Abstract
Background and purpose
Post‐viral olfactory dysfunction is well established and has been shown to be a key symptom of COVID‐19 with more than 66% of European and US patients reporting some degree of loss of smell. Persistent olfactory dysfunction appears to be commonplace and will drive the demand for general practitioner, otolaryngology or neurology consultation in the next few months – evidence regarding recovery will be essential in counselling our patients.
Methods
This was a prospective survey‐based data collection and telemedicine follow‐up.
Results
In total, 751 patients completed the study, of whom 477 were females and 274 males. The mean age of the patients was 41 ± 13 years (range 18–60). There were 621 patients (83%) who subjectively reported a total loss of smell and 130 (17%) a partial loss. After a mean follow‐up of 47 ± 7 days (range 30–71) from the first consultation, 277 (37%) patients still reported a persistent subjective loss of smell, 107 (14%) reported partial recovery and 367 (49%) reported complete recovery. The mean duration of the olfactory dysfunction was 10 ± 6 days (range 3–31) in those patients who completely recovered and 12 ± 8 days (range 7–35) in those patients who partially recovered.
Conclusions
According to our results, at this relatively early point in the pandemic, subjective patterns of recovery of olfactory dysfunction in COVID‐19 patients are valuable for our patients, for hypothesis generation and for treatment development.
Keywords: anosmia, coronavirus, COVID‐19, olfaction, recovery, smell
Introduction
As of 10 May 2020, nearly 4 million citizens globally across 215 countries have tested positive for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) [1]. Post‐viral olfactory dysfunction (OD) is well established [2] and has been shown to be a key symptom of COVID‐19, with more than 66% European and US patients reporting some degree of loss of smell [3, 4, 5]. Apparently the worst part of the initial outbreak has been overcome. However, persistent OD appears to be commonplace and will drive the demand for general practitioner, otolaryngology or neurology consultation in the next few months. Evidence regarding recovery will be essential in counselling our patients.
Method
In order to evaluate patterns of olfactory recovery, data from patients with confirmed COVID‐19 were collected prospectively from three university hospitals. Adults (>18 years old) with a positive test for SARS‐CoV‐2 via reverse transcription polymerase chain reaction (RT‐PCR) or a positive immunoglobulin G/immunoglobulin M (IgG/IgM) were included. Those with symptom duration <14 days were tested with a nasopharyngeal swab; in the case of negative RT‐PCR or patients with symptoms for ≥14 days, serology testing was performed. Only patients with a positive RT‐PCR or with positive IgG/IgM were included (Fig. 1). All patients had at least 30 days of follow‐up after their last negative COVID‐19 test (Fig. 1).
Figure 1.
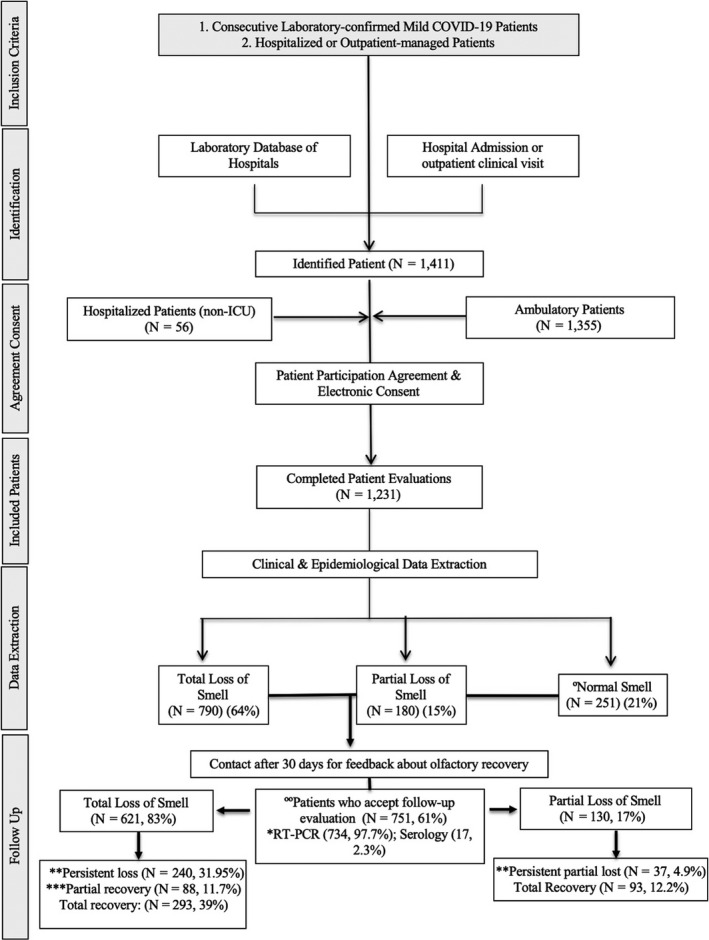
Study flowchart. Eleven patients initially considered in the group of normal smell developed an olfactory dysfunction. Four hundred and eighty patients were not included due to incomplete follow‐up data (362, 75.4%), lost to follow‐up by impossibility to contact the patient (61, 12.7%), because they refused to participate for personal reasons (48, 10%) or due to the need for intensive care unit admission (9, 1.9%). *To consider COVID‐19 negative patients were tested almost three times. **Persistent loss was considered in those patients who did not report any improvement. ***Partial recovery was considered in those patients who subjectively started to smell some odours.
Patients with pre‐existing olfactory or gustatory dysfunction, without a laboratory‐confirmed COVID‐19 infection diagnosis and those requiring intensive care at the time of the study were excluded. Information was collected using an online questionnaire created with Professional Survey Monkey (San Mateo, CA, USA). Informed consent was obtained.
Relevant epidemiological and clinical features contained within the questionnaire were collected by the COVID‐19 Study Group of the Young Otolaryngologists of the International Federation of Oto‐rhino‐laryngological Societies (YO‐IFOS) and consisted of four subsets (demographic data, medical background, ear, nose and throat symptoms, and olfactory and gustatory dysfunction). All patients completed the short version of the Questionnaire of Olfactory Disorders – Negative Statements (sQOD‐NS) [6]. The remaining olfactory and taste questions were based on the smell and taste component of the National Health and Nutrition Examination Survey [7]. Physical examination (rhinoscopy, nasal endoscopy or objective olfactory testing) was not performed in this study due to the risk of nosocomial infection.
The Statistical Package for the Social Sciences for Windows (SPSS version 21.0; IBM Corp., Armonk, NY, USA) was used to perform the statistical analyses. The potential associations between epidemiological, clinical and olfactory and gustatory outcomes were assessed through cross‐tab generation between two variables (binary or categorical variables) and the chi‐squared test. Incomplete responses were excluded from analysis. The differences in sQOD‐NS scores between patients with regard to olfactory dysfunction during the first evaluation and after almost 30 days of follow‐up were made through the Kruskal–Wallis test. A level of P < 0.05 was used to determine statistical significance. A multivariate analysis was performed to address possible confounders.
Results
All told, 1411 patients identified in the emergency room or primary care consultation were invited to participate in the study. A total of 1231 patients agreed to participate, and 751 patients completed the study (Supplementary material). The mean age of patients was 41 ± 13 years (range 18–60). There were 477 females and 274 males. The groups were comparable according to age, sex ratio, comorbidities and addiction (P = 0.273, Wilcoxon). There were 621 patients (83%) who subjectively reported a total loss of smell and 130 (17%) a partial loss. After a mean follow‐up of 47 ± 7 days (range 30–71) from the first consultation, 277 (37%) patients still reported a persistent subjective loss of smell, 107 (14%) reported partial recovery and 367 (49%) reported complete recovery. The mean duration of the OD was 10 ± 6 days (range 3–31) in those patients who completely recovered and 12 ±8 days (range 7–35) in those patients who partially recovered (Table S1 and Fig. 1).
Treatments used during the follow‐up period varied, with 71 patients (9%) using a nasal corticosteroid spray, 58 (8%) using oral steroids and 149 (20%) using nasal saline irrigation. There was no significant correlation between the use of nasal spray (P = 0.324), oral steroids (P = 0.211) or nasal irrigation (P = 0.453) and olfactory recovery. A significant difference was found about initial nasal symptoms and sQOD‐NS score, being significantly lower in patients with a total loss of smell compared with those patients with a partial loss of smell or normosmic during the first consultation and after almost 30 days of follow‐up (P = 0.001). There was no significant association between comorbidities and the development or persistence of OD (Table S2).
Discussion
Hopkins et al. [8] recently found that nearly 80% of patients experienced improvement in loss of smell within a few weeks of onset, with recovery rates appearing to plateau after 3 weeks. It was found that nearly 63% of patients reported improvement in their subjective loss of sense of smell after at least 4 weeks. However, the frequency of residual OD after 30 days of follow‐up was significant and, despite the possibility of a later recovery, it is necessary to highlight that the higher incidence of COVID‐19 patients affected allows the inference to be made that a large number of patients will suffer from long‐term OD.
Currently the mechanism for anosmia is not clear; some evidence suggests viral spread through the neuroepithelium of the olfactory cleft, with the consequent infiltration of the olfactory bulb and the central nervous system as the main cause. This theory is supported by the increasing evidence about nasal respiratory epithelial cells and olfactory epithelial support cells that may express moderate to high levels of angiotensin converting enzyme‐2 (ACE2) proteins used as a carrier by the SARS‐CoV‐2 to infect cells [9]. However, more evidence is necessary to elucidate the real mechanism for the OD.
The limitations of this study are the exclusion of patients with severe disease, the small proportion of older patients, the higher proportion of female respondents, loss to follow‐up and recruitment from ear, nose and throat clinics, potentially introducing a selection bias. Lack of objective testing to confirm anosmia is also a limitation. However, at this relatively early point in the pandemic, subjective patterns of recovery of OD in COVID‐19 patients are valuable for our patients, for hypothesis generation and for treatment development.
Disclosure of conflicts of interest
The authors have no conflicts of interest.
Ethical approval and consent to participate
Four ethics committees approved the current study protocol (HAP2020‐011; CHUSP20032020; EpiCURA‐2020‐2303; CHU‐Charleroi: B32522020).
Supporting information
Table S1. Demographic and clinical data
Table S2. Short version of the Questionnaire of Olfactory Disorders – Negative Statements of patients (initial and follow‐up evaluation)
*Joint first authors.
†Joint senior authors.
Data availability statement
Data may be available upon reasonable request and an approval from the originating university hospitals.
References
- 1. Coronavirus disease 2019 (COVID‐19) Situation Report–111. 2019. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200410‐sitrep‐81‐covid‐19.pdf?sfvrsn=ca96eb84_2 (accessed 10 May 2020)
- 2. Suzuki M, Saito K, Min WP, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope 2007; 117: 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020; 277(8): 2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID‐19 pandemic. Rhinology 2020; 58: 295–298. [DOI] [PubMed] [Google Scholar]
- 5. Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS. Self‐reported olfactory loss associates with outpatient clinical course in COVID‐19. Int Forum Allergy Rhinol 2020; 10: 821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mattos JL, Edwards C, Schlosser RJ, et al. A brief version of the questionnaire of olfactory disorders in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 2019; 9: 1144–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhattacharyya N, Kepnes LJ. Contemporary assessment of the prevalence of smell and taste problems in adults. Laryngoscope. 2015; 125: 1102–1106. [DOI] [PubMed] [Google Scholar]
- 8. Hopkins C, Surda P, Whitehead E, Kumar BN. Early recovery following new onset anosmia during the COVID‐19 pandemic – an observational cohort study. J Otolaryngol Head Neck Surg 2020; 49: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu C, Zheng S, Chen Y, Zheng M. Single‐cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019‐nCoV, in the nasal tissue. medRxiv 2020. 10.1101/2020.02.11.20022228. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographic and clinical data
Table S2. Short version of the Questionnaire of Olfactory Disorders – Negative Statements of patients (initial and follow‐up evaluation)
Data Availability Statement
Data may be available upon reasonable request and an approval from the originating university hospitals.


