To the Editor,
Coronavirus Disease 2019 (COVID‐19) has become a pandemic and the number of infected cases continues to rise. As of 8 July 2020, a total of 11 669 259 laboratory‐confirmed cases were reported worldwide, with a mortality rate of 4.6%. 1 Previous meta‐analyses have shown that chronic obstructive pulmonary disease (COPD), cerebrovascular disease, hypertension, diabetes, and cardiovascular disease were risk factors for disease progression in patients with COVID‐19. 2 , 3 However, to be the best of our knowledge, no meta‐analysis has yet evaluated the impact of tuberculosis on COVID‐19 severity and mortality. Therefore, we performed a systematic review and meta‐analysis to assess whether tuberculosis is associated with an increased risk of severe disease and death in patients with COVID‐19.
This meta‐analysis was conducted following the Preferred Reporting in Systematic Reviews and Meta‐Analyses (PRISMA) statement. 4 We have registered this review protocol in the International Prospective Register of Systematic Reviews (PROSPERO, CRD42020185896). A comprehensive literature search was conducted using EMBASE.com, PubMed, Web of Science, the Cochrane Central Register of Controlled Trials (CENTRAL), Chinese Biomedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI), and Wanfang Database on May 12, 2020, with the search terms: “coronavirus disease‐19”, “COVID‐19”, “new coronavirus”, “2019‐nCoV”, “novel corona virus”, “novel coronavirus”, “nCoV‐2019”, “2019 novel coronavirus”, “coronavirus disease 2019”, “SARS‐CoV‐2”, “severe acute respiratory syndrome coronavirus 2”, “tuberculosis”, “comorbidities”, “clinical characteristic”, “clinical feature”, “risk factor”, and “prognosis”. The search strategy of Web of Science is presented in Appendix Word 1. We further reviewed reference lists of eligible studies and relevant systematic reviews to identify potentially eligible studies. Two reviewers independently conducted study selection. Disagreements were resolved by consensus or by a discussion with a third reviewer.
Our meta‐analysis included clinical studies that met the following criteria: (a) patients have a laboratory‐confirmed diagnosis of COVID‐19; (b) written in English or Chinese; (c) reported prevalence of tuberculosis among patients; (d) compared patients with the severe and non‐severe disease or between non‐survivors and survivors. Severe cases were defined as patients experiencing acute respiratory distress syndrome (ARDS), needing mechanical ventilation, requiring intensive care unit (ICU) admission support, or requiring vital life support. 5 , 6 We excluded studies with following characteristics: (a) involved suspected cases; (b) did not provide the prevalence of tuberculosis; (c) with a sample size of lower than 20 patients; (d) without comparisons (eg, severe vs nonsevere patients); (e) studies that were not peer‐reviewed, reviews, abstracts, letters, and editorials.
We used a predefined form to extract data from included studies. The data included first author, publication year, publication language, country of the corresponding author, recruitment time frame, age and sex of patients, sample size, the prevalence of tuberculosis, and outcomes of interest. The Newcastle‐Ottawa Quality Assessment Scale (NOS) was used to assess the quality of included studies. The data abstraction was conducted by one reviewer and verified by another.
The primary outcome was the association between tuberculosis and the risk of severe disease in patients with COVID‐19. The secondary outcome was the association between tuberculosis and COVID‐19 mortality. We adopted the Review Manager (version 5.3) to estimate pooled odds risk (OR) and its 95% confidence interval (CI) for primary and secondary outcomes using the inverse variance statistical method. Owing to heterogeneity within and between studies, we performed meta‐analyses using the random‐effects model. We assessed the heterogeneity using Cochran's Q test and I 2 statistic, and I 2 values of less than 25%, 26% to 50%, and more than 50% were considered as low, moderate, and high degrees of heterogeneity, respectively. Stata 13.0 (Stata Corporation, College Station, TX) was used to conduct a meta‐regression analysis to explore whether the primary outcome or the heterogeneity was associated with the mean age of patients.
We identified 2932 records through systematic electronic searches. After screening titles, abstracts, and full texts, six studies 7 , 8 , 9 , 10 , 11 , 12 were included for analysis. All the included studies 7 , 8 , 9 , 10 , 11 , 12 were from China and published in 2020. Three studies 7 , 11 , 12 published in English and three studies 8 , 9 , 10 published in Chinese. Six studies included a total of 2765 patients and the sample of individual study ranged from 143 to 1350. The prevalence of tuberculosis among COVID‐19 patients of included studies varied from 0.37% to 4.47%. The included studies were rated six to eight stars according to the NOS scale (Table 1).
Table 1.
Characteristics of included studies
| Reference | Country | Language | Recruitment time frame | Sample | Sex (Male) | Age* | Tuberculosis | NOS |
|---|---|---|---|---|---|---|---|---|
| Li et al 7 | China | English | 2020.1.26‐2020.2.5 | 548 | 279(50.91%) | 60 (48‐69) | 9(1.64%) | 7 |
| Liu et al 8 | China | Chinese | 2020.1.23‐2020.2.12 | 342 | 183(53.51%) | 56 (45‐67) | 2(0.58%) | 6 |
| Xiao et al 9 | China | Chinese | 2020.1.23‐2020.2.8 | 143 | 73(51.05%) | 45.13 ± 1.04 | 4(2.80%) | 7 |
| Zhang et al 10 | China | Chinese | 2020.1.15‐2020.3.4 | 1350 | 664(49.19%) | 44.1 ± 17.9 | 5(0.37%) | 6 |
| Chen et al 11 | China | English | 2020.1.1‐2020.2.10 | 203 | 108(53.20%) | 54 (41‐68) | 1(2.33%) | 6 |
| Du et al 12 | China | English | 2019.12.25‐2020.2.7 | 179 | 97(54.19%) | 57.6 ± 13.7 | 8(4.47%) | 8 |
Abbreviations: IQR, interquartile range; NOS, Newcastle‐Ottawa Quality Assessment Scale; SD, standard deviation.
Age data presented as median (IQR) or mean ± SD.
Four studies 7 , 8 , 9 , 10 involving 2,383 patients reported the prevalence of tuberculosis between severe and non‐severe COVID‐19 patients. The meta‐analysis indicated the prevalence of tuberculosis in severe patients (1.47%, 10/680) was higher than that in non‐severe patients (0.59%; 10/1703) (OR = 2.10, 95%CI: 0.61 to 7.18; P = .24; I 2 = 36%), although the statistical difference was not significant (Figure 1A). The univariate meta‐regression analysis indicated the mean age of patients was not the source of heterogeneity or the factor affecting the correlation between tuberculosis and COVID‐19 severity (P = .469; Figure 2). Two studies 11 , 12 provided the prevalence of tuberculosis between surviving and dying COVID‐19 patients. Chen et al 's study 11 revealed that non‐survivors had a higher prevalence of tuberculosis than survivors (5.26% vs. 0%). However, Du et al 's study 12 showed non‐survivors had a lower prevalence of tuberculosis than survivors (0% vs 5.06%). These inconsistent results may be due to differences in the follow‐up time of included studies and the treatment regimens of the patients. Our meta‐analysis indicated tuberculosis was not associated with the increased risk of mortality in patients with COVID‐19 (OR = 1.40, 95%CI: 0.10 to 18.93, P = .80; I 2 = 31%), Figure 1B.
Figure 1.
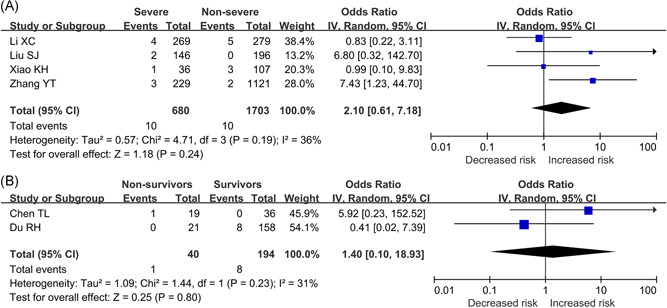
A, Association between tuberculosis and COVID‐19 severity. B, Association between tuberculosis and COVID‐19 mortality. COVID‐19, corona virus 2019
Figure 2.
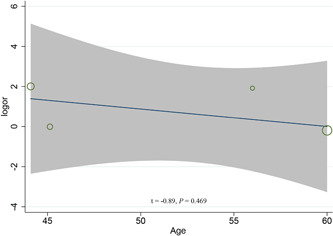
Univariate meta‐regression analysis on the mean age of patients for the association between tuberculosis and COVID‐19 severity. COVID‐19, corona virus 2019
Having a reliable estimate of the association between tuberculosis and COVID‐19 severity and mortality is crucial to ensure specific successful global preventive and treatment strategies for tuberculosis patients. Our study revealed that tuberculosis was associated with a 2.10‐fold increased risk of severe COVID‐19 disease, although the statistical difference was not significant. When a patient suffers from a previous respiratory disease, the patient's lung function is impaired, and their resistance to viruses is low and they tend to develop ARDS. 2 Therefore, tuberculosis may be a risk factor for disease progression. This study highlights the need for effective preventive measures and treatment strategies to reduce the risk of COVID‐19 severity in tuberculosis patients. However, our study did not suggest that tuberculosis was associated with an increased risk of mortality. This may be due to the small number of samples used in the analysis. Further analysis is needed to validate this result in the future.
Our study also had some limitations. First, only six studies were included in our analysis and all of them are from China. The results should be interpreted with caution. Second, due to limited data, we did not conduct subgroup analysis and assess the publication bias. As more evidence available, it will be interesting to assess whether the duration and type of tuberculosis are associated with increased complications in patients diagnosed with COVID‐19.
In conclusion, people with tuberculosis are not more likely to get COVID‐19, but pre‐existing tuberculosis has a higher chance of developing serious complications from COVID‐19. More high‐quality studies from different countries are needed to better understand the association between tuberculosis and COVID‐19 prognosis.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
YG, ML, and JHT conceived the study protocol. YG, ML, YMC, SZS, and JG participated in the literature search and the data collection. YG, ML, and JHT analyzed the data. YG and JHT drafted the manuscript. YG, ML, and JHT revised the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the Emergency Research Project of Key Laboratory of Evidence‐based Medicine and Knowledge Translation of Gansu Province (Grant No. GSEBMKT‐2020YJ01).
Ya Gao and Ming Liu contributed equally to this study.
REFERENCES
- 1. World Health Organization . Coronavirus disease (COVID‐2019) situation reports. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200708-covid-19-sitrep-170.pdf?sfvrsn=bca86036_2. Accessed July 9, 2020.
- 2. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID‐19 cases: a systematic literature review and meta‐analysis. J Infect. 2020. 10.1016/j.jinf.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID‐19: evidence from meta‐analysis. Aging. 2020;12(7):6049‐6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ (Clinical research ed) . 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao Y, Chen Y, Liu M, Shi S, Tian J. Impacts of immunosuppression and immunodeficiency on COVID‐19: systematic review and meta‐analysis. J Infect. 2020. 10.1016/j.jinf.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu M, Gao Y, Shi S, Chen Y, Yang K, Tian J. Drinking no‐links to the severity of COVID‐19: a systematic review and meta‐analysis. J Infect. 2020. 10.1016/j.jinf.2020.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu SJ, Cheng F, Yang XY, et al. A study of laboratory confirmed cases between laboratory indexes and clinical classification of 342 cases with corona virus disease 2019 in Ezhou. Lab Med. 2020. 10.3969/j.issn.1673-8640.2019.00.000 [DOI] [Google Scholar]
- 9. Xiao KH, Shui LL, Pang XH, et al. The clinical features of the 143 patients with COVID‐19 in North‐East of Chongqing. J Third Milit Med Univ. 2020. 10.16016/j.1000-5404.202002097 [DOI] [Google Scholar]
- 10. Zhang YT, Deng AP, Hu T, et al. Clinical outcomes of COVID‐19 cases and influencing factors in Guangdong province. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:E057. [DOI] [PubMed] [Google Scholar]
- 11. Chen T, Dai Z, Mo P, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID‐19) in Wuhan, China (2019): a single‐centered, retrospective study. J Gerontol A Biol Sci Med Sci. 2020. 10.1093/gerona/glaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID‐19 pneumonia caused by SARS‐CoV‐2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524. [DOI] [PMC free article] [PubMed] [Google Scholar]


