Abstract
We describe a case in which a 29‐year‐old male with no medical history presented with ST‐segment elevation myocardial infarction as his presentation of coronavirus disease. During cardiac catheterization, he was found to have total occlusion of his left anterior descending artery by thrombus. Laboratory testing revealed markedly elevated inflammatory markers as well as evidence of a hypercoagulable state in the setting of severe acute respiratory syndrome coronavirus 2 infection, which was suspected to be the inciting factor for his acute coronary event.
Keywords: COVID‐19, myocardial infarction, STEMI
Abbreviations
- COVID‐19
coronavirus disease
- EKG
electrocardiogram
- LAD
left anterior descending artery
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- STEMI
ST‐segment elevation myocardial infarction
- TTE
transthoracic echocardiography
1. INTRODUCTION
Coronavirus disease (COVID‐19) has developed into a global health crisis, with over 6.1 million confirmed cases worldwide and greater than 370,000 deaths due to the disease. 1 Caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the disease frequently leads to fever and respiratory symptoms. However, patients present with varying degrees of severity of the disease, and many patients, particularly those with severe cases, have shown cardiovascular complications. 2
2. CASE DESCRIPTION
The patient is a 29‐year‐old male who presented to the emergency department with 1 day of abdominal pain, nausea, and vomiting. Following an episode of emesis, he developed chest tightness. Due to recurrent emesis and chest tightness, he sought medical attention. He denied fevers, cough, and shortness of breath. The patient had no past medical history and had no history of tobacco or illicit drug use. His occupational history was notable for employment at a healthcare institution with known COVID‐19 patients. He endorsed a family history of heart failure in his father at age 40 but did not know the etiology of his heart failure.
His presenting vital signs were normal (temperature 98.4 °F, heart rate 75 beats per minute, and oxygen saturation 100% on room air). Initial laboratory testing showed an elevated WBC count of 12,900/μl, C‐reactive protein of 149.0 mg/L, ferritin of 1,342 ng/ml, fibrinogen of 693 mg/dl, and D‐dimer of 4.18 mg/L (see Table I for relevant laboratory data). His initial troponin T was 0.15 ng/ml (normal ≤ range: <0.01 ng/ml). Initial electrocardiogram (EKG) showed normal sinus rhythm without ST‐segment deviations (Figure 1a). Chest X‐ray was normal. Reverse‐transcription polymerase chain reaction for SARS‐CoV‐2 was immediately sent and returned positive. He was initially started on hydroxychloroquine for treatment, but upon recommendations of the infectious disease team, this was stopped after two doses due to lack of evidence supporting use in patients with his clinical presentation.
TABLE I.
Laboratory values of the patient during admission
| Reference values | Patient values (initial) | Patient values (peak) | |
|---|---|---|---|
| WBC | 4.0–10.0 × 1000/μl | 12.9 | 12.9 |
| Troponin T | <0.01 ng/ml | 0.15 | 5.03 |
| B‐type natriuretic peptide, Pro | <125.0 pg/ml | 362 | 2016 |
| d‐dimer | ≤0.50 mg/L FEU | 4.18 | 7.60 |
| Fibrinogen | 209–444 mg/dl | 693 | 832 |
| C‐reactive protein | <3.0 mg/L | 149.0 | 178.9 |
| Ferritin | 30–400 ng/ml | 1,342 | 1,413 |
| Cholesterol | <200 mg/dl | 195 | N/A |
| HDL cholesterol | ≥40 mg/dl | 49 | N/A |
| LDL cholesterol | <100 mg/dl | 132 | N/A |
| Triglycerides | <150 mg/dl | 70 | N/A |
| Lipoprotein (a) | <75 nmol/L | 74 | N/A |
| Hemoglobin A1c | 4.0–5.6% | 5.4 | N/A |
Abbreviations: HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; WBC, white blood cell.
FIGURE 1.
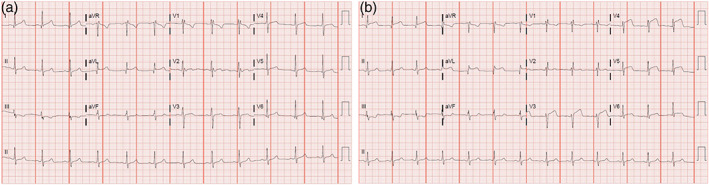
Presenting and follow‐up EKGs. (a) Initial EKG on day of admission showing normal sinus rhythm with no ST‐segment elevations or depressions. (b) Subsequent EKG on hospital day 2 showing anterolateral ST‐segment elevations consistent with acute myocardial infarction in the left anterior descending artery territory. EKG, electrocardiogram [Color figure can be viewed at wileyonlinelibrary.com]
Serial EKGs and troponins were obtained. Fourteen hours after admission, the patient developed severe chest pain and a repeat EKG (Figure 1b) showed the development of acute ST‐segment elevations in the anterolateral leads.
The patient was taken for emergent cardiac catheterization. Loading doses of aspirin, prasugrel, and intravenous heparin were administered. Coronary angiography revealed total occlusion of the mid left anterior descending artery (LAD) (Figure 2a). Upon wiring of the LAD lesion and predilation with a compliant balloon, thrombolysis in myocardial infarction (TIMI) 2 flow was restored and significant thrombus was visualized at the index lesion (Figure 2b,c). Aspiration thrombectomy was performed with retrieval of a small amount of white thrombus. Intravascular ultrasound was performed, which showed significant thrombus burden as well as nonobstructive atherosclerotic plaque in the mid‐LAD (Figure 3). Additional tirofiban load and drip was administered. The index lesion was then treated with a 3.5×18 mm drug‐eluting stent and postdilated with a 4.0 × 12 mm noncompliant balloon. A final angiogram showed excellent result at the index lesion with TIMI 3 flow but with distal embolization at the apical LAD (Figure 2d).
FIGURE 2.
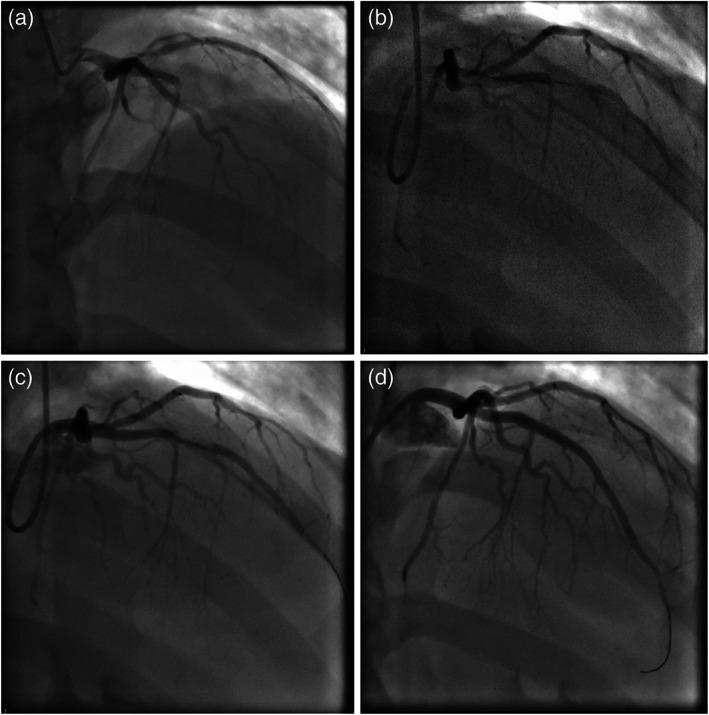
Coronary angiogram. (a) Coronary angiography showing total occlusion of the left anterior descending artery. (b) Partial antegrade flow through the left anterior descending artery (LAD) with wiring of the lesion. (c) Improved flow through the LAD after predilation with a balloon; thrombus is visualized in the LAD. (d) Final results after a drug‐eluting stent was deployed and postdilated in the LAD. LAD, left anterior descending artery
FIGURE 3.
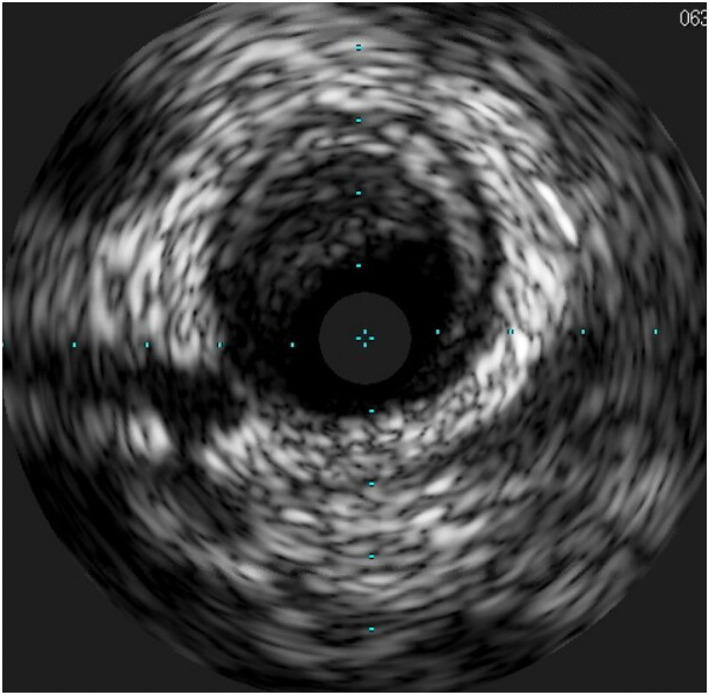
Intravascular ultrasound of the LAD. Intravascular ultrasound of the mid‐LAD showing significant thrombus burden remaining even after predilation with a balloon and aspiration thrombectomy; there is also nonobstructive atherosclerotic plaque noted. LAD, left anterior descending artery [Color figure can be viewed at wileyonlinelibrary.com]
After completion of his cardiac catheterization, the patient was chest pain free and was managed on dual antiplatelet therapy with aspirin 81 mg daily and prasugrel 10 mg daily. Systemic anticoagulation was continued for 18 hr with intravenous heparin and tirofiban. Hematology was consulted and he was then started on therapeutic dose enoxaparin.
The patient's symptoms resolved and he was ultimately discharged from the hospital on Day 5 of admission. A transthoracic echocardiogram (Figure 4) performed after revascularization showed left ventricular ejection fraction of 35%, with akinesis of the entire apex, mid anterior, mid inferoseptal, and mid anteroseptal segments. He remains on dual‐antiplatelet therapy, as well as full‐dose anticoagulation with enoxaparin 1 mg/kg BID, with plans to follow up with cardiology and hematology prior to deciding upon the duration of anticoagulation.
FIGURE 4.
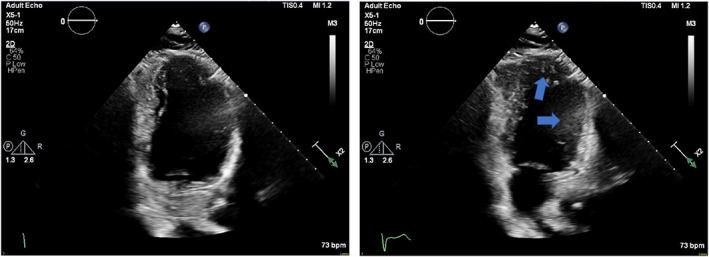
Transthoracic echocardiogram. Apical two chamber views in diastole (left) and systole (right) demonstrating hypokinesis of the anterior wall and apex (arrows) [Color figure can be viewed at wileyonlinelibrary.com]
3. DISCUSSION
Cardiovascular complications have been seen in many cases of COVID‐19, and frequently patients show evidence of myocardial injury. Elevated cardiac enzymes are associated with more severe cases of COVID‐19 and a higher mortality rate. The mechanism of cardiac injury is thought to be multifactorial. In the setting of respiratory infection associated with severe hypoxia and acute respiratory distress syndrome, supply–demand mismatch would be expected to lead to ischemia and myocardial damage. 3
However, there are also several cases in which the pattern of cardiac injury suggests viral myocarditis or stress cardiomyopathy. One case report from China described a man who developed left ventricular dysfunction (ejection fraction = 27%) and elevated troponin T to >10 ng/ml). His EKG was remarkable for inferior ST‐segment elevations but coronary angiography found no coronary obstruction. Following treatment with steroids and intravenous immunoglobulin, his left ventricular ejection fraction normalized and cardiac biomarkers decreased. 4
COVID‐19 also appears to be associated with a marked systemic inflammatory response particularly in severe cases. C‐reactive protein, erythrocyte sedimentation rate, and ferritin are elevated, and those with more fulminant cases of the disease have significantly higher elevations of these markers compared to those with milder cases. 5 In severe COVID‐19, cytokine storm with a profile suggestive of secondary hemophagocytic lymphohistiocytosis has also been observed, with elevated increased interleukin (IL)‐2, IL‐7, granulocyte colony‐stimulating factor, interferon‐γ inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1‐α, and tumor necrosis factor‐α. 6 Cardiac injury from this inflammatory response, as well as from a subsequent hypercoagulability, has been proposed as a mechanism of the cardiovascular complications that can been seen in COVID‐19. A number of hemostatic abnormalities have been described with COVID‐19, including increased d‐dimer levels, prolongation of prothrombin time and activated partial thromboplastin time, and increased levels of fibrin degradation products. 7 Microvascular injury and thrombosis were described in a case series in which skin and lung tissues from five patients with severe COVID‐19 were examined. Inflammatory capillary injury with complement deposition was noted in both lung tissue and in skin lesions. Complement‐mediated endothelial injury and fibrin deposition and subsequent thrombosis were observed. 8 A separate case series also describes endothelial inflammation across the vascular beds of multiple organs, including the lungs, heart, kidneys, and intestines, of COVID‐19 patients. A predominantly lymphocytic endotheliitis is described in this series. 9 The endothelial dysfunction observed in many cases can be expected to be the precipitant of a hypercoagulable state.
The overall picture is one of hypercoagulability and a predisposition to thrombotic events, perhaps due to both direct effect of the virus itself and the systemic inflammatory response that SAR‐CoV‐2 elicits. In this particular case, although the patient had underlying plaque in his LAD, he was noted to have markedly elevated inflammatory markers and d‐dimer, suggesting that the prothrombotic and inflammatory state related to COVID‐19 was the precipitant of the thrombotic occlusion of his LAD.
4. CONCLUSIONS
Cardiovascular complications of COVID‐19 remain incompletely described, and it is still unclear how to approach patients with evidence of myocardial injury due to COVID‐19. Although there appear to be a number of ways in which SARS‐CoV‐2 may affect the cardiovascular system, suspicion for acute coronary syndrome must remain high especially in those patients who have markers suggestive of a systemic inflammatory and hypercoagulable response. It remains unclear what the role of systemic anticoagulation should play in the treatment of COVID‐19 patients who may be at higher risk of thrombosis, coronary, and otherwise, and further investigation is needed.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
We would like to thank Eric Brandt, MD, and Yanting Wang, MD, for their participation in the care of this patient and for their contribution to the manuscript.
Ong E, Castro‐Dominguez Y, Brennan J, Oen‐Hsiao J. COVID‐19 complicated by ST‐segment elevation myocardial infarction in a 29‐year‐old patient. Catheter Cardiovasc Interv. 2021;97:267–271. 10.1002/ccd.29102
REFERENCES
- 1. WHO . Coronavirus disease 2019 (COVID‐19) situation report—134. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200602‐covid‐19‐sitrep‐134.pdf?sfvrsn=cc95e5d5_2. Accessed June 2, 2020.
- 2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thygesen K, Alpert JS, Jaffe AS, et al. The executive group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) task force for the universal definition of myocardial infarction. Fourth universal definition of myocardial infarction. Circulation. 2018;138:e618‐e651. [DOI] [PubMed] [Google Scholar]
- 4. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020;ehaa190. 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res. 2020;220:1‐13. 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417‐1418. 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


