Abstract
We report a case series of five patients affected by SARS‐CoV‐2 who developed neurological symptoms, mainly expressing as polyradiculoneuritis and cranial polyneuritis in the 2 months of COVID‐19 pandemic in a city in the northeast of Italy. A diagnosis of Guillain‐Barré syndrome was made on the basis of clinical presentation, cerebrospinal fluid analysis, and electroneurography. In four of them, the therapeutic approach included the administration of intravenous immunoglobulin (0.4 g/kg for 5 days), which resulted in the improvement of neurological symptoms. Clinical neurophysiology revealed the presence of conduction block, absence of F waves, and in two cases a significant decrease in amplitude of compound motor action potential compound muscle action potential (cMAP). Four patients presented a mild facial nerve involvement limited to the muscles of the lower face, with sparing of the forehead muscles associated to ageusia. In one patient, taste assessment showed right‐sided ageusia of the tongue, ipsilateral to the mild facial palsy. In three patients we observed albuminocytological dissociation in the cerebrospinal fluid, and notably, we found an increase of inflammatory mediators such as the interleukin‐8. Peripheral nervous system involvement after infection with COVID‐19 is possible and may include several signs that may be successfully treated with immunoglobulin therapy.
Keywords: COVID‐19, cranial polyneuritis, immunoglobulin, interleukins, polyradiculonevritis
Highlights
Neurological symptoms may be common in COVID‐19 patients
Neurophysiological assessment is fundamental for a correct diagnosis
Peripheral nervous system involvement is possibile in people with COVID‐19
In these patients, intravenous immunoglobulin administration is a safe and efficient therapy
1. INTRODUCTION
Since December 2019, the novel coronavirus (SARS‐CoV‐2) has rapidly spread worldwide, causing an increased number of hospitalization and intensive care admissions, due to severe respiratory distress. Even though respiratory symptoms play a critical role in the clinical picture, in the last few weeks a variety of systemic manifestations has been increasingly described, including neurological symptoms. Neurological complications reported so far in patients affected by new coronavirus infectious disease (COVID‐19) suggest a possible neurotropism of the virus and its potential ability to induce auto‐immunity reactions. Several neurological complications have been described, including cerebrovascular accidents, polyradiculoneuritis (Guillain‐Barré syndrome), and other inflammatory diseases. 1 Among the peripheral nervous system manifestations, the most frequently observed are hyposmia, hypogeusia, and Guillain‐Barrè syndrome (GBS). 2 , 3 GBS is a heterogeneous condition with several variant forms: the most common presentation is the progressively ascending tetraparesis (acute inflammatory demyelinating polyneuropathy), but other localized clinical variants are also recognized. Miller‐Fisher syndrome (MFS), a regional variant characterized by the triad of ophthalmoplegia, ataxia, and areflexia, has also been linked to COVID‐19. 4 According to a new classification, autoimmune neuropathies can also include forms with central nervous system involvement (Bickerstaff brainstem encephalitis). 5 About 60% of the above‐mentioned autoimmune syndromes can be infection‐related by humoral and cellular cross‐reactivity, 6 , 7 most frequently gastrointestinal (Campylobacter jejuni) or respiratory tract infections, including flu syndrome and pneumonia. 8 , 9 Clinical neurophysiology represents a fundamental tool for the diagnosis of acute inflammatory neuropathies. Neurophysiological investigations, however, require close contact with the patient and may result in an increased risk of infection, therefore, only partial data have been collected so far in COVID‐19 patients.
Here we report a case series of five patients affected by COVID‐19 who developed a spectrum of autoimmune polyneuropathies during hospitalization. We describe their clinical features, laboratory testing as well as treatment response. Particular attention has been paid to neurophysiological findings and cerebrospinal fluid analysis.
2. MATERIALS AND METHODS
This case series described five patients admitted to the hospital affected by bilateral pneumonia due to SARS‐CoV‐2 infection from March to April 2020. Symptoms on admission were fever and cough, and in four out of five patients significant impairment of taste and smell was also reported (Table 1). Due to respiratory failure patients were admitted in the COVID‐19 protected areas of the University Hospital of Trieste. COVID‐19 diagnosis was then confirmed by means of nasopharyngeal swab. COVID‐19 management included a variety of treatments, including antiviral drugs (Lopinavir/Ritonavir, Darunavir), hydroxychloroquine, antibiotic therapy, and oxygen support (Table 1). Two patients received Tocilizumab, a monoclonal antibody targeting the interleukin (IL)‐6 receptor. Two out of the five patients remained in COVID‐dedicated internal medicine units, whereas three of them required mechanical ventilation in the intensive care unit (ICU) for a prolonged time (from 11 to 20 days).
Table 1.
Demographic, clinical, and laboratory features of the patients
| Patient | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age | 72 y | 72 y | 49 y | 94 y | 76 y |
| Sex | Male | Male | Female | Male | Male |
| Early symptoms of COVID‐19 | Fever, dyspnea, hyposmia, and ageusia | Fever, cough, dyspnea, hyposmia, and ageusia | Fever, cough, dyspnea, hyposmia, and ageusia | Fever, cough, gastrointestinal symptoms, | Fever, cough, dysuria, hyposmia, and ageusia |
| Need for mechanical ventilation | Yes | Yes | No | No | Yes |
| Latency of neurological symptoms a | 18 d | 30 d b | 14 d | 33 d | 22 d |
| Neurological signs and symptoms | Flaccid tetraparesis, with proximal upper limb predominance | Flaccid tetraparesis with lower limbs predominance | Ophthalmoplegia with diplopia in the vertical and lateral gaze, limb ataxia | Lower limbs weakness | Proximal weakness of lower and upper limb, with upper limb predominance |
| Deep tendon reflexes | Diffusely absent | Diffusely absent | Diffusely absent | Diffusely weak | Diffusely absent |
| Sensory disturbances | Tingling of distal lower extremities | Sense of having a tight bandage on legs and feet | Right‐sided hypoesthesia of the face | Unassessable due to agitation status | None |
| Cranial nerve involvement | Mild right‐sided lower face facial weakness, with sparing of the forehead muscles | None | Bilateral ophthalmoplegia; | None | Mild left‐sided lower facial deficit; |
| Hypoesthesia in the territory of maxillary and mandibular trigeminal branches; | Reported mild transient diplopia fully recovered at the time of evaluation | ||||
| Mild right‐sided lower facial deficit | |||||
| CSF findings | Protein level 52 mg/dL; 1 cell/mm3 | Normal protein level (40 mg/dL); 1 cell/mm3 | Protein level 72 mg/dL; 5 cells/mm3 | Not performed | Protein level 53 mg/dL; 2 cell/mm3 |
| PCR for SARS‐CoV‐2: negative | PCR for SARS‐CoV‐2: negative | PCR for SARS‐CoV‐2: negative | PCR for SARS‐CoV‐2: negative | ||
| Antiganglioside antibodies | Negative | Negative | Negative | Not performed | Negative |
| Serum interleukin level c | IL‐1β: 0.2 pg/mL ↑ | IL‐1β: 0.5 pg/mL ↑ | Not performed | Not performed | IL‐1β: 0.2 pg/mL ↑ |
| IL‐6: 113.0 pg/mL ↑↑↑ | IL‐6: 9.8 pg/mL ↑ | IL‐6: 32.7 pg/mL ↑↑ | |||
| IL‐8: 20.0 pg/mL ↑ | IL‐8: 55.0 pg/mL ↑↑ | IL‐8: 17.8 pg/mL ↑ | |||
| TNF‐α: 16.0 pg/mL ↑ | TNF‐α: 16.0 pg/mL ↑ | TNF‐α: 11.1 pg/mL | |||
| IL‐2R: 1203.0 pg/mL | |||||
| IL‐10: 4.6 pg/mL | |||||
| IP‐10: 94.8 pg/mL | |||||
| INF‐γ: 0.8 pg/mL | |||||
| CSF interleukin level d | IL‐1β: 0.12 pg/mL | IL‐1β: 0.1 pg/mL | Not performed | Not performed | IL‐1β: 0.52 pg/mL |
| IL‐6: 9.6 pg/mL | IL‐6: 1.4 pg/mL | IL‐6: 5.9 pg/mL | |||
| IL‐8: 22.7 pg/mL | IL‐8: 96.0 pg/mL | IL‐8: 42.6 pg/mL | |||
| TNF‐α: 0.3 pg/mL | TNF‐α: 0.7 pg/mL | TNF‐α: 0.25 pg/mL | |||
| IL‐2R: 24.6 pg/mL | |||||
| IL‐10: 0.55 pg/mL | |||||
| IP‐10: 60.8 pg/mL | |||||
| INF‐γ: 0.63 pg/mL | |||||
| IL‐8 CSF/serum ratio | 1.1 | 1.74 | Not performed | Not performed | 2.39 |
| Brain MRI | Not performed | Normal findings | Normal findings | Not performed | Not performed |
| Treatment of the neurological syndrome | IVIG cycle (0.4 g/kg for 5 d) | IVIG cycle (0.4 g/kg for 5 d) | IVIG cycle (0.4 g/kg for 5 d) | Methylprednisolone 60 mg for 5 d | IVIG cycle (0.4 g/kg for 5 d) |
| Other therapies | COVID‐19 management included administration of hydroxychloroquine, oseltamivir, darunavir, methylprednisolone, and tocilizumab | COVID‐19 management included administration of hydroxychloroquine, lopinavir‐ritonavir, methylprednisolone | COVID‐19 management included administration of hydroxychloroquine, lopinavir‐ritonavir, methylprednisolone | COVID‐19 management included administration of methylprednisolone | COVID‐19 management included administration of hydroxychloroquine, oseltamivir, darunavir, methylprednisolone, |
| Tocilizumab, | |||||
| meropenem, linezolid, | |||||
| clarithromycin, | |||||
| doxycycline and fluconazole | |||||
| Outcome | Improvement of tetraparesis | Improvement of weakness | Progressive improvement | Stationary | Progressive improvement |
Abbreviations: CDF, cerebrospinal fluid; IL, interleukin; PCR, polymerase chain reaction; TNF, tumor necrosis factor.
Days between early respiratory symptoms and neurological syndrome onset.
It is possible that symptoms appeared earlier in the course of disease but were not evident as the patient was intubated and sedated.
Laboratory reference values for serum interleukins—IL‐β: <0.001 pg/mL; IL‐6: 0.8‐6.4 pg/mL; IL‐8: 6.7‐16.2 pg/mL; TNF‐α: 7.8‐12.2 pg/mL; IL‐2R: 440.0‐1435.0 pg/mL; IL‐10: 1.8‐3.8 pg/mL; IP‐10: 37.2‐222.0 pg/mL; INF‐γ: <0.99 pg/mL.
Reference values for CDF interleukins were assumed equal to serum values, as standardized cut‐off values are not yet recognized.
Patients developed progressive weakness of the upper and lower limbs, in a distoproximal fashion; the latency between the onset of the respiratory symptoms and neurological involvement ranged from 14 to 30 days. However, the longest latency (30 days) has been observed in a patient who came under prolonged sedation in ICU, therefore it is likely that symptoms appeared earlier in the course of the disease, but were not assessable at that time.
All the patients received neurological examination at symptoms development, routine blood chemistry analyses, and a panel of antiganglioside antibodies, including anti‐GM1, ‐GM2, ‐GM3, ‐GD1a, ‐GD1b, ‐GT1b, and ‐GQ1b, were performed according to standard procedures. Cerebrospinal fluid (CSF) was collected and processed for standard analyses including pressure, cell count, proteins, and glucose. CSF culture and polymerase chain reaction (PCR) for possible organisms, such as bacteria, Mycobacterium tuberculosis, fungi, Herpes viruses, Enteroviruses, Japanese B virus, and Dengue viruses, were performed, including analysis for SARS‐CoV‐2.
2.1. Clinical neurophysiology
Motor and sensory nerve conduction studies were performed in the upper and lower limbs following standard international guidelines. The neurophysiological evaluation included electroneurography (ENG) and electromyography (EMG) that were performed in the COVID‐dedicated area for all the patients. F waves were recorded from lower and upper limbs. Facial nerve conduction velocity was studied by using as recording points the Orbicularis Oris and the Orbicularis oculi muscles and using as stimulation point the tragus on both sides. A blink reflex testing was also carried out to the patient with suspect MFS. Needle EMG was performed in four patients. The physician and the technician wore personal protective equipment including appropriate masks, face shields, gowns, and gloves following the guidelines American Association of Clinical Neurophysiology website has guidance (https://www.acns.org/practice/covid-19‐ resources).
3. RESULTS
Neurological examination revealed a flaccid paresis in four patients, with variable lower or upper limb predominance; interestingly, in these patients, we observed a unilateral mild facial nerve involvement limited to the muscles of the lower face, with sparing of the forehead muscles. Two patients also reported paresthesia, described as tingling or a sense of “tight bandage” located at the lower extremities. Deep tendon reflexes were diffusely absent. The fifth patient developed a slightly different clinical picture, characterized by bilateral ophthalmoplegia, hypoesthesia in the territory of maxillary and mandibular trigeminal branches, mild right lower facial nerve palsy, and limb ataxia. Generalized areflexia was also present. All patients have been complaining of taste and smell impairment since the beginning of the respiratory symptoms. In the latter patient described we performed a taste assessment which showed right‐sided ageusia of the tongue, ipsilateral to the mild facial palsy (Figure 1).
Figure 1.
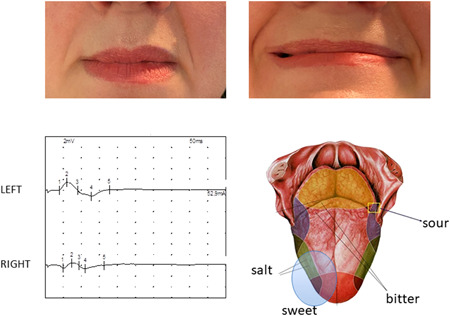
a 50‐year‐old female with initial ophthalmoplegia and diplopia, left upper arm cerebellar dysmetria, generalized areflexia, mild defect in right lower branch of facial nerve. The facial defect persisted after the general improvement with immunoglobulins therapy. Asymmetry of the latency and amplitude of facial nerve conduction of the right lower branch was associated with asymmetrical ageusia for salt and sweet taste on the right side of the tongue
In all patients the results of routine blood chemistry tests, human immunodeficiency virus, Hepatitis B Virus, Hepatatis C Virus, as well as a panel of serological tests for autoimmune disorders were unremarkable. A panel of antiganglioside antibodies, including anti‐GM1, ‐GM2, ‐GM3, ‐GD1a, ‐GD1b, ‐GT1b, and ‐GQ1b, was negative. In four out of five patients. CSF analysis revealed clear CSF, normal pressure, and no blood cells (Table 1). The CSF/serum glucose ratio was normal in all patients. In three of them, CSF revealed a mild albumin‐cytologic dissociation (ranging from 52 to 72 mg/dL of proteins; normal protein level <45 mg/dL). CSF culture and PCR for possible organisms yielded negative results. PCR for SARS‐CoV‐2 was also carried out and resulted in negative in all the patients tested. A remarkable increase of ILs (mainly IL‐8) was found in three patients suggesting an active inflammatory process inside the cerebrospinal fluid (Table 1).
3.1. Clinical neurophysiology
Three patients showed the presence of conduction block mainly in lower limbs, two of them mainly in the upper limbs (Table 2). All the patients showed either an increase in latency of F wave or the dispersion and the decrease in amplitude of F wave. In two of them, we noted the presence of denervation signs related to the marked decrease amplitude of peroneal and tibial nerve suggesting axonal damage. In particular, the patients had a decrease in amplitude of the facial nerve conduction recorded from orbicularis oris and stimulated from the tragus site ipsilateral to the facial weakness. Notably, the nerve conduction recorded from orbicularis oculi was normal. The blink reflex was performed in one patient and was normal.
Table 2.
Neurophysiological study of patients
| Patients | Nerve | Stimulation point | Record point | Distal latency, ms | Amplitude, mV | Velocity, m/s | F wave minimal latency, ms |
|---|---|---|---|---|---|---|---|
| 1 | Motor NCS | ||||||
| Median (L) | Elbow | Wrist | 13.70 | 4.10 | 45.0 | 26.6 | |
| Median (R) | Elbow | Wrist | 8.80 | 5.30 | 67.0 | 31.3 | |
| Ulnar (L) | Elbow | Wrist | 6.56 | 3.60 | ⋯ | 34.4 | |
| Ulnar (R) | Elbow | Wrist | 7.38 | 4.20 | 67.0 | 29.5 | |
| Tibial (L) | Ankle | Adb hal | 6.17 | 2.30 | ⋯ | 67.0 | |
| Peroneal (L) | Ankle | EDB | 4.42 | 1.80 | ⋯ | 62.0 | |
| Head of fibula | Ankle | 4.60 | 1.10 | 34.4 | 73.0 | ||
| Peroneal (R) | Ankle | EDB | 5.21 | 1.05 | ⋯ | ⋯ | |
| Head of fibula | Ankle | 16.10 | 0.13 | 34.0 | ⋯ | ||
| Antidromic sensory NCS | |||||||
| Median (R) | Wrist | 2rd Digit | 3.80 | 8.50 | ⋯ | ⋯ | |
| Ulnar (R) | Wrist | 5th Digit | 3.70 | 7.20 | ⋯ | ⋯ | |
| Sural (L‐R) | Lateral malleolus | Calf | ⋯ | ⋯ | ⋯ | ⋯ | |
| 2 | Motor NCS | ||||||
| Median (L) | Wrist | APB | 3.74 | 1.31 | ⋯ | 39.0 | |
| Elbow | 8.20 | 0.69 | 23.7 | ⋯ | |||
| Median (R) | Wrist | APB | 5.37 | 2.00 | ⋯ | 29.0 | |
| Elbow | 11.30 | 0.88 | 23.7 | ⋯ | |||
| Ulnar (L) | Wrist | ADM | 5.85 | 1.93 | ⋯ | 34.0 | |
| Elbow | 11.30 | 1.62 | 49.5 | ⋯ | |||
| Peroneal (L) | Ankle | EDB | 6.69 | 0.18 | ⋯ | Absent | |
| Head of fibula | 8.54 | 0.13 | 10.8 | ⋯ | |||
| Peroneal (R) | Ankle | Abd hal | 25.90 | 0.60 | ⋯ | Absent | |
| Knee | 24.10 | 0.54 | ⋯ | ⋯ | |||
| Head of fibula | Ankle | 27.10 | 0.06 | ⋯ | ⋯ | ||
| Antidromic sensory NCS | |||||||
| Median (L) | Wrist | 2nd Digit | 3.79 | 15.40 | ⋯ | ⋯ | |
| Median (R) | Wrist | 2nd Digit | 4.18 | 4.80 | ⋯ | ⋯ | |
| Ulnar (R) | Wrist | 5th Digit | 3.36 | 13.10 | ⋯ | ⋯ | |
| Sural (L) | Lateral malleolus | Calf | 7.65 | 15.00 | 24.8 | ⋯ | |
| 3 | Motor NCS | ||||||
| Median (R) | 2nd Digit | 4.51 | 9.10 | 54.0 | 25.5 | ||
| Ulnar (R) | 5th Digit | 4.32 | 8.20 | 55.1 | 27.4 | ||
| Peroneal R | 4.70 | 9.50 | 50.2 | 40.3 | |||
| Facial (L) | Postauricular | Orbicular oris | 3.88 | 1.60 | ⋯ | ⋯ | |
| Orbicular oculi | 2.88 | 1.50 | ⋯ | ⋯ | |||
| Facial (R) | Postauricular | Orbicular oris | 4.85 | 0.50 | ⋯ | ⋯ | |
| Orbicular oculi | 3.25 | 1.60 | ⋯ | ⋯ | |||
| Antidromic sensory NCS | |||||||
| Median (R) | 3.50 | 11.30 | ⋯ | ⋯ | |||
| Ulnar (R) | 3.80 | 12.00 | ⋯ | ⋯ | |||
| Sural (R) | 6.30 | 20.00 | ⋯ | ⋯ | |||
| 4 | Motor NCS | ||||||
| Peroneal (L) | Ankle | Abd hal | 23.60 | 1.50 | ⋯ | Absent | |
| Peroneal (R) | Ankle | EDB | 7.90 | 0.10 | ⋯ | Absent | |
| Ankle | EDB | 15.70 | 0.16 | ⋯ | Absent | ||
| Head of fibula | Ankle | 17.50 | 0.75 | ⋯ | ⋯ | ||
| 4.50 | ⋯ | ⋯ | |||||
| Facial (R) | Postauricular | Orbicular oris | 5.73 | 1.10 | ⋯ | ⋯ | |
| Facial (L) | Postauricular | Orbicular oris | 4.50 | 1.99 | ⋯ | ⋯ | |
| Antidromic sensory NCS | |||||||
| Not tested | |||||||
| 5 | Motor NCS | ||||||
| Median (R) | Wrist | APB | Not detectable | ⋯ | ⋯ | ⋯ | |
| Ulnar (R) | Wrist | ADM | Not detectable | ⋯ | ⋯ | ⋯ | |
| Tibial (L) | 49.8 | ||||||
| Tibial (R) | 50.7 | ||||||
| Peroneal (L) | Ankle | 3.00 | 0.76 | ⋯ | 47.6 | ||
| Head of fibula | EDB | 9.13 | 0.74 | 45.7 | ⋯ | ||
| Knee | Ankle | 11.50 | 0.79 | 46.4 | ⋯ | ||
| Peroneal (R) | Ankle | 3.32 | 1.07 | ⋯ | 49.9 | ||
| Head of fibula | EDB | 9.44 | 0.62 | 46.6 | ⋯ | ||
| Knee | Ankle | 11.30 | 0.68 | 43.0 | ⋯ | ||
| Facial (R) | Postauricular | Orbicular oris | 2.10 | 3.40 | ⋯ | ⋯ | |
| Facial (L) | Postauricular | Orbicular oris | 2.70 | 1.10 | ⋯ | ⋯ | |
| Antidromic sensory NCS | |||||||
| Not tested |
Note: “⋯” = not measured.
Abbreviations: Abd Hal, abductor halluces; ADM, abductor digiti minini; APB, abductor pollicis brevis; EDB, extensor digitorum brevis; L, left side of the body; NCS, nerve conduction study; R, right side of the body.
Based on the clinical presentation, neurophysiological and CSF findings, intravenous immunoglobulins (IVIG) therapy was initiated in four patients at a dose of 0.4 g/kg for 5 days. The neurological symptoms improved and partially resolved after the initiation of IVIG treatment in four of the patients. No side effects were reported after the use of IVIG therapy.
4. DISCUSSION
The clinical presentation, the neurophysiology findings, and CSF analysis showing albumin‐cytological dissociation suggested a diagnosis of GBS in these patients during and after COVID‐19 infection. The neurophysiology examination, although complicated by the protective equipment and protocols to reduce the risk of infection, was extremely useful to detect subclinical findings, better defining the diagnosis and encouraging the start of the appropriate therapy with IVIG, as previously suggested. 5 In fact, during COVID‐19 infection the weakness of the upper and lower limbs could be underestimated and misdiagnosed, especially in the first phase of the disease, when the emergency and the high risk of infection in COVID‐19 dedicated areas are the overriding concern. In our experience, ENG was mandatory for the correct diagnosis despite being complicated to perform in COVID‐19 areas, to respect the protocols of security and protection. 10 In two of our patients, the ENG has documented the presence of conduction block and increase in latency and dispersion of F waves typical of GBS, while in the other two cases a significant decrease in amplitude in distal and proximal compound muscle action potential as previously reported in axon motor acute neuropathy. 5 In contrast, the ENG in the fifth patient was able to clearly detect the facial involvement.
In this study, we observed an increase in serum ILs, particularly IL‐6 and IL‐8 in three patients; these findings are consistent with the current evidence that the overproduction of inflammatory cytokines may lead to severe forms of COVID‐19, increased risk of multiorgan failure, and eventually death. 11 Indeed, in three of our patients, mechanical ventilation was required due to the acute respiratory distress syndrome, while two received Tocilizumab to block the inflammatory cascade. As such, the autoimmune polyneuropathies observed in our patients may be considered as part of a systemic overactive inflammatory response, namely “cytokine storm.” Moreover, in two out of three patients tested, IL‐8 resulted markedly increased also in CSF, with a CSF/serum ratio more than 1, suggesting the presence of an acute inflammatory process specifically targeting the nervous system. Previous studies showed that the CSF/serum IL‐8 ratio was increased in GBS as compared to chronic neuropathies such as the chronic inflammatory demyelinating polyneuropathy, thus it has been proposed as a possible biomarker of acute immune reaction against the nervous system. 12 , 13
Neurological complications are common following respiratory infections, 8 therefore, a cross‐reactivity for SARS‐CoV‐2 was also speculated and reported. 3 , 14 This note is in agreement with other studies reporting GBS and cranial polyneuritis during the SARS‐CoV‐2 pandemic as neurological clinical complications of COVID‐19. We did not find the presence of any antiganglioside antibody as anti‐GM1, ‐GM2, ‐GM3, ‐GD1a, ‐GD1b, ‐GT1b, and ‐GQ1b. The GQ1b and other glycoproteins are located at the paranodal sites of the peripheral nerves, Ia‐afferent neurons of the spinal dorsal horn cells, proximal segments of the cranial nerves controlling ocular motion, muscle spindle afferents, cerebellar vermis, and hypothalamic nuclei, usually explaining the symptoms of the disease. 5 , 15 However, in some cases of MFS (10%‐15%) and MFS/GBS overlap syndrome (22%) negative results for anti‐GQ1b tests have been reported. 16 , 17
Despite the exact mechanisms linking SARS‐CoV‐2 infection to neurological symptoms need further investigation, it is not possible to exclude a direct penetration of the virus in the peripheral and central nervous system. 18 , 19 In our patients, an autoimmune mechanism may be speculated, for the typical features of clinical neurophysiology, the albuminocytologic dissociation in CSF, and mainly for the significant response to immunoglobulins. A peculiar note is the rapid onset of neurological symptoms of neuropathy in these cases after the onset of COVID infection, indicative of a rapid and dramatic inflammatory reactivity not common in the typical GBS. 5 The timing of neurological signs in one of our patients seemed too rapid to imply a typical heterologous immune reaction to a first viral exposure and it could rather resemble a form of acute parainfectious paralysis that has already been associated with some viruses, such as ZIKV. 20 , 21 This paralysis usually develops from 5 to 10 days after infection and has higher morbidity, more frequently with cranial neuropathies and concurrent immune‐mediated disorders such as thrombocytopenic purpura 9 ; antiglycolipids antibodies typically involved in cross‐reactive mechanisms are not common. 9 , 21 The hypothesis of neurological manifestations of COVID‐19 is supported by the evidence of neurotropic and neuroinvasive characteristics of coronaviruses in humans 19 ; moreover, other strains of human coronaviruses have already been involved with peripheral nervous system manifestations 3 , 14 and with pediatric cases of GBS. 22
A novelty of this study is represented by the subclinical involvement of facial nerve detected by ENG in patients associated with ageusia. Facial nerve involvement is intriguing and should be considered as the real novelty of this study since its implication aligns with other cranial nerves involvement in these forms of GBS. Detecting facial nerve impairment with the ENG underlines the importance of clinical neurophysiology as a precious tool to detect mild demyelinating nerve damage; it also helps us investigate the mechanism behind ageusia, as direct involvement of facial nerve and not only as central dysfunction. In particular, the patient presenting a unilateral involvement with ageusia affecting the same side confirms this hypothesis, already described in some other cases. 22 , 23 Finally, IVIG therapy was found to be effective and safe to treat the reported peripheral neurological symptoms, with a good recovery in most of the patients. Most patients with COVID‐19 and lower/upper limbs related weakness are still not recognized or are not investigated by ENG and do not receive any specific treatment, therefore increasing the risk of permanent damage. In conclusion, this study describes the clinical and neurophysiological characteristics of GBS polyneuropathy in patients affected by COVID‐19, and the clinical responses to IVIG therapy, encouraging the use of clinical neurophysiology to better detect abnormalities, define the diagnosis, and promote the use of specific therapies, such as IVIG, to treat these important neurological symptoms.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTION
PM contributed to conceptualization, study design, data collection, wrote the draft and approved the final version of the manuscript. GB contributed to study design, data collection, manuscript draft, and approved the final version of the manuscript. LD contributed to study design, data collection, reviewed the manuscript, and approved the final version. VT contributed to data collection and reviewed the manuscript, and approved the final version. MF contributed to data collection and reviewed the manuscript, and approved the final version. AS and LB contributed to data collection and reviewed the manuscript, and approved the final version. AB contributed to data analysis, wrote the draft, and approved the final version of the manuscript. VP contributed to conceptualization, study design, data collection, reviewed, and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank all the staff of the COVID‐19 dedicated area and the neurophysiology technicians.
Manganotti P, Bellavita G, D'Acunto L, et al. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain‐Barré syndrome and polyneuritis cranialis in COVID‐19 patients: A case series. J Med Virol. 2021;93:766–774. 10.1002/jmv.26289
REFERENCES
- 1. Ahmad I, Rathore FA. Neurological manifestations and complications of COVID‐19: A literature review. J Clin Neurosci. 2020;77:8‐12. 10.1016/j.jocn.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med. 2020;382:2268‐2270. 10.1056/NEJMc2008597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain‐Barré syndrome associated with SARS‐CoV‐2 infection: causality or coincidence? Lancet Neurol. 2020;19:383‐384. 10.1016/S1474-4422(20)30109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manganotti P, Pesavento V, Stella AB, et al. Miller Fisher syndrome diagnosis and treatment in a patient with SARS‐CoV‐2 [published online ahead of print June 11, 2020]. J Neurovirol. 2020:1‐2. 10.1007/s13365-020-00858-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wakerley BR, Uncini A, Yuki N. Guillain‐Barré and Miller‐Fisher syndromes—new diagnostic classification. Nat Rev Neurol. 2014;10(9):537‐544. 10.1038/nrneurol.2014.138 [DOI] [PubMed] [Google Scholar]
- 6. Lehmann HC, Hartung H‐P, Kieseier BC, Hughes RAC. Guillain‐Barré syndrome after exposure to influenza virus. Lancet Infect Dis. 2010;10(9):643‐651. 10.1016/S1473-3099(10)70140-7 [DOI] [PubMed] [Google Scholar]
- 7. Pusch E, Renz H, Skevaki C. Respiratory virus‐induced heterologous immunity: part of the problem or part of the solution? Allergo J Int. 2018;27(3):79‐96. 10.1007/s40629-018-0056-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sellers SA, Hagan RS, Hayden FG, Fischer WA 2nd. The hidden burden of influenza: a review of the extra‐pulmonary complications of influenza infection. Influenza Other Respi Viruses. 2017;11(5):372‐393. 10.1111/irv.12470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim JE, Heo JH, Kim HO, et al. Neurological complications during treatment of Middle East respiratory syndrome. J Clin Neurol. 2017;13(3):227‐233. 10.3988/jcn.2017.13.3.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kassardjian CD, Desai U, Narayanaswami P. Practical guidance for managing EMG requests and testing during the COVID‐19 pandemic. Muscle Nerve. 2020;62:30‐33. 10.1002/mus.26891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jose RJ, Manuel A. COVID‐19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8:46. 10.1016/S2213-2600(20)30216-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sainaghi PP, Collimedaglia L, Alciato F, et al. The expression pattern of inflammatory mediators in cerebrospinal fluid differentiates Guillain‐Barré syndrome from chronic inflammatory demyelinating polyneuropathy. Cytokine. 2010;51(2):138‐143. 10.1016/j.cyto.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 13. Breville G, Lascano AM, Roux‐Lombard P, Lalive PH. IL‐8 as a potential biomarker in Guillain‐Barre Syndrome. Eur Cytokine Netw. 2019;30(4):130‐134. 10.1684/ecn.2019.0436 [DOI] [PubMed] [Google Scholar]
- 14. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China [published online ahead of print April 10, 2020]. JAMA Neurol 2020;77(6):1–9. 10.1001/jamaneurol.2020.1127, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaida K, Kanzaki M, Morita D, et al. Anti‐ganglioside complex antibodies in Miller‐Fisher syndrome. J Neurol Neurosurg Psychiatry. 2006;77(9):1043‐1046. 10.1136/jnnp.2006.087940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sekiguchi Y, Mori M, Misawa S, et al. How often and when Fisher syndrome is overlapped by Guillain‐Barré syndrome or Bickerstaff brainstem encephalitis? Eur J Neurol. 2016;23(6):1058‐1063. 10.1111/ene.12983 [DOI] [PubMed] [Google Scholar]
- 17. Wattanasit P, Sathirapanya P. Anti‐ganglioside antibody‐negative Miller Fisher and AMSAN variant Guillain‐Barré Overlap Syndrome. Case Rep Neurol. 2020;12(1):92‐96. 10.1159/000506191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID‐19 and other coronaviruses. Brain Behav Immun. 2020;87:18‐22. 10.1016/j.bbi.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID‐19 virus targeting the CNS: tissue distribution, host‐virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995‐998. 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
- 20. Musso D, Ko AI, Baud D. Zika virus infection—after the pandemic. N Engl J Med. 2019;381(15):1444‐1457. 10.1056/NEJMra1808246 [DOI] [PubMed] [Google Scholar]
- 21. Uncini A, Shahrizaila N, Kuwabara S. Zika virus infection and Guillain‐Barré syndrome: a review focused on clinical and electrophysiological subtypes. J Neurol Neurosurg Psychiatry. 2017;88(3):266‐271. 10.1136/jnnp-2016-314310 [DOI] [PubMed] [Google Scholar]
- 22. Sharma K, Tengsupakul S, Sanchez O, Phaltas R, Maertens P. Guillain‐Barré syndrome with unilateral peripheral facial and bulbar palsy in a child: acase report. SAGE Open Med Case Rep. 2019;7:2050313X19838750. 10.1177/2050313X19838750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polo A, Manganotti P, Zanette G, De Grandis D. Polyneuritis cranialis: clinical and electrophysiological findings. J Neurol Neurosurg Psychiatry. 1992;55(5):398‐400. 10.1136/jnnp.55.5.398 [DOI] [PMC free article] [PubMed] [Google Scholar]


