CoV‐2 infection in vitro. Cell Discov, 6, 16 10.1038/s41421-020-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y. , Wu, L. , Shaw, N. , Gao, Y. , Wang, J. , Sun, Y. , … Rao, Z. (2015). Structural basis and functional analysis of the SARS coronavirus nsp14–nsp10 complex. Proceedings of the National Academy of Sciences of the United States of America, 112, 9436–9441. 10.1073/pnas.1508686112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase, E. (2020). Covid‐19: Six million doses of hydroxychloroquine donated to US despite lack of evidence. BMJ, 368, m1166 10.1136/bmj.m1166 [DOI] [PubMed] [Google Scholar]
- Ma‐Lauer, Y. , Carbajo‐Lozoya, J. , Hein, M. Y. , Müller, M. A. , Deng, W. , Lei, J. , … von Brunn, A. (2016). p53 down‐regulates SARS coronavirus replication and is targeted by the SARS‐unique domain and PLpro via E3 ubiquitin ligase RCHY1. Proceedings of the National Academy of Sciences of the United States of America, 113, E5192–E5201. 10.1073/pnas.1603435113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret, W. (2013). Zinc biochemistry: From a single zinc enzyme to a key element of life. Advances in Nutrition, 4, 82–91. 10.3945/an.112.003038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywald, M. , Wessels, I. , & Rink, L. (2017). Zinc signals and immunity. International Journal of Molecular Sciences, 18, 2222 10.3390/ijms18102222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle, D. , Sharif, K. , O'Regan, A. , & Bridgewood, C. (2020). The role of cytokines including interleukin‐6 in COVID‐19 induced pneumonia and macrophage activation syndrome‐like disease. Autoimmunity Reviews, 19, 102537 10.1016/j.autrev.2020.102537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, P. , McAuley, D. F. , Brown, M. , Sanchez, E. , Tattersall, R. S. , & Manson, J. J. (2020). COVID‐19: Consider cytokine storm syndromes and immunosuppression. Lancet, 395, 1033–1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullard, A. (2017). First in vivo gene‐editing drugs enter the clinic. Nature Reviews. Drug Discovery, 17, 7 10.1038/nrd.2017.268 [DOI] [PubMed] [Google Scholar]
- Paschon, D. E. , Lussier, S. , Wangzor, T. , Xia, D. F. , Li, P. W. , Hinkley, S. J. , … Rebar, E. J. (2019). Diversifying the structure of zinc finger nucleases for high‐precision genome editing. Nature Communications, 10, 1133 10.1038/s41467-019-08867-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez, D. R. , Sklar, L. A. , & Chigaev, A. (2019). Clioquinol: To harm or heal. Pharmacology & Therapeutics, 199, 155–163. 10.1016/j.pharmthera.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, J. M. , Gallagher, T. , & Weiss, S. R. (2017). Neurovirulent murine coronavirus JHM.SD uses cellular zinc metalloproteases for virus entry and cell–cell fusion. Journal of Virology, 91, e01564–e01516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz, B. J. , Seal, G. , & Loeb, L. A. (1974). Reverse transcriptase: Correlation of zinc content with activity. Proceedings of the National Academy of Sciences of the United States of America, 71, 4892–4896. 10.1073/pnas.71.12.4892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad, A. S. (2012). Discovery of human zinc deficiency: 50 years later. Journal of Trace Elements in Medicine and Biology, 26, 66–69. 10.1016/j.jtemb.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Rahman, M. T. , & Idid, S. Z. (2020). Can Zn be a critical element in COVID‐19 treatment? Biological Trace Element Research, 26, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read, S. A. , Obeid, S. , Ahlenstiel, C. , & Ahlenstiel, G. (2019). The role of zinc in antiviral immunity. Advances in Nutrition, 10, 696–710. 10.1093/advances/nmz013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder, N. L. , Kaplan, J. , Xu, J. , Youngquist, R. S. , Wallace, J. , Hu, P. , … Saunders, C. W. (2011). Zinc pyrithione inhibits yeast growth through copper influx and inactivation of iron–sulfur proteins. Antimicrobial Agents and Chemotherapy, 55, 5753–5760. 10.1128/AAC.00724-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, W. G. , Turpin, J. A. , Huang, M. , Clanton, D. , Buckheit, R. W. Jr. , Covell, D. G. , … Sausville, E. A. (1997). Azodicarbonamide inhibits HIV‐1 replication by targeting the nucleocapsid protein. Nature Medicine, 3, 341–345. 10.1038/nm0397-341 [DOI] [PubMed] [Google Scholar]
- Riddell, F. G. (2002). Structure, conformation, and mechanism in the membrane transport of alkali metal ions by ionophoric antibiotics. Chirality, 14, 121–125. 10.1002/chir.10052 [DOI] [PubMed] [Google Scholar]
- Risch, H. A. (2020). Early outpatient treatment of symptomatic, high‐risk Covid‐19 patients that should be ramped‐up immediately as key to the pandemic crisis. American Journal of Epidemiology, kwaa093 10.1093/aje/kwaa093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargsyan, K. , Lin, C.‐C. , Chen, T. , Grauffel, C. , Chen, Y.‐P. , & Yang, W.‐Z. (2020). Multi‐targeting of functional cysteines in multiple conserved SARS‐CoV‐2 domains by clinically safe Zn‐ejectors. ChemRxiv. 10.26434/chemrxiv.12179037.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar, A. , Balakrishnan, K. , Chen, J. , Patel, V. , Neelapu, S. S. , McMurray, J. S. , & Gandhi, V. (2016). Molecular evidence of Zn chelation of the procaspase activating compound B‐PAC‐1 in B cell lymphoma. Oncotarget, 7, 3461–3476. 10.18632/oncotarget.6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi, S. L. , Granzotto, A. , Siotto, M. , & Squitti, R. (2018). Copper and zinc dysregulation in Alzheimer's disease. Trends in Pharmacological Sciences, 39, 1049–1063. 10.1016/j.tips.2018.10.001 [DOI] [PubMed] [Google Scholar]
- Shang, J. , Ye, G. , Shi, K. , Wan, Y. , Luo, C. , Aihara, H. , … Li, F. (2020). Structural basis of receptor recognition by SARS‐CoV‐2. Nature, 581, 221–224. 10.1038/s41586-020-2179-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar, A. H. , & Prasad, A. S. (1998). Zinc and immune function: The biological basis of altered resistance to infection. The American Journal of Clinical Nutrition, 68, 447S–463S. 10.1093/ajcn/68.2.447S [DOI] [PubMed] [Google Scholar]
- Shittu, M. O. , & Afolami, O. I. (2020). Improving the efficacy of chloroquine and hydroxychloroquine against SARS‐CoV‐2 may require zinc additives—A better synergy for future COVID‐19 clinical trials. Le Infezioni in Medicina, 28, 192–197. [PubMed] [Google Scholar]
- Sims, A. C. , Burkett, S. E. , Yount, B. , & Pickles, R. J. (2008). SARS‐CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium. Virus Research, 133, 33–44. 10.1016/j.virusres.2007.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalny, A. V. , Rink, L. , Ajsuvakova, O. P. , Aschner, M. , Gritsenko, V. A. , Alekseenko, S. I. , … Tinkov, A. A. (2020). Zinc and respiratory tract infections: Perspectives for COVID‐19 (review). International Journal of Molecular Medicine, 46, 17–26. 10.3892/ijmm.2020.4575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater, A. F. (1993). Chloroquine: Mechanism of drug action and resistance in Plasmodium falciparum . Pharmacology & Therapeutics, 57, 203–235. 10.1016/0163-7258(93)90056-J [DOI] [PubMed] [Google Scholar]
- Sodhi, M. , & Etminan, M. (2020). Therapeutic potential for tetracyclines in the treatment of COVID‐19. Pharmacotherapy, 40, 487–488. 10.1002/phar.2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X. , Wang, T. , Cai, D. , Hu, Z. , Chen, J. , Liao, H. , … Wang, A. (2020). Cytokine storm intervention in the early stages of COVID‐19 pneumonia. Cytokine & Growth Factor Reviews, 53, 38–42. 10.1016/j.cytogfr.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supuran, C. T. , Innocenti, A. , Mastrolorenzo, A. , & Scozzafava, A. (2004). Antiviral sulfonamide derivatives. Mini Reviews in Medicinal Chemistry, 4, 189–200. 10.2174/1389557043487402 [DOI] [PubMed] [Google Scholar]
- Tebas, P. , Stein, D. , Tang, W. W. , Frank, I. , Wang, S. Q. , Lee, G. , … June, C. H. (2014). Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. The New England Journal of Medicine, 370, 901–910. 10.1056/NEJMoa1300662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velthuis, A. J. , van den Worm, S. H. , Sims, A. C. , Baric, R. S. , Snijder, E. J. , & van Hemert, M. J. (2010). Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathogens, 6, e1001176 10.1371/journal.ppat.1001176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumoorthy, N. , Shyam, S. A. , Manisenthil, K. K. , Senthil, K. M. , Ganesh, G. , & Chatterjee, M. (2011). A review of metallothionein isoforms and their role in pathophysiology. World Journal of Surgical Oncology, 9, 54–59. 10.1186/1477-7819-9-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijms, M. A. , van Dinten, L. C. , Gorbalenya, A. E. , & Snijder, E. J. (2001). A zinc finger‐containing papain‐like protease couples subgenomic mRNA synthesis to genome translation in a positive‐stranded RNA virus. Proceedings of the National Academy of Sciences of the United States of America, 98, 1889–1894. 10.1073/pnas.98.4.1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touret, F. , & de Lamballerie, X. (2020). Of chloroquine and COVID‐19. Antiviral Research, 177, 104762 10.1016/j.antiviral.2020.104762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, A. J. , Hiscox, J. A. , & Hooper, N. M. (2004). ACE2: From vasopeptidase to SARS virus receptor. Trends in Pharmacological Sciences, 25, 291–294. 10.1016/j.tips.2004.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaden, R. M. , Guillen, K. P. , Salvant, J. M. , Santiago, C. B. , Gibbons, J. B. , Pathi, S. S. , … Welm, B. E. (2019). A cancer‐selective zinc ionophore inspired by the natural product naamidine A. ACS Chemical Biology, 14, 106–117. 10.1021/acschembio.8b00977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemert, M. J. , van den Worm, S. H. , Knoops, K. , Mommaas, A. M. , Gorbalenya, A. E. , & Snijder, E. J. (2008). SARS‐coronavirus replication/transcription complexes are membrane‐protected and need a host factor for activity in vitro . PLoS Pathogens, 4, e1000054 10.1371/journal.ppat.1000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Cao, R. , Zhang, L. , Yang, X. , Liu, J. , Xu, M. , … Xiao, G. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Research, 30, 269–271. 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weekley, C. M. , & He, C. (2017). Developing drugs targeting transition metal homeostasis. Current Opinion in Chemical Biology, 37, 26–32. 10.1016/j.cbpa.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, A. R. , Du, T. , Laughton, K. M. , Volitakis, I. , Sharples, R. A. , Xilinas, M. E. , … Masters, C. L. (2006). Degradation of the Alzheimer disease amyloid β‐peptide by metal‐dependent up‐regulation of metalloprotease activity. The Journal of Biological Chemistry, 281, 17670–17680. 10.1074/jbc.M602487200 [DOI] [PubMed] [Google Scholar]
- Wiggins, H. L. , Wymant, J. M. , Solfa, F. , Hiscox, S. E. , Taylor, K. M. , Westwell, A. D. , & Jones, A. T. (2015). Disulfiram‐induced cytotoxicity and endo‐lysosomal sequestration of zinc in breast cancer cells. Biochemical Pharmacology, 93, 332–342. 10.1016/j.bcp.2014.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C. , Liu, Y. , Yang, Y. , Zhang, P. , Zhong, W. , Wang, Y. , … Li, H. (2020). Analysis of therapeutic targets for SARS‐CoV‐2 and discovery of potential drugs by computational methods. Acta Pharmaceutica Sinica B, 10, 766–788. 10.1016/j.apsb.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, J. , Moyer, A. , Peng, B. , Wu, J. , Hannafon, B. N. , & Ding, W. Q. (2014). Chloroquine is a zinc ionophore. PLoS ONE, 9, e109180 10.1371/journal.pone.0109180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, X. , Ye, F. , Zhang, M. , Cui, C. , Huang, B. , Niu, P. , … Liu, D. (2020). In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clinical Infectious Diseases, ciaa237 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, K. , Zheng, X. , Wang, G. , Liu, M. , Li, Y. , Yu, P. , … Wang, C. (2019). Immunonutrition vs standard nutrition for cancer patients: A systematic review and meta‐analysis (part 1). JPEN Journal of Parenteral and Enteral Nutrition, 44, 742–767. 10.1002/jpen.1736 [DOI] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X. L. , Wang, X. G. , Hu, B. , Zhang, L. , Zhang, W. , … Shi, Z. L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579, 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


