Abstract
Background
COVID‐19 pandemic has affected healthcare systems worldwide. Resources are being shifted and potentially jeopardize safety of non‐COVID‐19 patients with comorbidities. Our aim was to investigate the impact of national lockdown and SARS‐CoV‐2 pandemic on percutaneous treatment of coronary artery disease in Poland.
Methods
Data on patients who underwent percutaneous coronary procedures (angiography and/or percutaneous coronary intervention [PCI]) were extracted for March 13–May 13, 2020 from a national PCI database (ORPKI Registry) during the first month of national lockdown and compared with analogous time period in 2019.
Results
Of 163 cardiac catheterization centers in Poland, 15 (9.2%) were indefinitely or temporarily closed down due to SARS‐CoV‐2 pandemic. There were nine physicians (9 of 544; 1.7%) who were infected with SARS‐CoV‐2. There were 13,750 interventional cardiology procedures performed in Poland in the analyzed time period. In 66% of cases an acute coronary syndrome was diagnosed, and in the remaining 34% it was an elective procedure for the chronic coronary syndrome in comparison to 50% in 2019 (p < .001). There were 362 patients (2.6% of all) with COVID‐19 confirmed/suspected who were treated in interventional cardiology centers and 145 with ST‐Elevation Myocardial Infarction (STEMI) diagnosis (6% of all STEMIs).
Conclusions
Due to SARS‐CoV‐2 pandemic there was an absolute reduction in the number of interventional procedures both acute and elective in comparison to 2019 and a significant shift into acute procedures. COVID‐19 confirmed/suspected patients do not differ in terms of procedural and baseline characteristics and reveal similar outcomes when treated with percutaneous coronary interventions.
Keywords: acute coronary syndrome, COVID‐19, interventional cardiology
1. INTRODUCTION
Poland has had a highly efficient and widely accessible system of percutaneous treatment of coronary artery disease (CAD) with evenly distributed interventional cardiology facilities around the country and a high number of annual percutaneous coronary intervention (PCI) and primary percutaneous coronary intervention (pPCI) procedures per million inhabitants. 1 , 2 , 3 The distribution of cardiac catheterization centers has for many years met the requirements of ESC (European Society of Cardiology) guidelines with one cath lab for <300,000 of inhabitants. 4 The development of the hospital network system for the interventional treatment of myocardial infarction has taken over two decades to complete. 5 However, with national lockdown being implemented on March 13, 2020 following the SARS‐CoV‐2 pandemic and shifting resources to the treatment of only acute cases, the principal assumptions of the existing hospital network for the percutaneous treatment of CAD in Poland has been jeopardized. Moreover, by administrative decisions, entire hospitals regardless of their primary specialization are being now transformed into dedicated centers for infectious diseases, in order to accommodate COVID‐19 patients. This also applies to the cardiology departments.
Our aim was to investigate based on the nationwide registry the impact of national lockdown and SARS‐CoV‐2 pandemic on the percutaneous treatment of CAD in Poland, as well as to provide a characteristic of COVID‐19 positive or suspected patients treated in interventional cardiology centers and their immediate procedural outcomes.
2. METHODS
The ORPKI Registry (Polish National PCI Registry) in Poland gathers data on all percutaneous diagnostic and therapeutic procedures since 2004. A detailed description of the registry and annual reports has been published previously. 1 , 6 There are currently 163 catheterization laboratories in Poland reporting data for the ORPKI registry online everyday with 544 board‐certified PCI operators.
For this analysis, data on all percutaneous procedures (angiography or PCI) were extracted for the two‐month period (March 13–May 13, 2020). Data were gathered from the date of nationwide lockdown was administered (closed schools, nurseries, and universities, only essential workers allowed to commute to work, travel restrictions with the closing of national country borders) due to SARS‐CoV2 pandemic. The recommendations for the unified proceedings with COVID‐19 positive or suspected cases requiring percutaneous diagnostic and treatment have been issued by the Polish Association of Cardiovascular Interventions on March 19, 2020. 7 The ACC‐SCAI recommendations were also endorsed. 8 Patients with COVID‐19 suspicion (according to tho the triage recommendations of the National Institute of the Public Health and Ministry of Health) were treated as potential COVID‐19 positive. The working or established diagnosis of COVID‐19 was always available prior to any interventional procedure (angiography, PCI) and recorded in the web‐based ORPKI database. Swabs for molecular RT‐PCR testing were obtained always before any procedure. Analysis of COVID‐19 positive or suspected patients versus non‐COVID patients was performed. Additionally, a comparison of contemporary two‐month data versus analogous period for 2019 procedures (March 13–May 13, 2019) was carried out. Only peri‐procedural outcomes and complications were recorded in the database.
All patients provided informed consent. The study complied with the ethical principles for clinical research based on the Declaration of Helsinki with later amendments. No external funding was used to support this analysis.
3. STATISTICAL ANALYSIS
Continuous variables are presented as mean with SD or median with the first and the third quartile where applicable. Categorical variables are presented as numbers and percentages. Normality was assessed by Shapiro–Wilk test. Equality of variances was assessed using the Levene's test. Differences between two groups were compared using the Student's or the Welch's t test depending on the equality of variances for normally distributed variables. The Mann–Whitney U test was used for non‐normally distributed continuous variables. Categorical variables were compared by the Pearson's chi‐squared test or Fisher's exact test if at least 20% of cells in the contingency table had an expected count of less than 5 (Monte Carlo simulation for the Fisher test was used tables of dimensions higher than 2×2).
All of the baseline/demographic characteristics were included in the logistic regression model used in propensity score (PS) matching: age, weight, gender, history of stroke, history of MI, history of PCI, history of CABG, smoking status, presence of diabetes, presence of psoriasis, presence of hypertension, presence of any kidney disease, presence of COPD (chronic obstructive pulmonary disease) and indication for the procedure. Each COVID‐19 positive/suspected case was matched with 10 COVID‐19 negative cases, which showed the best balance between analyzed groups. PS matching was performed using the nearest neighbor algorithm. The groups were considered balanced if standardized differences for each of the analyzed baseline/demographic characteristics were lower than 10%.
Comparison of the total amount of contrast used during the procedure, total radiation dose during procedure, and odds for death between groups after PS matching was performed using a generalized linear mixed‐effects model to account for matching.
A similar sub‐analysis was performed only among patients with STEMI (ST‐Elevation Myocardial Infarction) indication with additional time from pain to first medical contact (FMC), time from pain to the balloon inflation or angiogram (in case of no pPCI was performed), and time from the FMC to the balloon inflation or angiogram after PS matching.
All confidence intervals are 95%, and all p‐values shown are two‐sided.
Statistical analysis was performed using the JMP version 14.3.0 (SAS Institute Inc., Cary, NC, 2019) and R, version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria, 2019) with the following packages: “MatchIt”, version 3.0.2, “lme4”, version 1.1–21 and “stddiff”, version 3.0.
Analysis was based on all cases introduced into the database within specific timeframe. Due to small number of missing data PS, matching was performed on completed cases only.
4. RESULTS
Of 163 cardiac catheterization centers in Poland, 12 (7.4%) were indefinitely closed down due to SARS‐CoV‐2 pandemic, and an additional 3 (1.8%) temporarily suspended (from 1 to 20 days) their operation. There were nine physicians (9 of 544; 1.7%) during a 2‐month period who had confirmed SARS‐CoV‐2 infection after performing coronary procedures in COVID‐19 positive/suspected patients.
There were 13,750 interventional cardiology procedures performed in Poland between March 13 and May 13, 2020, reported into the national ORPKI Registry. In 9093 (66%) cases, an acute coronary syndrome (ACS) was the primary diagnosis, and in the remaining 4,657 (34%) cases, it was an elective procedure for the chronic coronary syndrome. A comparison to the analogous period for the year 2019 is shown in Figure 1. Mean times (in minutes) from pain to FMC, from pain to inflation/angiography and from FMC to inflation/angiography were: 217 (95% CI 207–227) versus 228 (95% CI 215–242) with p = .364; 312 (95% CI 302–323) versus 313 (95% CI 299–326) with p = .4639 and 127 (95% CI 121–132) versus 114 (95% CI 107–120) with p = .0142 for STEMI patients treated with pPCI in 2019 versus 2020, respectively.
FIGURE 1.
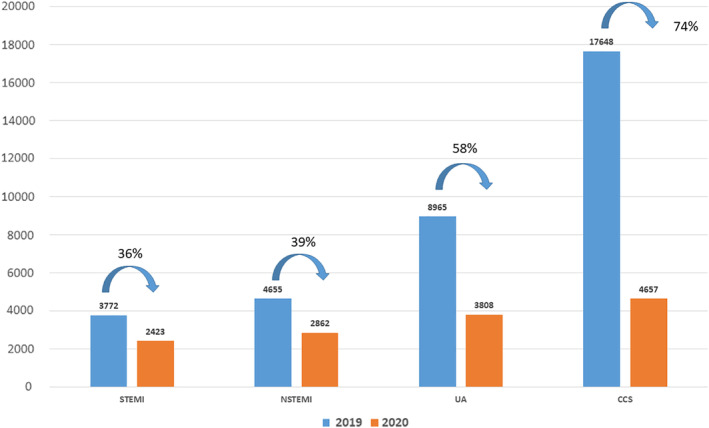
Change in absolute number of patients admitted for interventional cardiology procedures in two‐month time periods in 2019 and 2020 in Poland (p < .001) [Color figure can be viewed at wileyonlinelibrary.com]
Of all patients who underwent invasive diagnostic or treatment during 2‐month period, 362 (2.6%) were COVID‐19 (suspected or confirmed by an approved RT‐PCR test). Baseline characteristic and procedural details and outcomes of COVID‐19 positive/suspected versus non‐COVID‐19 patients are shown in Table 1 with PS matching adjustment on outcomes in Table 2.
TABLE 1.
Comparison of COVID‐19 versus non‐COVID‐19 patients who underwent percutaneous coronary diagnostic and treatment in Poland
| Variable | Measure/levels | Non‐COVID‐19 | COVID‐19 | p |
|---|---|---|---|---|
| n | 13,388 | 362 | ||
| Age, years | Mean (±SD) | 66.93 (±10.88) | 64.84 (±12.48) | .0003* |
| Gender | n | 13,316 | 358 | .3807 |
| Female | 4,423 (33.22%) | 111 (31.01%) | ||
| Male | 8,893 (66.78%) | 247 (68.99%) | ||
| Diabetes mellitus | n | 13,404 | 362 | |
| 1 | 2,974 (22.19%) | 65 (17.96%) | .0623 | |
| Previous stroke | n | 13,404 | 362 | |
| 1 | 428 (3.19%) | 15 (4.14%) | .2903 | |
| Previous MI | n | 13,404 | 362 | |
| 1 | 3,989 (29.76%) | 68 (18.78%) | .0000* | |
| Previous PCI | n | 13,404 | 362 | |
| 1 | 4,707 (35.12%) | 69 (19.06%) | .0000* | |
| Previous CABG | n | 13,404 | 362 | |
| 1 | 663 (4.95%) | 16 (4.42%) | .8051 | |
| Smoking | n | 13,404 | 362 | |
| 1 | 2,901 (21.64%) | 100 (27.62%) | .0081* | |
| Hypertension | n | 13,404 | 362 | |
| 1 | 9,232 (68.87%) | 184 (50.83%) | .0000* | |
| Chronic kidney disease | n | 13,404 | 362 | |
| 1 | 873 (6.51%) | 23 (6.35%) | 1.0000 | |
| COPD | n | 13,404 | 362 | |
| 1 | 492 (3.67%) | 18 (4.97%) | .2021 | |
| Access site during angiogram | n | 11,354 | 345 | .0000* |
| Femoral | 1,299 (11.44%) | 70 (20.29%) | ||
| Radial | 9,933 (87.48%) | 269 (77.97%) | ||
| Other | 122 (1.07%) | 6 (1.74%) | ||
| Killip class on admission | n | 9,346 | 290 | .0000* |
| 1 | 8,409 (89.97%) | 225 (77.59%) | ||
| 2 | 582 (6.23%) | 32 (11.03%) | ||
| 3 | 189 (2.02%) | 17 (5.86%) | ||
| 4 | 166 (1.78%) | 16 (5.52%) | ||
| Angiography result | n | 11,354 | 345 | .0000* |
| No critical lesions | 2,484 (21.88%) | 41 (11.88%) | ||
| No atherosclerosis | 903 (7.95%) | 19 (5.51%) | ||
| Single‐vessel disease | 3,221 (28.37%) | 105 (30.43%) | ||
| Multivessel disease | 3,809 (33.55%) | 143 (41.45%) | ||
| Multivessel disease with LMCA | 905 (7.97%) | 33 (9.57%) | ||
| LMCA only | 32 (0.28%) | 4 (1.16%) | ||
| Decision based on angiography | n | 4,835 | 81 | .0187* |
| Further assessment | 58 (1.20%) | 4 (4.94%) | ||
| CABG | 333 (6.89%) | 2 (2.47%) | ||
| PCI ad hoc | 39 (0.81%) | 0 (0.00%) | ||
| Elective PCI | 113 (2.34%) | 0 (0.00%) | ||
| Heart team consultation | 539 (11.15%) | 8 (9.88%) | ||
| Conservative treatment | 3,753 (77.62%) | 67 (82.72%) | ||
| FFR during PCI | n | 8,518 | 279 | |
| 1 | 190 (2.23%) | 1 (0.36%) | .0330* | |
| IVUS during PCI | n | 8,518 | 279 | |
| 1 | 264 (3.10%) | 0 (0.00%) | .0004* | |
| OCT during PCI | n | 8,518 | 279 | |
| 1 | 9 (0.11%) | 1 (0.36%) | .2756 | |
| Total amount of contrast used during procedure, ccm | n | 12,836 | 354 | .0000* |
| Mean (±SD) | 126.93 (±76.56) | 145.55 (±77.14) | ||
| Mean 95% CI | (125.60; 128.25) | (137.48; 153.61) | ||
|
Total radiation dose during procedure, mGy |
n | 12,835 | 353 | .0002* |
| Mean (±SD) | 597.21 (±634.18) | 670.11 (±588.90) | ||
| Mean 95% CI | (586.24; 608.18) | (608.46; 731.75) | ||
| Stent type | n | 8,518 | 279 | .6094 |
| BMS | 7 (0.08%) | 0 (0.00%) | ||
| BVS | 7 (0.08%) | 0 (0.00%) | ||
| DES | 7,037 (82.61%) | 239 (85.66%) | ||
| DES, BMS | 2 (0.02%) | 0 (0.00%) | ||
| No stent used | 1,465 (17.20%) | 40 (14.34%) | ||
| Death during procedure | n | 13,404 | 362 | |
| 1 | 56 (0.42%) | 6 (1.66%) | .0056* | |
| MI during PCI | n | 8,518 | 279 | |
| 1 | 7 (0.08%) | 0 (0.00%) | 1.0000 | |
| No‐reflow during PCI | n | 8,518 | 279 | |
| 1 | 69 (0.81%) | 2 (0.72%) | 1.0000 | |
| Bleeding at the puncture site during PCI | n | 8,518 | 279 | |
| 1 | 12 (0.14%) | 1 (0.36%) | .3425 | |
| Coronary artery perforation during PCI | n | 8,518 | 279 | |
| 1 | 18 (0.21%) | 0 (0.00%) | 1.0000 |
p<.05 was considered meaningful.
TABLE 2.
Comparison of COVID‐19 versus non‐COVID‐19 patient's outcome after propensity score adjustment
| Variable | Measure/levels | Non‐COVID‐19 | COVID‐19 | p |
|---|---|---|---|---|
| Contrast volume ccm | n | 3,470 | 347 | .1282 |
| Mean (±SD) | 139.74 (±76.01) | 145.69 (±77.65) | ||
| Mean 95% CI | (137.21; 142.27) | (137.49; 153.89) | ||
| Radiation in Gy | n | 3,470 | 347 | .1321 |
| Mean (±SD) | 650.14 (±656.96) | 668.17 (±584.83) | ||
| Mean 95% CI | (628.27; 672.00) | (606.42; 729.92) | ||
| Death during procedure/PCI | n | 3,470 | 347 | .1862 |
| Yes | 32 (0.92%) | 6 (1.73%) |
There were 2,421 patients treated with pPCI for STEMI during the 2‐month timeframe, of which 145 (6%) were COVID‐19 (suspected or confirmed by an approved RT‐PCR test). Baseline characteristic and procedural details and outcomes of COVID‐19 positive/suspected versus non‐COVID‐19 patients with PS matching adjustment on outcomes are shown in Tables 3 and 4.
TABLE 3.
Comparison of COVID‐19 versus non‐COVID‐19 STEMI patients who underwent percutaneous coronary diagnostic and treatment in Poland
| Variable | Measure/levels | Non‐COVID‐19 | COVID‐19 | p |
|---|---|---|---|---|
| n | 2,276 | 145 | ||
| Age, years | Mean (±SD) | 65.43 (±12.23) | 63.19 (±12.55) | .0332* |
| Gender | n | 2,260 | 143 | .3570 |
| Female | 731 (32.35%) | 41 (28.67%) | ||
| Male | 1,529 (67.65%) | 102 (71.33%) | ||
| Diabetes mellitus | n | 2,278 | 145 | .4496 |
| 1 | 384 (16.86%) | 21 (14.48%) | ||
| Previous stroke | n | 2,278 | 145 | .8516 |
| 1 | 69 (3.03%) | 4 (2.76%) | ||
| Previous MI | n | 2,278 | 145 | .2449 |
| 1 | 363 (15.94%) | 18 (12.41%) | ||
| Previous PCI | n | 2,278 | 145 | .1550 |
| 1 | 400 (17.56%) | 19 (13.10%) | ||
| Previous CABG | n | 2,278 | 145 | .0844 |
| 1 | 24 (1.05%) | 0 (0.00%) | ||
| Smoking | n | 2,278 | 145 | .1265 |
| 1 | 708 (31.08%) | 54 (37.24%) | ||
| Hypertension | n | 2,278 | 145 | .0078 |
| 1 | 1,311 (57.55%) | 67 (46.21%) | ||
| Chronic kidney disease | n | 2,278 | 145 | .5952 |
| 1 | 75 (3.29%) | 6 (4.14%) | ||
| COPD | n | 2,278 | 145 | .2986 |
| 1 | 61 (2.68%) | 2 (1.38%) | ||
| Killip class | n | 1,756 | 124 | .2357 |
| 1 | 1,446 (82.35%) | 98 (79.03%) | ||
| 2 | 190 (10.82%) | 12 (9.68%) | ||
| 3 | 42 (2.39%) | 7 (5.65%) | ||
| 4 | 78 (4.44%) | 7 (5.65%) | ||
| Arterial access site | N | 2,055 | 143 | .0388* |
| Other | 15 (0.73%) | 2 (1.40%) | ||
| Radial left | 241 (11.73%) | 12 (8.39%) | ||
| Radial right | 1,501 (73.04%) | 96 (67.13%) | ||
| Femoral | 298 (14.50%) | 33 (23.08%) | ||
| Angiography finding | n | 2,055 | 143 | .4308 |
| No critical lesions | 58 (2.82%) | 4 (2.80%) | ||
| No atherosclerosis | 21 (1.02%) | 2 (1.40%) | ||
| Single vessel disease | 940 (45.74%) | 60 (41.96%) | ||
| Multivessel disease | 914 (44.48%) | 63 (44.06%) | ||
| Multivessel disease with LMCA | 116 (5.64%) | 12 (8.39%) | ||
| LMCA only | 6 (0.29%) | 2 (1.40%) | ||
| Time from pain to FMC, min | n | 1,618 | 105 | .8922 |
| Mean (±SD) | 226.77 (±282.07) | 249.13 (±306.70) | ||
| Mean 95% CI | (213.02; 240.53) | (189.78; 308.49) | ||
| Time from pain to inflation or angiogram, min | n | 1,560 | 101 | .1338 |
| Mean (±SD) | 309.90 (±279.09) | 354.33 (±313.35) | ||
| Mean 95% CI | (296.04; 323.76) | (292.47; 416.19) | ||
| Time from FMC to inflation or angiogram, min | n | 1,710 | 123 | .0323* |
| Mean (±SD) | 111.57 (±133.46) | 141.08 (±179.11) | ||
| Mean 95% CI | (105.24; 117.90) | (109.11; 173.05) | ||
| Death during procedure | n | 2,278 | 145 | .0441* |
| 1 | 26 (1.14%) | 5 (3.45%) | .0345* | |
| No‐reflow during PCI | n | 2,160 | 136 | .3522 |
| 1 | 36 (1.67%) | 1 (0.74%) | .7223 | |
| Bleeding at the puncture site during PCI | n | 2,160 | 136 | .6211 |
| 1 | 2 (0.09%) | 0 (0.00%) | 1.0000 |
p<.05 was considered meaningful.
TABLE 4.
Comparison of COVID‐19 versus non‐COVID‐19 STEMI patient's outcome after propensity score adjustment
| Variable | Measure/levels | Non‐COVID‐19 | COVID‐19 | p |
|---|---|---|---|---|
| Time from pain to FMC, min | n | 970 | 97 | .9723 |
| Mean (±SD) | 201.69 (±237.99) | 224.38 (±283.09) | ||
| Mean 95% CI | (186.70; 216.69) | (167.33; 281.44) | ||
| Time from pain to inflation or angiogram, min | n | 970 | 97 | .1018 |
| Mean (±SD) | 301.27 (±268.40) | 346.98 (±305.24) | ||
| Mean 95% CI | (284.36; 318.18) | (285.46; 408.50) | ||
| Time from first contact to inflation or angiogram, min | n | 970 | 97 | .0282 |
| Mean (±SD) | 99.57 (±105.40) | 122.60 (±120.90) | ||
| Mean 95% CI | (92.93; 106.22) | (98.23; 146.96) | ||
| Death during procedure | n | 970 | 97 | .9259 |
| Yes | 11 (1.13%) | 1 (1.03%) |
5. DISCUSSION
Best to our knowledge, this is one of the first detailed national analysis on the impact of SARS‐CoV‐2 pandemic on interventional cardiology procedures as well as the first presentation of baseline characteristics and immediate outcome of over 300 COVID‐19 confirmed/suspected patients. Some studies and preliminary reports have suggested that a substantial decrease in interventional cardiology procedures has been observed with longer time delays to intervention, especially among STEMI COVID‐19 patients 9 , 10 and a shift toward acute cases. 11 , 12
Poland represents a high volume country for interventional cardiology procedures with a well‐established hospital referral network program for ACS. The magnitude of SARS‐CoV‐2 pandemic places Poland among moderately affected countries with over 21,000 confirmed cases so far and over 1,000 deaths with governmental lockdown measures being implemented early on. 13 Yet, the negative impact on the healthcare system seems substantial. The shift toward acute cases (ACS) is evident, but what raises even more concern is an almost 40% decrease in the absolute number of interventional procedures in acute setting (STEMI, NSTEMI) when compared with the analogous time frame in 2019 and over 70% decrease instable angina procedures. The well‐functioning network of catheterization labs has been shattered by closures and temporary suspensions (almost 10% of all labs) and transformation into COVID‐19 hospitals/departments in order to accommodate the infectious patients. Resources are now predominantly redirected into the treatment of COVID‐19 patients.
On the other hand, no significant delays have been observed in a STEMI cohort in 2020 when comparing with 2019. Moreover, a significantly shorter time of a mean 13 min from FMC to angiography was observed in 2020. Maybe this is associated with an overall fewer cases and EMS and cath labs running on half empty which facilitates patient transfer and management.
Although COVID‐19 patients in 2020 were more often those with critical lesions in baseline angiography (17 vs 29%) versus those non‐COVID‐19, they were less frequently referred for invasive treatment (78 vs 83%). Modern imaging techniques, which prolong PCI procedure like intravascular ultrasound and fractional flow reserve, were also less frequently used in COVID‐19 patients. Total amount of contrast and radiation used during interventional procedures were similar in COVID‐19 and non‐COVID‐19 patients after PS adjustment.
While we have observed a low rate of periprocedural complications and similar periprocedural outcome in COVID‐19 versus non‐COVID‐19 patients with STEMI after PS adjustment, there was a significantly longer delay in COVID‐19 patients from FMC until intervention (~ mean of 30 and 23 min after PS adjustment). The proper preparation of the cathlab staff and dealing with potentially infected patient certainly generates additional delays, which cannot be overcome at least at the initial phase of the pandemic when we all learn how to cope with new issues. The long‐term impact of this finding on survival; however, remains unknown. The main issue is if all who have indications make it to the cathlab. Moreover, we may see consequences of these events in the near future mainly as an increase of heart failure incidence and hospitalizations.
The chance to perform interventional procedure in a COVID‐19 confirmed/suspected case is 1 in 39 in general or 1 in 17 when dealing with STEMI patients in Poland and the risk of becoming infected as a PCI operator is currently 1.7% per 2 months and seems low but that numbers will certainly vary in the end.
It is vital to provide recommendations on a national/international level for the management of COVID‐19 and non‐COVID‐19 patients; however, these days, this might not be the case anymore since anyone may be infected. Therefore, cathlab staff needs to adhere to the new reality that will probably stay with us for months if not years and considering every patient as a potential COVID‐19 infection. Constant monitoring of the number of procedures and outcomes of all patients should be undertaken. Elective patients should be carefully screened and monitored, preferably using telemedicine for the indications for urgent or accelerated coronary diagnostic and treatment. Patients with ongoing rest chest pain should be educated not to delay emergency calls.
The study has limitations which include the lack of further follow‐up of patients beyond catheterization room and the analysis represents only situation in one country (Poland) which may not be universal due to various presentation of SARS‐CoV‐2 pandemic worldwide.
6. CONCLUSIONS
The SARS‐CoV‐2 pandemic has had a tremendous effect on interventional cardiology procedures in Poland so far. There is an absolute reduction in the number of interventional procedures both in acute and elective settings in comparison to 2019 and in a significant shift into acute procedures in terms of fraction of all procedures. COVID‐19 confirmed/suspected patients do not differ in terms of procedural and baseline characteristics and reveal similar outcomes when treated with percutaneous coronary interventions. Longer time delays from FMC until intervention have been observed in a STEMI cohort of COVID‐19 patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Siudak Z, Grygier M, Wojakowski W, et al. Clinical and procedural characteristics of COVID‐19 patients treated with percutaneous coronary interventions. Catheter Cardiovasc Interv. 2020;96:E568–E575. 10.1002/ccd.29134
REFERENCES
- 1. Dudek D, Siudak Z, Legutko J, et al. Percutaneous interventions in cardiology in Poland in the year 2017. Summary report of the Association of Cardiovascular Interventions of the polish cardiac society AISN PTK and Jagiellonian University Medical College. Postepy Kardiol Interwencyjnej. 2018;14:422‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kristensen SD, Laut KG, Fajadet J, et al. Reperfusion therapy for ST elevation acute myocardial infarction 2010/2011: current status in 37 ESC countries. Eur Heart J. 2014;35:1957‐1970. [DOI] [PubMed] [Google Scholar]
- 3. Legutko J, Siudak Z, Parma R, Ochała A, Dudek D. Poland: coronary and structural heart interventions from 2010 to 2015. EuroIntervention. 2017;13:Z51‐Z54. [DOI] [PubMed] [Google Scholar]
- 4. Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119‐177. [DOI] [PubMed] [Google Scholar]
- 5. Siudak Z, Ochała A, Lesiak M, et al. Temporal trends and patterns in percutaneous treatment of coronary artery disease in Poland in the years 2005‐2011. Kardiol Pol. 2015;73:485‐492. [DOI] [PubMed] [Google Scholar]
- 6. Siudak Z, Tokarek T, Dziewierz A, et al. Reduced periprocedural mortality and bleeding rates of radial approach in ST‐segment elevation myocardial infarction. Propensity score analysis of data from the ORPKI polish National Registry. EuroIntervention. 2017;13:843‐850. [DOI] [PubMed] [Google Scholar]
- 7.AISN PTK guidelines. http://www.aisn.pl/aktualnosci/index/Postepowanie-z-chorym-SARS-Cov-2-i-OZW/idn:153. Accessed May 26, 2020.
- 8. Welt FGP, Shah PB, Aronow HD, et al.; American College of Cardiology's Interventional Council and the Society for Cardiovascular Angiography and InterventionsCatheterization laboratory considerations during the coronavirus (COVID‐19) pandemic. From ACC's Interventional Council and SCAI J Am Coll Cardiol. 2020;75:2372‐2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST‐segment elevation cardiac catheterization laboratory activations in the United States during COVID‐19 pandemic. J Am Coll Cardiol. 2020;75(22):2871–2872. 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tam CF, Cheung KS, Lam S, et al. Impact of coronavirus disease 2019 (COVID‐19) outbreak on ST‐segment‐elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13(4):e006631. 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID‐19 era. Eur Heart J. 2020;41(22):2083–2088. 10.1093/eurheartj/ehaa409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID‐19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41(19):1852‐1853. 10.1093/eurheartj/ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. https://coronavirus.jhu.edu/map.html. Accessed May 26, 2020.


