In order to quantify the levels of SARS‐CoV‐2‐specific IgM, IgA, and IgG antibodies, identify changes in them based on COVID‐19 severity, and establish the significance of combined antibody detection, a chemiluminescence method was used to detect the levels of SARS‐CoV‐2‐specific antibodies in COVID‐19 patients with different severity. We concluded that detection of SARS‐CoV‐2‐specific combined IgA–IgG antibodies is advantageous in diagnosing COVID‐19 and more attention should be paid to specific IgA.
![]()
Keywords: antibody, COVID‐19, diagnosis, SARS‐CoV‐2, severity
Summary
The diagnosis of coronavirus 19 (COVID‐19) relies mainly upon viral nucleic acid detection, but false negatives can lead to missed diagnosis and misdiagnosis; severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐specific antibody detection is convenient, safe and highly sensitive. Immunoglobulin (Ig)M and IgG are commonly used to serologically diagnose COVID‐19; however, the role of IgA is not well known. We aimed to quantify the levels of SARS‐CoV‐2‐specific IgM, IgA and IgG antibodies, identify changes in them based on COVID‐19 severity, and establish the significance of combined antibody detection. COVID‐19 patients, divided into a severe and critical group and a moderate group, and non‐COVID‐19 patients with respiratory disease were included in this study. A chemiluminescence method was used to detect the levels of SARS‐CoV‐2‐specific IgM, IgA and IgG in the blood samples from the three groups. Epidemiological characteristics, symptoms, blood test results and other data were recorded for all patients. Compared to the traditional IgM–IgG combined antibodies, IgA–IgG combined antibodies are more effective for diagnosing COVID‐19. During the disease process, IgA appeared first and disappeared last. All three antibodies had significantly higher levels in COVID‐19 patients than in non‐COVID‐19 patients. IgA and IgG were also higher for severe and critical disease than for moderate disease. All antibodies were at or near low levels at the time of tracheal extubation in critical patients. Detection of SARS‐CoV‐2‐specific combined IgA–IgG antibodies is advantageous in diagnosing COVID‐19. IgA detection is suitable during early and late stages of the disease. IgA and IgG levels correspond to disease severity.
Introduction
A novel coronavirus pneumonia outbreak commenced in Wuhan, China, in late December 2019 [1] and spread rapidly throughout the country and overseas. In February 2020, the World Health Organization named the virus severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and the disease coronavirus 19 (COVID‐19). Thus far, more than 3·3 million people have been infected and 230 000 have died worldwide; these numbers are expected to rise further. The diagnosis of COVID‐19 depends upon the detection of the viral nucleic acid; however, the detection of SARS‐CoV‐2 nucleic acid has a high false negative rate, making the disease easy to misdiagnose. Reverse transcription–polymerase chain reaction (RT–PCR) was used to detect SARS‐CoV‐2 in 4880 patients with COVID‐19 in a hospital in Wuhan. While fewer than 50% of nasopharynx swabs and sputum were positive, bronchoalveolar lavage fluid had a 100% positivity rate [2]. The acquisition of alveolar lavage fluid, however, is invasive and not suitable for most patients with mild disease. Current COVID‐19 diagnosis guidelines suggest the combined use of nucleic acid detection and clinical symptomology [3]. However, the large number of asymptomatic people positive for SARS‐CoV‐2 make this method flawed. Thus, it is necessary to increase the detection of the SARS‐CoV‐2‐specific antibody.
Detection of the SARS‐CoV‐2‐specific antibody is convenient, safe and highly sensitive. It has certain advantages in the auxiliary diagnosis of COVID‐19. The moderately used SARS‐CoV‐2‐specific antibodies include immunoglobulin (Ig)M, which signifies the primary immune response and indicates a recent infection, and IgG, which is the main antibody produced by the secondary immune response. IgA is often ignored in the diagnosis of COVID‐19. Guo et al. [4] found that IgA and IgM appear simultaneously, which is important for diagnosing patients with acute or asymptomatic infection. The specific IgA antibody, therefore, should be considered in the diagnosis of COVID‐19. The diagnostic efficacy of specific IgA antibody and the levels of these specific antibodies depending on disease severity are currently unclear.
The rise times for specific IgM and IgG levels are different, and combined detection could be more advantageous in the diagnosis of COVID‐19 [5]. Large‐scale detection of SARS‐CoV‐2‐specific IgM and IgG has been carried out nationwide, but combined evaluation is rare. The benefit of combining the detection of specific IgA with that of combined specific IgM–IgG is still uncertain. In this study, SARS‐CoV‐2‐specific IgM, IgA, and IgG levels were measured in patients with varying severities of COVID‐19, the relationship between specific antibody levels and disease severity was classified and the significance of combined antibody detection was clarified, providing a reference for the clinical diagnosis of COVID‐19.
Materials and methods
Patient and sample collection
All COVID‐19 patients tested positive for the SARS‐CoV‐2 viral nucleic acid. The clinical classification of COVID‐19 was determined according to the Guidelines of the Diagnosis and Treatment of New Coronavirus Pneumonia (version 7) published by the National Health Commission of China [6]. Moderate disease was characterized by fever, respiratory and other symptoms and the manifestation of viral pneumonia on computed tomography (CT) imaging. Severe disease met at least one of the additional following conditions: (1) shortness of breath with respiratory rate ≥ 30 times/min, (2) oxygen saturation at rest ≤ 93% and (3) oxygenation index ≤ 300 mmHg. The critical classification met at least one of the additional following conditions: (1) respiratory failure requiring mechanical ventilation, (2) shock and (3) other organ failure possibly requiring admission to the intensive care unit. Between February and April 2020, 19 patients with severe and critical COVID‐19 from the First Affiliated Hospital of Guangzhou Medical University were included into this study. Twenty‐four patients with moderate COVID‐19 were included from the Yangjiang and Qingyuan People’s Hospital in the Guangdong Province. Meanwhile, 61 non‐COVID‐19 patients with respiratory diseases were included as controls from the respiratory clinic of the First Affiliated Hospital of Guangzhou Medical University. Epidemiological characteristics, symptoms, blood test results and other data were collected from all patients. The study was approved by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University (Medical Research Ethics no. 44, 2020). Written informed consent was waived in light of this emerging infectious disease of high clinical relevance. All healthy control subjects signed written informed consent prior to the collection of peripheral blood.
Antibody against SARS‐CoV‐2 measurement
Forty‐nine days after symptom onset, 298 serum samples from 43 COVID‐19 patients were collected. At the first visit in Respiratory Clinic of the 61 non‐COVID‐19 patients, 61 serum samples were collected. All serum samples were inactivated in a water bath at 56ºC for 30 min. The Kaeser 6600 automatic chemiluminescence immunoanalyzer and matching reagents kit (Guangzhou Kangrun Biotech Co. Ltd, Guangzhou, China) was used to detect the SARS‐CoV‐2‐specific IgM, IgA and IgG levels using a two‐step indirect detection method. A sequence‐encoding receptor binding region of spike protein (S protein) was cloned into pTT5 vector, and the constructed expression vector was used to transiently transfect HEK293F cells. The carboxyl group on the magnetic beads is activated by an activator, and the amino group on the S protein is then coupled with the carboxyl group of the magnetic beads to form an amide bond, and the antigen is fixed on the magnetic beads. Anti‐human IgA, IgM and IgG antibodies were coupled with acridine ester derivatives. The specific antibody in the testing sample was combined with a magnetic bead coating (S protein recombinant antigen) to form a magnetic bead coating–specific antibody complex. After the unbound substances were separated and washed with magnetic beads, the acarithrate marker was added to form the magnetic bead coating material–SARS‐CoV‐2‐specific antibody–acarithrate labeling complex. A photomultiplier was used to detect light signals from acridine ester that were converted to obtain the corresponding signal value. The relative light signal values, expressed in relative light units (RLU), indicated IgM, IgA and IgG levels. The relative light signal value is equivalent to the original signal value over the specific antibody cut‐off value. The cut‐off values of IgM, IgA and IgG are 11 300, 56 492 and 42 213, respectively. A relative luminescence value (RLV) greater than or equal to 1·0 is positive for specific IgM, IgA and IgG.
Statistical analysis
Moderately distributed continuous data are represented as means and standard deviations, while non‐moderately distributed data are indicated by medians and interquartile ranges (IQR). The χ2 test or Fisher’s exact probability test was used to compare qualitative data. The Mann–Whitney U‐test was used for independent‐sample comparison between two groups of non‐parametric data. The Kruskal–Wallis H test was used for comparison between multiple groups. For statistical purposes, we grouped severe and critical patients. P < 0·05 was considered statistically significant. spss version 23.0 (IBM Corporation, Armonk, NY, USA) and Graphpad Prism version 8.0.1 (©1995–2020; GraphPad Software, LLC, San Diego, CA, USA) were used for data analyses.
Result
Patient characteristics
Among the severe and critical COVID‐19 patients, nine were severe and 10 were critical. Of all included patients, 28 were male and 15 were female. The average ages of the patients with severe and critical disease and moderate disease were 50 and 60 years, respectively. There was no statistically significant difference between the ages of the two groups. The severe and critical group had significantly more exposure history in Wuhan than the moderate group (P < 0·05). COVID‐19 patients had significantly more symptoms of fever, wheezing and fatigue than non‐COVID‐19 patients (P < 0·01). The leukocyte count of the severe and critical and non‐COVID‐19 groups were significantly higher than that of the moderate group (P < 0·01). The lymphocyte and basophil count of the severe and critical group were significantly lower than those of the non‐COVID‐19 group (P < 0·01). The chest computerized tomographies (CTs) of COVID‐19 patients all revealed suspected viral pneumonia. Interestingly, 10 non‐COVID‐19 patients reported the same CT findings. Throughout the entire disease course, specific IgM, IgA and IgG were detected in almost all patients with COVID‐19. In addition, 10 non‐COVID‐19 patients had positive IgM and two had positive IgA (Table 1).
Table 1.
Comparison of patient characteristics between groups
| Severe and critical COVID‐19 | Moderate COVID‐19 | Non‐COVID‐19 | P‐value | |
|---|---|---|---|---|
| Number of patients | 19 (severe: 9, critical: 10) | 24 | 61 | – |
| Demographic information | ||||
| Male/female | 15/4 | 13/11 | 42/19 | P = 0·210** |
| Age, median (IQR) | 50 (27, 58) | 60 (50, 67) | 47 (32, 64) | P = 0·069** |
| Epidemiology | ||||
| Wuhan exposure history | 13/19 | 9/24 | 0/61 | P < 0·05*, ** |
| History of confirmed case exposure | 7/19 | 10/24 | 5/61 | P = 0·748*, ** |
| Symptoms at the onset, n/total | ||||
| Fever | 17/19a | 17/24a | 24/61b | P < 0·01*, ** |
| Cough | 14/19 | 13/24 | 32/61 | P = 0·254*, ** |
| Wheezing | 13/19a | 13/24a | 16/61b | P < 0·01*, ** |
| Fatigue | 12/19a | 7/24a | 5/61b | P < 0·01*, ** |
| Blood cell analysis, median (IQR) | ||||
| Leukocyte | 8.71 (7.42, 9.70)a | 4.73 (3.87, 5.28)b | 7.75 (6.41, 10.24)a | P < 0·01** |
| Lymphocyte | 1.01 (0.77, 1.23)a | 1.40 (1.14, 1.53)ab | 1.52 (1.16, 2.11)b | P < 0·01** |
| Hemoglobin | 0.18 (0.07, 0.26) | 0.09 (0.08, 0.10) | 0.10 (0.05, 0.20) | P = 0·355** |
| Eosinophil | 0.02 (0.01, 0.04) | 0 (0, 0.01) | 0.02 (0, 0.05) | P = 0·261** |
| Basophil | 91.80 (87.41, 111.82)a | 117.43 (101.60, 120.89)ab | 132.08 (113.20, 149.25)b | P < 0·01** |
| Other laboratory tests, n/total | ||||
| Suspected viral pneumonia by CT | 19/19 | 24/24 | 10/61 | – |
| Antibody‐positive rate at peak time, n/total | ||||
| IgM antibody against SARS‐CoV‐2 | 19/19 | 24/24 | 10/61 | – |
| IgG antibody against SARS‐CoV‐2 | 19/19 | 22/24 | 0/61 | – |
| IgA antibody against SARS‐CoV‐2 | 19/19 | 24/24 | 2/61 | – |
P‐value significant between the three groups.
P‐value significant between severe and critical coronavirus 19 (COVID‐19) and moderate COVID‐19 groups. If the same letter (a versus a or ab) is included between two groups, no significant difference was found. IQR = interquartile range; Ig = immunoglobulin; CT = computerized tomography; SARS‐CoV‐2 = severe acute respiratory syndrome coronavirus 2.
Diagnostic roles of combined antibodies
A scatter diagram of pairwise combinations of IgA, IgG and IgM is shown in Fig. 1. The four quadrants can be divided by the minimum positive relative luminescence value of the antibodies in the diagram. The serum numbers of each quadrant are shown in Table 2. Of the patients with COVID‐19, 98·66% (100% of the severe and critical group and 95·06% of the moderate group) were correctly diagnosed with antibodies; however, 16·39% (10 of 61) of non‐COVID‐19 patients were misdiagnosed by the IgA–IgM combined antibodies (Fig. 1a). One hundred per cent of the patients with severe and critical and 96·3% of the patients with moderate COVID‐19 were correctly diagnosed by IgA–IgG combined antibodies; however, 3·28% (2 of 61) of non‐COVID‐19 patients were IgA‐positive and IgG‐negative (Fig. 1b). Of the patients with COVID‐19, 97·99% (100% of the severe and critical patients and 92·59% of moderate patients) were correctly diagnosed by IgM–IgG combined antibodies, but 16·39% (10 of 61) of non‐COVID‐19 patients were misdiagnosed (Fig. 1c). IgA–IgG combined antibodies play a better role than the traditional IgM–IgG combined antibodies in the diagnosis of COVID‐19.
Fig. 1.
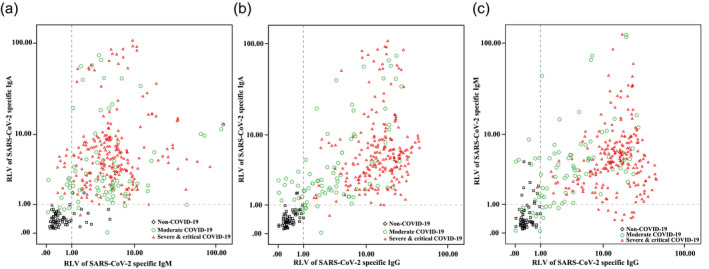
Scatter diagram of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐specific immunoglobulin (Ig)A, IgG and IgM antibodies. Both minimum positive relative luminescence values are 1·0; 218 serum samples from 19 severe and critical coronavirus 19 (COVID‐19) patients, 81 serum samples from 24 moderate COVID‐19 patients and 61 serum samples from non‐COVID‐19 patients were used.
Table 2.
Positive/negative rates and consistency in pairwise combinations of IgA, IgG and IgM
| IgA versus IgM | IgA versus IgG | IgM versus IgG | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Severe and critical | Moderate | Non‐COVID‐19 | Severe and critical | Moderate | Non‐COVID‐19 | Severe and critical | Moderate | Non‐COVID‐19 | |
| A (Po‐Po) | 207 | 66 | 2 | 216 | 62 | 0 | 207 | 62 | 0 |
| B (Po‐Ne) | 10 | 7 | 0 | 1 | 11 | 2 | 10 | 5 | 0 |
| C (Ne‐Po) | 1 | 4 | 8 | 1 | 5 | 0 | 1 | 8 | 10 |
| D (Ne‐Ne) | 0 | 4 | 51 | 0 | 3 | 59 | 0 | 6 | 51 |
| Consistency | 94·95% | 86·42% | 86·89% | 99·08% | 80·25% | 96·72% | 94·95% | 83·95% | 83·61% |
| Positive rate*, ** | 100·00% | 95·06% | – | 100·00% | 96·30% | – | 100·00% | 92·59% | – |
| Negative rate* | – | 83·61% | – | – | 96·72% | – | – | 83·61% | |
The numbers of both or any one antibody‐positive/total numbers.
The numbers of both of the two antibody‐negative/total numbers. Po = positive; Ne = negative; Ig = immunoglobulin; COVID‐19 (coronavirus 19).
Dynamic changes of IgA, IgG and IgM antibody levels in severe and critical and moderate COVID‐19
We tracked the changes in IgA, IgG and IgM antibodies in 19 severe and critical COVID‐19 patients for 7 weeks and in 24 moderate COVID‐19 patients for 5 weeks. Positive IgA levels (RLV = 3·71) were detected in almost all severe and critical COVID‐19 patients by the second week after symptom onset (10–14 days). The peak value (RLV = 6·88) was reached in the third week (15–21 days), and then gradually decreased throughout the observation period (> 49 days). After the IgA levels began to decrease there was no rebound in the IgA curve. During the first week (1–7 days), positive IgA levels (median RLV = 1·42) were detected in two‐thirds of the moderate COVID‐19 patients; the peak point (RLV = 4·83) was reached during the third week (Fig. 2a). Very high IgG levels were detected in the severe and critical COVID‐19 patients during the second week after symptom onset and gradually decreased by the fourth week (22–28 days). The IgG levels, however, increased again after 36–42 days. For moderate COVID‐19 patients, IgG levels (median RLV = 0·97) were below the reference value during the first week and peaked during the third week (Fig. 2b). Positive IgM levels were detected in severe and critical COVID‐19 patients during the first week and in moderate COVID‐19 patients during the second week. The peak point was reached during the fourth week before gradually decreasing (Fig. 2c). Higher levels of all three antibodies were observed in the severe and critical patients than in the moderate patients. The difference of IgA, IgG and IgM antibody levels of severe and critical patients and moderate patients in each period was compared. The results showed that the IgG level of severe and critical patients was significantly higher than that of moderate patients during the 2–5‐week period and the IgM level of severe and critical patients was higher than that of moderate patients during the second week (P < 0·05).
Fig. 2.
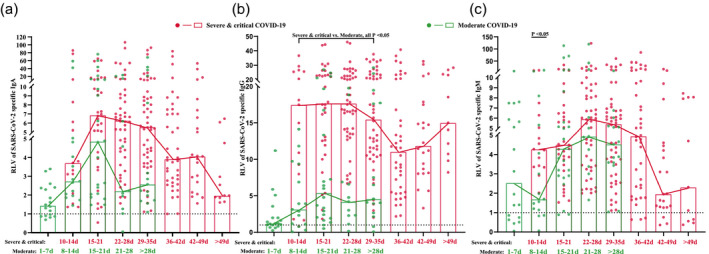
Dynamic changes of immunoglobulin (Ig)A, IgG and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Solid dots represent each patient in each time period, while rectangles represent the median values in the corresponding periods. Lines show the changing trends with onset of time. Green markers indicate moderate coronavirus 19 (COVID‐19) patients, while red markers indicate severe and critical COVID‐19 patients.
In addition, we analyzed the positive rate of the antibodies in all COVID‐19 patients at each time‐period. In severe and critical COVID‐19 patients, IgA positivity reached 100% during the second week and continued until the seventh week, IgG positivity reached 100% during the third week and continued until the seventh week and IgM reached 100% positivity during the second week and continued until weeks 4 and 5. In moderate COVID‐19 patients, IgA positivity reached 100% during the second week and remained after the fifth week, IgG positivity did not reach 100% during the observation period and IgM levels reached 100% during the fourth week and remained after the fourth and fifth weeks.
Distribution difference of IgA, IgG and IgM antibodies between COVID‐19 and non‐COVID‐19 patients
We compared the distribution of IgA, IgG and IgM antibodies between COVID‐19 and non‐COVID‐19 patients (Fig. 3). The levels of the three antibodies were significantly higher in COVID‐19 patients than in non‐COVID‐19 patients (P < 0·01). In addition, the level of IgA [5·27 (3·14, 8·74)] in severe and critical COVID‐19 patients was higher than that in moderate COVID‐19 patients [2·37 (1·70, 6·13)]. The level of IgG [15·49 (10·13, 22·68)] in severe and critical COVID‐19 patients was higher than that [3·50 (1·19, 7·17)] in moderate COVID‐19 patients. All differences were statistically significant (P < 0·01). There was no statistically significant difference in the level of IgM, however, between the severe and critical and moderate groups (Table 3).
Fig. 3.
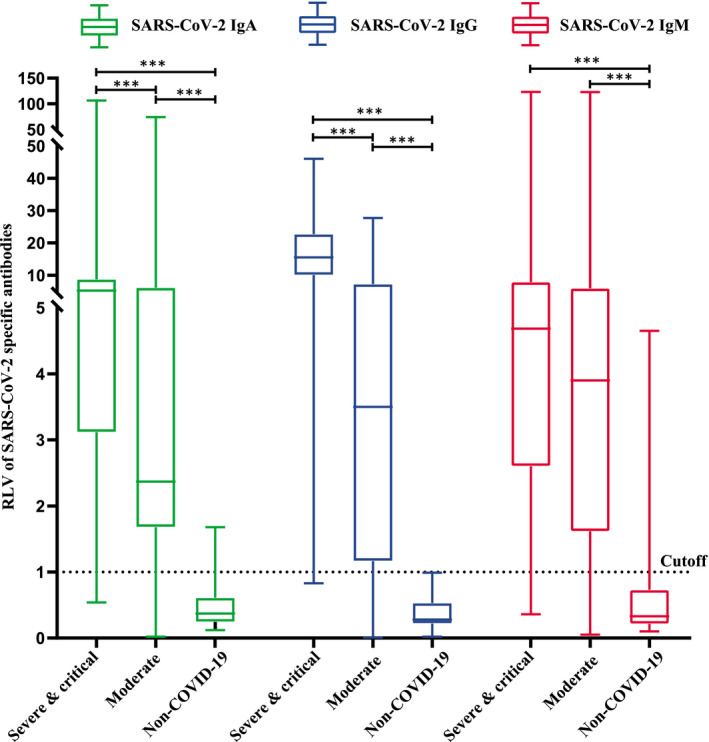
Distribution difference of immunoglobulin (Ig)A, IgG and IgM between coronavirus 19 (COVID‐19) and non‐COVID‐19 groups.
Table 3.
Comparison of positive rate in COVID‐19 patients at different time‐periods
| SARS‐CoV‐2 IgA | SARS‐CoV‐2 IgG | SARS‐CoV‐2 IgM | ||||
|---|---|---|---|---|---|---|
| Severe and critical | Moderate | Severe and critical | Moderate | Severe and critical | Moderate | |
| 1–7d | – | 66·67% | – | 66·67% | – | 66·67% |
| 10–14 days/8–14 days** | 100·00% | 100·00% | 93·33% | 80·00% | 100·00% | 80·00% |
| 15–21 days | 96·88% | 100·00% | 100·00% | 90·91% | 100·00% | 90·91% |
| 22–28 days | 100·00% | 81·82% | 100·00% | 90·91% | 100·00% | 100·00% |
| 29–35 days/> 28 days* | 100·00% | 100·00% | 100·00% | 86·67% | 100·00% | 100·00% |
| 36–42 days | 100·00% | – | 100·00% | – | 92·50% | – |
| 42–49 days | 100·00% | – | 100·00% | – | 86·36% | – |
| > 49 days | 100·00% | – | 100·00% | – | 63·64% | – |
10–14 days = time‐period of severe and critical coronavirus 19 (COVID‐19), 8–14 days = time‐period of moderate COVID‐19;
29–35 days = time period of severe and critical COVID‐19; > 28 days = time‐period of moderate COVID‐19. The positive rate is the ratio of the numbers of relative luminescence value (RLV) ≥ 1 to the total numbers in the corresponding time‐period. Ig = immunoglobulin; SARS‐CoV‐2 = severe acute respiratory syndrome coronavirus 2.
Levels of IgA, IgG and IgM at the time of symptom relief in critical COVID‐19 patients
Ten critical COVID‐19 patients were included into our study. Using data collection, six of the patients had been extubated. Tracheal extubation was used as a marker of symptom improvement in critical COVID‐19 patients. We found that the six extubated patients had undergone endotracheal tube removal on an average of 30 days after symptom onset. IgA, IgG and IgM were at or near low levels at the time of tracheal extubation (Fig. 4).
Fig. 4.
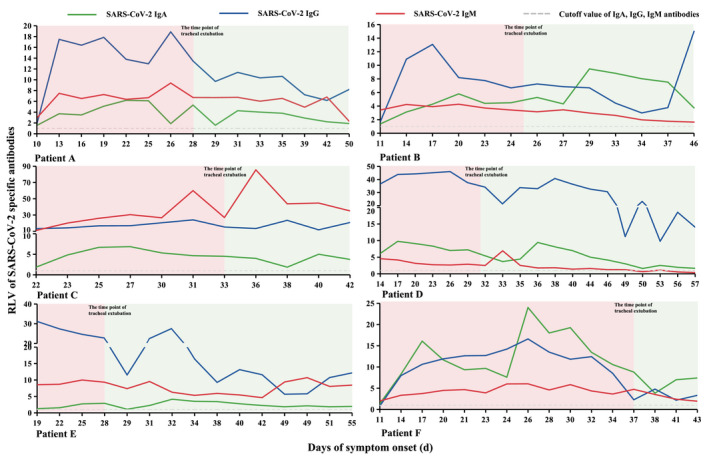
Tracheal extubation and antibody levels in critical coronavirus 19 (COVID‐19) patients. The curve of each patient was divided into two parts based on the time of tracheal extubation.
Discussion
The COVID‐19 outbreak has caused a great loss of life throughout the world. The gold standard of diagnosis, detection of SARS‐CoV‐2 nucleic acid by RT–PCR, is prone to missed diagnoses due to its false negative rate. To some extent, the detection of the SARS‐CoV‐2‐specific antibody can make up for a deficiency in nucleic acid detection. Studies [7] have shown that in suspected COVID‐19 cases with negative nucleic acid, the specific antibody is an effective supplementary indicator of SARS‐CoV‐2 infection and can be combined with nucleic acid testing to confirm infection. Xie et al. also believe that the combination of nucleic acid and the IgM–IgG antibody test is the optimal method for diagnosing SARS‐CoV‐2 infection [8]. This provides a more convenient and fast method for the diagnosis and avoidance of missed diagnoses of COVID‐19.
In our study, the included patients were divided into three groups: severe and critical COVID‐19, moderate COVID‐19 and non‐COVID‐19 with respiratory diseases. Compared with moderate COVID‐19 patients, severe and critical COVID‐19 patients were more likely to have a history of Wuhan exposure, consistent with the clinical characteristics of COVID‐19 reported in the Hunan and Anhui provinces [9, 10]. COVID‐19 patients were more likely than non‐COVID‐19 patients to develop fever, wheezing, and fatigue, the same COVID‐19 symptoms initially reported in Wuhan [11] and now established as the typical symptoms. In patients with viral infection, blood work often showed normal or decreased leukocytes or lymphopenia. In patients with COVID‐19, normal or reduced leukocytes and inhibited lymphocytes were significant laboratorial characteristics [12]. Our study found that severe and critical patients and non‐COVID‐19 patients had higher leukocyte counts than moderate COVID‐19 patients. This was reflective of the results from Zhongnan Hospital of Wuhan University [13]. This may be due to the higher proportion of bacterial infections in these groups than in the moderate group. Chen et al. [14] found that severe cases more frequently had lymphopenia. We also found that the lymphocyte count of the severe and critical COVID‐19 patients was lower than that of the moderate COVID‐19 and non‐COVID‐19 patients, consistent with the results by Chen et al. A descriptive study by Tan et al. [15] suggested that lymphopenia could predict the severity of COVID‐19 and that the lower the lymphocyte count the more severe the disease, which was consistent with our conclusion. Currently, the literature rarely focuses on basophil counts in COVID‐19 patients. In an analysis of the immune response in COVID‐19 patients, Qin et al. [16] pointed out that severe cases tended to have lower basophil percentages. We found that basophil levels were lower in severe and critical COVID‐19 patients, confirming that basophil reduction may be an important characteristic of critical disease.
Imaging is important for COVID‐19 diagnosis. Under the premise of lagging diagnosis caused by falsely negative nucleic acid, lung CT images provided an important reference for a clinical diagnosis [17]. During the surge of infected cases in Wuhan, nucleic acid detection was replaced by lung CT. Among the included patients, lung CT of 10 of the 61 non‐COVID‐19 patients revealed viral pneumonia, but these patients were eventually excluded by nucleic acid and antibody testing. Similarly, 10 of the 61 non‐COVID‐19 patients had positive IgM and two had positive IgA. These patients were finally excluded by multiple detections of nucleic acid and antibodies.
IgM and IgG have a reciprocal relationship; therefore, the simultaneous detection of IgM and IgG antibodies is more suitable for COVID‐19 patients with an unclear infection stage. In a study evaluating the sensitivity and specificity of IgG and IgM combined antibodies [18], the combined antibodies showed 88·66% sensitivity and 90·63% specificity in the diagnosis of COVID‐19. This was superior to IgM or IgG testing alone. We found that IgA–IgG combined detection, however, is more effective than the traditional IgM–IgG combined detection, preventing missed diagnosis and misdiagnosis to a greater extent.
In all included COVID‐19 patients, IgA increased during the first week after symptom onset, peaked during the third week and gradually decreased thereafter. IgM levels in severe and critical COVID‐19 patients remained high during the second week, and in moderate COVID‐19 patients it remained high during the first week after symptom onset and peaked during the fourth week before slowly decreasing. IgG levels in severe and critical COVID‐19 patients remained high during the second week, peaked during the fourth week, then began to decline, and increased again during the sixth week. In moderate COVID‐19 patients, IgG levels increased during the first week and peaked during the third week. Guo et al. [4] found that after SARS‐CoV‐2 infection in humans, specific antibodies are produced in 1–5 days, specific IgM and IgA are detected in 3–6 days, and IgM levels rise to the highest level in 8–14 days after symptoms appear. The IgA level continues to rise for 0–14 days following symptom onset and thereafter ceases to increase. The IgG can be detected 14 days after the onset of symptoms, rises during days 8–21, stabilizes after 21 days and remains present in the later stages of infection. Andrea et al. [19] found that the IgA response appears early, peaks at week 3 and it is stronger and more persistent than the IgM response, which was similar to our results.
In our study, however, the peak IgM, IgA and IgG levels were detected 1 week after they were detected in the study by Guo et al. [4], but in the study by Hou et al [20] IgM levels increased during the first week, peaked during 2 weeks and then reduced, IgG was detected during 1 week and was maintained at a high level for a long period. The difference of time in antibody appearance and reaching the peak may need more research for confirmation. After an observation period of 49 days, we found that all severe and critical COVID‐19 patients had high IgA and IgG levels, while only some severe and critical patients had high IgM levels. Twenty‐eight days after symptom onset, all moderate COVID‐19 patients had high IgA and IgM levels, while only some moderate COVID‐19 patients maintained a high IgG level. Thus, we speculated that the course of COVID‐19 is at least 4 weeks or longer. Therefore, determination of changes in SARS‐CoV‐2‐specific antibodies throughout the disease process may require a larger sample size and longer monitoring time.
Currently, IgM and IgG antibody detection is widely used in clinical practice but IgA, a mucosal immune antibody, has not received attention for the diagnosis of COVID‐19. In a study of antibody detection in patients infected with SARS‐CoV [21], IgM and IgG sensitivity were 60·4% and IgA specificity was 96·6%. While this does not show an obvious advantage, patients infected with the influenza virus can produce a protective‐type secretory IgA in asymptomatic or mild infection [22]. In the detection of hepatitis B, IgA has proved more sensitive than IgM and its level is associated with the severity of liver disease [23]. In the study by Guo et al., specific SARS‐CoV‐2 IgA was detected in 92·7% of the samples in the early stages of COVID‐19, while IgM and IgG were detected in only 85·4 and 77·9%, respectively. This indicates that IgA may play an important role in the early diagnosis of SARS‐CoV‐2 infection, which is significantly different from IgA in the SARS‐CoV infection in 2003 [21]. Bene et al. also called for the monitoring of SARS‐CoV‐2‐specific IgA levels and emphasized the role of IgA in COVID‐19 [24]. In our study, IgA was 100% positive by the second week after symptom onset, regardless of the COVID‐19 severity, and was higher than the positive rates of IgG and IgM. This indicates that IgA is more suitable than IgM or IgG in the early diagnosis of SARS‐CoV‐2 infection and may be important for screening latent or asymptomatic SARS‐CoV‐2 infections. IgA and IgG in all severe and critical COVID‐19 patients remained 100% positive 49 days after symptom onset, while IgA positivity was higher than IgG positivity in patients with moderate COVID‐19 28 days after symptom onset. This indicates that IgA is also useful in the later stages of COVID‐19.
We also found that while IgA and IgG levels were significantly higher in the severe and critical patients than in moderate patients, there was no difference in IgM between the two groups. These results suggest that while IgA and IgG could reflect disease severity, IgM did not have the same quality. In order to further observe the relationship between SARS‐CoV‐2‐specific antibodies and COVID‐19 severity, we considered tracheal extubation to be an index of improvement in critical COVID‐19 patients. We found that the specific antibodies were at or near low levels at the time of tracheal extubation, indicating that dynamic monitoring of SARS‐CoV‐specific antibodies may help to determine the optimal time for extubation and help to guide the treatment of critical patients. More cases must be studied and other factors that may contribute to bias should be ruled out before accurate conclusions can be drawn.
There are some limitations to our study. First, only a small number of cases were included with limited clinical data in moderate COVID‐19 and non‐COVID‐19 patients. Secondly, almost all severe and critical COVID‐19 patients were transferred from other hospitals; therefore, antibody levels during the first week after symptom onset could not be monitored. As we were also unable to monitor the antibody levels of patients after discharge, we did not fully understand the dynamic trend of antibody levels 4 and 7 weeks after symptom onset in moderate and severe and critical COVID‐19 patients, respectively.
Conclusions
In conclusion, compared to the traditional detection of IgM–IgG combined antibodies, the detection of SARS‐CoV‐2‐specific IgA–IgG combined antibodies is more advantageous in the diagnosis of COVID‐19. SARS‐CoV‐2‐specific IgA detection is even more suitable than IgM detection in the early stages of COVID‐19 and has important reference value in the later stages of the disease. Levels of IgA and IgG were higher in severe and critical COVID‐19 patients than in moderate COVID‐19 patients, while IgM levels were no different between the two groups. This suggests that IgA and IgG levels are associated COVID‐19 severity; therefore, in the serological diagnosis of COVID‐19 using SARS‐CoV‐2‐specific IgM and IgG, we suggest that more attention should be paid to specific IgA levels.
Disclosures
None of the authors have any conflicts of interest to declare.
Acknowledgements
This study received Zhejiang University special scientific research funding for COVID‐19 prevention and control (2020XGZX001, 2020XGZX025). We thank all the patients we included, and Qingyuan and Yangjiang People’s Hospital for providing us with blood samples and data of COVID‐19 patients.
Contributor Information
B. Sun, Email: sunbaoqing@vip.163.com.
N. Zhong, Email: nanshan@vip.163.com.
References
- 1. Zhu N, Zhang D, Wang W et al A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu R, Han H, Liu F et al Positive rate of RT–PCR detection of SARS‐CoV‐2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta 2020; 505:172–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang Y, Kang H, Liu X, Tong Z. Combination of RT–qPCR testing and clinical features for diagnosis of COVID‐19 facilitates management of SARS‐CoV‐2 outbreak. J Med Virol 2020; 92:538–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo L, Ren L, Yang S et al Profiling early humoral response to diagnose novel coronavirus disease (COVID‐19). Clin Infect Dis 2020. 10.1093/cid/ciaa310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao J, Yuan Q, Wang H et al Antibody responses to SARS‐CoV‐2 in patients of novel coronavirus disease 2019. Clin Infect Dis 2020. 10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Health Commission of China . Guidelines for the Diagnosis and Treatment of Novel Coronavirus (2019‐nCoV) Infection by the National Health Commission (Trial Version 7). Beijing, China: National Health Commission of China, 2020. http://www.gov.cn/zhengce/zhengceku/2020‐03/04/content_5486705.htm [Google Scholar]
- 7. Yong G, Yi Y, Tuantuan L et al Evaluation of the auxiliary diagnostic value of antibody assays for the detection of novel coronavirus (SARS‐CoV‐2). J Med Virol 2020. 10.1002/jmv.25919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie J, Ding C, Li J et al Characteristics of patients with coronavirus disease (COVID‐19) confirmed using an IgM‐IgG antibody test. J Med Virol 2020. 10.1002/jmv.25930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zheng F, Tang W, Li H, Huang YX, Xie YL, Zhou ZG. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID‐19) in Changsha. Eur Rev Med Pharmacol Sci 2020; 24:3404–10. [DOI] [PubMed] [Google Scholar]
- 10. Zhu W, Xie K, Lu H et al Initial clinical features of suspected coronavirus disease 2019 in two emergency departments outside of Hubei, China. J Med Virol 2020. 10.1002/jmv.25763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang C, Wang Y, Li X et al Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu YH, Dong JH, An WM et al Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS‐CoV‐2. J Infect 2020; 80:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang G, Hu C, Luo L et al Clinical features and short‐term outcomes of 221 patients with COVID‐19 in Wuhan, China. J Clin Virol 2020; 127:104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen G, Wu D, Guo W et al Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan L, Wang Q, Zhang D et al Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther 2020; 5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qin C, Zhou L, Hu Z et al Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis 2020. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tenda ED, Yulianti M, Asaf MM et al The importance of chest CT scan in COVID‐19. Acta Med Indones 2020; 52:68–73. [PubMed] [Google Scholar]
- 18. Li Z, Yi Y, Luo X et al Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J Med Virol 2020. 10.1002/jmv.25727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Padoan A, Sciacovelli L, Basso D et al IgA‐Ab response to spike glycoprotein of SARS‐CoV‐2 in patients with COVID‐19: a longitudinal study. Clin Chim Acta 2020; 507:164–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hou H, Wang T, Zhang B et al Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. Clin Translat Immunol 2020; 9:e01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woo PC, Lau SK, Wong BH et al Detection of specific antibodies to severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein for serodiagnosis of SARS coronavirus pneumonia. J Clin Microbiol 2004; 42:2306–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Renegar KB, Small PJ, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol 2004; 173:1978–86. [DOI] [PubMed] [Google Scholar]
- 23. Lin S, Sun Q, Mao W, Chen Y. Serum immunoglobulin A (IgA) level is a potential biomarker indicating cirrhosis during chronic hepatitis B infection. Gastroenterol Res Pract 2016; 2016:2495073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bene MC, de Carvalho M, Eveillard M, Lebri Y. Good IgA bad IgG in SARS‐CoV‐2 infection? Clin Infect Dis 2020. 10.1093/cid/ciaa426 [DOI] [PMC free article] [PubMed] [Google Scholar]


