TO THE EDITOR
Red meat allergy (RMA) is characterized by urticaria, angioedema, gastrointestinal symptoms, and/or anaphylaxis occurring approximately 3 to 6 hours after ingesting mammalian meats that contain the antigen galactose-α−1,3-galactose (α-Gal).1 The molecular structure of α-Gal is similar to that of the ABO blood group B antigen, a self-antigen in patients with blood types B or AB.2 This molecular similarity provokes the hypothesis that patients expressing the B antigen might exhibit tolerance to α-Gal. Consistent with this hypothesis, a recent case series suggested that the B and AB blood groups are underrepresented in patients with RMA,3 although this study did not include controls. In addition, patients who express the B antigen produce less α-Gal-specific IgG and IgE than those without the B antigen.4,5 However, it remains unknown whether patients who express the B antigen are protected from developing RMA.
To address this, we employed a meta-analysis approach combining individual patient data from 4 institutions. Our previous systematic literature search6 identified 3 studies3,7,8 that reported ABO type for 77 individual patients with RMA. We then combined these cases with 15 patients with RMA (92 total) and 188 controls from Washington University School of Medicine (WashU), all of whom had known ABO types as determined by routine forward and reverse ABO typing. Details on the identification and selection of patients with RMA and controls at WashU are found in the Methods section of this article’s Online Repository at www.jaci-inpractice.org, and the characteristics of the 4 RMA cohorts are shown in Table E1 (available in this article’s Online Repository at www.jaci-inpractice.org).
TABLE E1.
The 4 red meat allergy cohorts included in this meta-analysis
| Characteristic | Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 |
|---|---|---|---|---|
| Source | Hamsten et al, 2013E2 | Martinez Arcediano et al, 2014E3 | Chinuki et al, 2016E1 | Washington University in St. Louis |
| Location | Sweden | Spain | Japan | United States |
| N | 39 | 10 | 28 | 15 |
| Women/men, %/% | 46/54 | 20/80 | 36/64 | 71/29 |
| Age, y | 52.8 ± 2.22 | 43.4 ± 3.86 | 68.7 ± 2.11 | 37.8 ± 6.59 |
| ABO blood types, n (%) | ||||
| O | 20 (51.3) | 8 (80.0) | 11 (39.3) | 9 (60.0) |
| A | 17 (43.6) | 2 (20.0) | 16 (57.1) | 5 (33.3) |
| B | 1 (2.6) | 0 (0) | 1 (3.6) | 0 (0) |
| AB | 1 (2.6) | 0 (0) | 0 (0) | 1 (6.7) |
| α-Gal IgE, IU/mL | 29.4 ± 4.93 (n = 39) | 32.3 ± 9.53 (n = 10) | 52.9 ± 7.81 (n = 28) | 11.8 ± 5.53 (n = 6) |
| Beef IgE, IU/mL | 6.57 ± 1.01 (n = 39) | 8.38 ± 4.89 (n = 10) | 10.2 ± 2.03 (n = 28) | 12.8 ± 6.7 (n = 8) |
| Pork IgE, IU/mL | 5.73 ± 1.06 (n = 39) | 3.41 ± 1.63 (n = 10) | 7.88 ± 1.49 (n = 28) | 15.0 ± 9.8 (n = 6) |
| Lamb IgE, IU/mL | Not reported | 2.10 ± 0.63 (n = 6) | Not reported | 1.33 (n = 1) |
Data expressed as mean ± standard error of the mean except for proportions that are expressed as percentages. For the antigen-specific IgE tests, the number of patients contributing data is shown in parentheses. In one study (Martinez Arcediano et al, 2014E3), beef IgE, pork IgE, and lamb IgE were reported as Radioallergosorbent test Categories only without quantification; therefore, the lowest value in that category was assigned.
The characteristics of controls and all patients with RMA included in this study are shown in Table E2 (available in this article’s Online Repository at www.jaci-inpractice.org). Patients with RMA were slightly older and more commonly men than women compared with controls, findings that are consistent with prior epidemiologic studies of α-Gal sensitization.9 Not surprisingly, patients with RMA had markedly increased concentrations of α-Gal-, beef-, pork-, and lamb-specific IgE in serum compared with the control patients (Table E2) (all comparisons P < .001). The ethnicity distributions of the control and patients with RMA differed because of the inclusion of previously reported patients from Japan, Spain, and Sweden.
TABLE E2.
Patient characteristics
| Characteristic | Subcategory | Control (n = 188) | Red meat allergy (n = 92) | P value |
|---|---|---|---|---|
| Age, y | 49.7 ± 1.5 | 54.2 ± 2.0 | .073 | |
| Sex, % | Women | 72.3 | 42.4 | <.001 |
| Men | 27.7 | 57.6 | ||
| Ethnicity, % | USA-Caucasian | 79.3 | 10.9 | <.001 |
| USA-African American | 17.6 | 5.4 | ||
| USA-Asian | 3.2 | 0 | ||
| Japan (Chinuki et al, 2016E1) | 0 | 30.4 | ||
| Sweden (Hamsten et al, 2013E2) | 0 | 42.4 | ||
| Spain (Martinez Arcediano et al, 2014E3) | 0 | 10.9 | ||
| Antigen-specific IgE, IU/mL | α-Gal IgE | 1.38 ± 1.20 (n = 18) | 36.4 ± 35.7 (n = 83) | <.001 |
| Beef IgE | 0.43 ± 0.04 (n = 158) | 8.55 ± 1.16 (n = 85) | <.001 | |
| Pork IgE | 0.49 ± 0.14 (n = 44) | 6.60 ± 1.02 (n = 83) | <.001 | |
| Lamb IgE | 0.36 ± 0.01 (n = 33) | 1.99 ± 0.54 (n = 7) | <.001 | |
Data are expressed as mean ± standard error of the mean (SEM) for age and antigen-specific IgE and are compared by Student’s t-tests. Sex and ethnicity and expressed percentages and are compared by χ2 tests.
The allelic frequency of the B antigen varies by population; therefore, we calculated expected frequencies of the O, A, B, and AB blood types using the population distributions of the 2 groups and well-characterized ABO allelic frequencies of the represented ethnicities or populations (Table E3, available in this article’s Online Repository at www.jaci-inpractice.org). We then compared expected and observed percentages of these blood types in the 2 groups. Among the controls, the expected and observed frequencies of all ABO blood types were similar (Table I). In contrast, among those with RMA, the observed frequencies of patients expressing the B antigen (type B or AB) were significantly lower than expected (Table I).
TABLE E3.
ABO blood type distributions by ethnicity and population
| ABO type (frequency) |
|||||
|---|---|---|---|---|---|
| Population | O | A | B | AB | Reference |
| USA-C | 0.450 | 0.400 | 0.110 | 0.040 | ARC, 2017E4 |
| USA-AA | 0.520 | 0.260 | 0.190 | 0.043 | ARC, 2017E4 |
| USA-AS | 0.400 | 0.275 | 0.254 | 0.071 | ARC, 2017E4 |
| Japan | 0.293 | 0.387 | 0.222 | 0.100 | Fujita et al, 1978E6 |
| Sweden | 0.390 | 0.460 | 0.107 | 0.043 | Beckman and Martensson, 1957E7 |
| Spain | 0.450 | 0.420 | 0.090 | 0.040 | FEDS, 2016E5 |
AA, African American; ARC, American Red Cross; AS, Asian; C, Caucasian; FEDS, Federación Española de Donantes de Sangre.
Data are expressed as proportions.
TABLE I.
Expected and observed frequencies of red meat allergy (RMA) according to ABO blood type or expression of the B antigen
| Control (n = 188) |
RMA (n = 92) |
||||
|---|---|---|---|---|---|
| Blood type | Expected (%) | Observed (%) | Expected (%) | Observed (%) | P value |
| O | 46.1 | 44.1 | 39.4 | 52.2 | .212 |
| A | 37.1 | 38.3 | 40.4 | 43.5 | .860 |
| B | 12.9 | 10.6 | 14.6 | 2.28 | .026 |
| AB | 4.15 | 6.91 | 5.74 | 2.17 | .126 |
| O or A | 83.2 | 82.4 | 79.8 | 95.7 | .321 |
| B or AB | 17.0 | 17.6 | 20.3 | 4.35 | .005 |
Expected frequencies are ethnicity-weighted according to population-based ABO distributions and representation of those populations in the control and RMA groups.
P values are generated by χ2 tests.
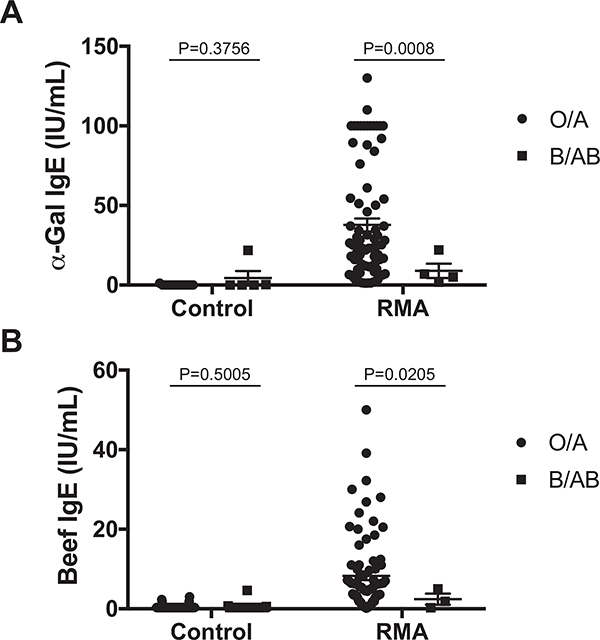
Next, we employed logistic regression and found that patients with blood type B were approximately 5 times less likely to have RMA than those with blood type O (Table II). Those with blood type AB also appeared to have a lower likelihood of RMA compared with those with blood type O, although this was not statistically significant (P = .109). In contrast, those with blood type A had a similar likelihood of RMA compared with those with blood type O. In a separate analysis, we found that individuals expressing the B antigen (blood type B or AB) were 5 times less likely than those without the B antigen (blood type O or A) to have been diagnosed with RMA (Table II). These associations were unaffected by adjustment for age and sex. Sensitivity analyses showed that removing any one RMA cohort did not affect the likelihood of RMA according to B antigen expression (Table E4, available in this article’s Online Repository at www.jaci-inpractice.org). In addition, we found that patients with the B antigen were less likely to produce α-Gal-specific IgE (odds ratio [OR] 0.19, 95% confidence interval [CI] 0.04–0.80, P = .023) or beef-specific IgE (OR 0.29, 95% CI 0.11–0.80, P = .016) compared with those without the B antigen. Finally, among patients with RMA, those expressing the B antigen appeared to produce less α-Gal or beef IgE than counterparts without the B antigen (Figure E1, available in this article’s Online Repository at www.jaci-inpractice.org).
TABLE II.
Odds ratios of red meat allergy according to ABO blood type or expression of the B antigen
| Unadjusted |
Adjusted |
|||
|---|---|---|---|---|
| Blood type | OR (95% CI) | P value | OR (95% CI) | P value |
| O | 1.00 (Ref.) | 1.00 (Ref.) | ||
| A | 0.96 (0.57–1.62) | .961 | 0.94 (0.54–1.65) | .840 |
| B | 0.17 (0.04–0.77) | .022 | 0.15 (0.06–0.70) | .016 |
| AB | 0.27 (0.06–1.23) | .090 | 0.27 (0.06–1.33) | .109 |
| O or A | 1.00 (Ref.) | 1.00 (Ref.) | ||
| B or AB | 0.21 (0.07–0.62) | .005 | 0.20 (0.07–0.61) | .004 |
CI, Confidence interval; OR, odds ratio of red meat allergy against the indicated reference group; Ref., reference group.
Bivariate logistic regression. Adjusted analyses include sex and age as covariates.
TABLE E4.
Sensitivity analyses for the effect of a cohort on the likelihood of red meat allergy
| OR for red meat allergy (95% CI) |
|||
|---|---|---|---|
| Sensitivity analysis | Blood type O/A | Blood type B/AB | P value |
| No study removed | 1.0 | 0.20 (0.07–0.61) | .004 |
| WashU cohort removed | 1.0 | 0.17 (0.05–0.61) | .006 |
| Martinez Arcediano et alE3 removed | 1.0 | 0.23 (0.08–0.69) | .009 |
| Chinuki et alE1 removed | 1.0 | 0.21 (0.06–0.74) | .015 |
| Hamsten et alE2 removed | 1.0 | 0.17 (0.04–0.76) | .020 |
CI, Confidence interval; OR, odds ratio.
Bivariate logistic regression adjusted for sex and age. Reference group, blood type O/A.
FIGURE E1.
Patients with red meat allergy (RMA) with the B antigen produce less α-Gal- and beef-specific IgE than counterparts without the B antigen. (A) Serum α-Gal-specific IgE and (B) serum beef-specific IgE concentrations were compared according to B antigen status, with separate analyses for controls and patients with RMA using unpaired Student’s t-tests assuming unequal variances (Welch’s correction). Disease status and statistical testing are described in additional detail in the Methods section of this article’s Online Repository at www.jaci-inpractice.org.
One limitation of this study is that oral food challenge was not used to definitively diagnose RMA. In addition, it is possible that patients with known ABO types differed systematically from those with unknown ABO types, potentially introducing selection bias. Furthermore, retrospective observational studies such as this one typically raise questions about the timing of an exposure relative to the outcome’s onset. However, in this study, the exposure (ABO type) is determined genetically and clearly precedes the outcome (disease).
Collectively, these findings suggest that expressing the B antigen may be protective against allergic sensitization to α-Gal and the development of RMA. Although patients who express the B antigen can undergo allergic sensitization to α-Gal and develop RMA, the likelihood of sensitization, degree of sensitization, and probability of disease appear to be markedly lower than in patients without the B antigen. To our knowledge, RMA is the first example where an ABO antigen may modulate the risk and perhaps the pathogenesis of a food allergy.
These observations have implications for laboratory testing of patients being evaluated for this disease. We suggest that patients being evaluated for RMA should have their ABO blood type determined, if not known already, early in the diagnostic evaluation. The positive predictive values and negative predictive values (ie, posttest probabilities of disease after a positive or negative result, respectively) of all laboratory tests strongly depend on the pretest probability of disease, including antigen-specific IgE tests commonly used to evaluate patients with RMA.6 The pretest probability of RMA is approximately 5 times lower for patients expressing the B antigen than for those who do not; therefore, a higher index of suspicion is necessary when using these tests to establish a diagnosis of RMA in patients with the B antigen.
ONLINE REPOSITORY
METHODS
Subjects
All test results for galactose-alpha-1,3-galactose (α-Gal)-specific IgE (Viracor-IBT, St. Louis, Mo) and beef-, pork-, and lamb-specific IgE (ThermoFisher, Phadia ImmunoCAP, Uppsala, Sweden) concentrations in serum were extracted from Cerner, a laboratory information database used at Barnes-Jewish Hospital (BJH) and St. Louis Children’s Hospital (SLCH), from January 1, 2009, until June 16, 2017. This search yielded results for 702 patients, of whom ABO blood type was also known for 203 patients who were included in subsequent analyses. At our institution, these antigen-specific IgE tests are used to rule in or rule out allergy to red meat or α-Gal in patients being evaluated for anaphylaxis, angioedema, urticaria, and/or other symptoms consistent with an allergic process. Two independent clinicians carefully reviewed the medical records of all patients with positive test results (≥0.35 IU/mL) for 1 or more of the antigen-specific IgE tests (n = 29) to identify patients who were diagnosed with α-Gal-mediated red meat allergy (RMA) (n = 15). Any discrepancies between the 2 reviewers were compared, and consensus was reached. To be classified as having RMA, the patient must have reported 1 or more episodes of anaphylaxis, angioedema, urticaria, rash, and/or gastrointestinal symptoms after red meat ingestion and have a positive α-Gal-, beef-, pork-, or lamb-specific IgE test result, or the patient must have had a documented allergy to red meat. Each of the 15 patients classified as having RMA was evaluated by an allergist at Washington University School of Medicine. The remaining 14 patients with a positive antigen-specific IgE test result and without associated symptoms consistent with RMA or a documented RMA were classified as controls. This cohort of 15 patients with RMA was merged with individual patient data from 77 previously reported patients with RMA and known ABO types.E1–E3 Subject characteristics are shown in Tables E1 and E2.
ABO typing
The patients with RMA and controls who were part of the Washington University School of Medicine cohort were ABO typed by routine forward and reverse typing. Forward and reverse ABO types had to match. Typing was performed as part of routine clinical care, and ABO types were extracted from the electronic medical record.
Expected frequencies of ABO blood types weighted by ethnicity
The ethnicities of all patients from BJH and SLCH were extracted from the electronic medical record system. The ethnicities from the 77 previously reported patients were assigned as Japanese,E1 Swedish,E2 or Spanish,E3 according to the locations of the respective studies. The frequencies of the A, O, B, and AB blood types in the United States for Caucasians, African Americans, and Asians were obtained from the American Red Cross,E4 in Spain from the Federación Española de Donantes de Sangre,E5 in Japan from a study of >56,000 patients,E6 and in Sweden from a study of >44,000 patients.E7 (Table E3). The known ABO blood type frequencies from these populations were multiplied by the relative proportions of the ethnicities in the control and RMA groups, yielding expected ABO blood type frequencies for the 2 groups weighted by ethnicity.
Statistics
Expected and observed frequencies of ABO type in the controls and patients with RMA were compared by χ2 tests. α-Gal IgE and beef IgE concentrations were compared separately in controls and patients with RMA according to the presence or absence of the B antigen using unpaired Student’s t-tests with Welch’s correction assuming unequal variance. Two-way analysis of variance was not possible because of nonindependence (ie, the disease state is partially dependent on antigen-specific IgE test results). Bivariate logistic regression was used to determine the odds ratios (OR) and 95% confidence intervals for sensitization to α-Gal IgE, sensitization to beef IgE, or diagnosis of RMA according to ABO blood type. Logistic regression analyses were performed with or without adjustment for age and sex. Sensitivity analyses were performed by removing one RMA cohort at a time from the dataset and performing bivariate logistic regression for the primary outcome (OR for RMA). Significance was set at P < .05. All analyses were performed using GraphPad Prism 7 (GraphPad Software, La Jolla, Calif) or SPSS Statistics version 24 for Mac (IBM Corporation, Armonk, NY).
Ethics
This study was reviewed and approved by the institutional review board (IRB) at BJH/SLCH/Washington University School of Medicine (IRB Protocol No. 201704012).
Clinical Implications.
Patients expressing the B antigen of the ABO blood group system are protected from allergic sensitization to galactose-α−1,3-galactose and the development of red meat allergy.
Acknowledgments
This work was supported by funds from the Department of Pathology and Immunology at the Washington University School of Medicine in St. Louis to JRB. This study was reviewed and approved by the Institutional Review Board (IRB) of the Barnes-Jewish Hospital/St. Louis Children’s Hospital/Washington University School of Medicine consortium (IRB Protocol No. 201704012). We thank Adam Bailey, MD, PhD, and Arjun Raman, MD, PhD (Washington University School of Medicine), for their helpful discussions, and we are grateful to the patients who contributed data to this study.
This work was supported by funds from the Department of Pathology and Immunology at the Washington University School of Medicine in St. Louis to JRB.
Conflicts of interest: J. R. Brestoff holds US patent 9754220 assigned to Intraspexion, Inc.; holds US patents 85958150, 0809312, and 8987245 assigned to Symmetry Therapeutics, Inc., a company that is now closed; received grant support from the National Institutes of Health (NIH); and received travel support from the Keystone Symposia. B. S. Kim receives grant support from the NIH (K08AR065577, R01AR070115), the Doris Duke Charitable Foundation Clinical Scientist Development Award, Celgene, and LEO Pharma, and serves as a consultant for Regeneron, Incyte, Sanofi, Celgene, and Concert. M. G. Scott serves on an advisory board for Becton Dickinson and Abbott; serves as a consultant for Alere, Roche, and Becton Dickinson; receives grant support from Abbott, Siemens, and Alere; and receives payment for lectures from the American Association for Clinical Chemistry and Abbott. A. M. Gronowski is a member of the scientific and medical advisory board of Theranos; serves as a consultant for Church and Dwight Co., Inc.; and receives grant support from BioRad and Abbott Laboratories. B. J. Grossman has served as a consultant for Fresenius Kabi USA and receives travel support from the National American Red Cross Medical Advisory Committee. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol 2009;123:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Platts-Mills TA, Schuyler AJ, Tripathi A, Commins SP. Anaphylaxis to the carbohydrate side chain alpha-gal. Immunol Allergy Clin North Am 2015;35: 247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamsten C, Tran TA, Starkhammar M, Brauner A, Commins SP, Platts-Mills TA, et al. Red meat allergy in Sweden: association with tick sensitization and B-negative blood groups. J Allergy Clin Immunol 2013;132:1431–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rispens T, Derksen NI, Commins SP, Platts-Mills TA, Aalberse RC. IgE production to alpha-gal is accompanied by elevated levels of specific IgG1 antibodies and low amounts of IgE to blood group B. PLoS One 2013;8:e55566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Posthumus J, James H, Wang X, Commins S, Platts-Mills TAE. Correlation of blood type with the presence of IgE antibodies to galactose-alpha-1,3-galactose (alpha-gal): is there a protective effect of blood group substance B? [Abstract 797]. J Allergy Clin Immunol 2010;125:AB203. [Google Scholar]
- 6.Brestoff JR, Zaydman MA, Scott MG, Gronowski AM. Diagnosis of red meat allergy with antigen-specific IgE tests in serum. J Allergy Clin Immunol 2017; 140:608–10. [DOI] [PubMed] [Google Scholar]
- 7.Chinuki Y, Ishiwata K, Yamaji K, Takahashi H, Morita E. Haemaphysalis longicornis tick bites are a possible cause of red meat allergy in Japan. Allergy 2016;71:421–5. [DOI] [PubMed] [Google Scholar]
- 8.Martinez Arcediano A, Audicana Berasategui MT, Longo Areso N, Ibanez EF, Balza de Vallejo OV, Velasco Azagra M, et al. Allergy to galactose-alpha-1,3-galactose: clinical features and the diagnostic value of cetuximab. J Investig Allergol Clin Immunol 2014;24:450–2. [PubMed] [Google Scholar]
- 9.Gonzalez-Quintela A, Dam Laursen AS, Vidal C, Skaaby T, Gude F, Linneberg A. IgE antibodies to alpha-gal in the general adult population: relationship with tick bites, atopy, and cat ownership. Clin Exp Allergy 2014;44: 1061–8. [DOI] [PubMed] [Google Scholar]
REFERENCES
- E1.Chinuki Y, Ishiwata K, Yamaji K, Takahashi H, Morita E. Haemaphysalis longicornis tick bites are a possible cause of red meat allergy in Japan. Allergy 2016;71:421–5. [DOI] [PubMed] [Google Scholar]
- E2.Hamsten C, Tran TA, Starkhammar M, Brauner A, Commins SP, Platts-Mills TA, et al. Red meat allergy in Sweden: association with tick sensitization and B-negative blood groups. J Allergy Clin Immunol 2013;132:1431–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Martinez Arcediano A, Audicana Berasategui MT, Longo Areso N, Ibanez EF, Balza de Vallejo OV, Velasco Azagra M, et al. Allergy to galactose-alpha-1,3-galactose: clinical features and the diagnostic value of cetuximab. J Investig Allergol Clin Immunol 2014;24:450–2. [PubMed] [Google Scholar]
- E4.The National American Red Cross. Blood Types; 2017. Available at: http://www.redcrossblood.org/learn-about-blood/blood-types.html. Accessed July 6, 2017.
- E5.Federacion Espanola de Donantes de Sangre. Datos Estadisticos Sobre la Donacion de Sangre en Espana; 2016. Available at: http://www.donantesdesangre.net/estadisticas_2017.pdf. Accessed July 6, 2017.
- E6.Fujita Y, Tanimura M, Tanaka K. The distribution of the ABO blood groups in Japan. Jinrui Idengaku Zasshi 1978;23:63–109. [DOI] [PubMed] [Google Scholar]
- E7.Beckman L, Martensson EH. Blood groups and anthropology in Dalecarlia (Sweden). Acta Genet Stat Med 1958;8:137–47. [DOI] [PubMed] [Google Scholar]