Abstract
Although not common, gastrointestinal and liver symptoms have reportedly been the initial presentation of coronavirus disease‐2019 (COVID‐19) in a large group of patients. Therefore, knowing the frequency and characteristics of these manifestations of COVID‐19 is important for both clinicians and health policy makers. A systematic review and meta‐analysis of the available data on the gastrointestinal and liver manifestations of patients with COVID‐19 was performed. PubMed and Scopus databases and Google Scholar search engine were searched for published and unpublished preprint articles up to 10 April 2020. Original studies providing information on clinical digestive symptoms or biomarkers of liver function in patients with polymerase chain reaction confirmed diagnosis of COVID‐19 were included. After quality appraisal, data were extracted. Prevalence data from individual studies were pooled using a random‐effects model. Overall, 67 studies were included in this systematic review and meta‐analysis, comprising a pooled population of 13 251 patients with confirmed COVID‐19. The most common gastrointestinal symptoms were anorexia (10.2%, 95% confidence interval [CI] = 6.2%‐16.4%), diarrhea (8.4%, 95% CI = 6.2%‐11.2%), and nausea (5.7%, 95% CI = 3.7%‐8.6%), respectively. Decreased albumin levels (39.8%, 95% CI = 15.3%‐70.8%), increased aspartate aminotransferase (22.8%, 95% CI = 18.1%‐28.4%), and alanine aminotransferase (20.6%, 95% CI = 16.7%‐25.1%) were common hepatic findings. After adjusting for preexisting gastrointestinal (5.9%) and liver diseases (4.2%), the most common gastrointestinal findings were diarrhea (8.7%, 95% CI = 5.4%‐13.9%), anorexia (8.0%, 95% CI = 3.0%‐19.8%), and nausea (5.1%, 95% CI = 2.2%‐14.3%). Gastrointestinal and liver manifestations are not rare in patients with COVID‐19, but their prevalence might be affected by preexisting diseases. Diarrhea and mild liver abnormalities seem to be relatively common in COVID‐19, regardless of comorbidities
Keywords: COVID‐19, digestive symptoms, gastrointestinal symptoms, hepatic abnormalities, hepatic injury
Highlights
Anorexia (10.2%), diarrhea (8.4%), and nausea (5.7%) were the most common gastrointestinal manifestations in confirmed COVID‐19 patients.
Serum albumin reduction (39.8%), AST elevation (22.8%), and ALT elevation (20.6%) were the most common hepatic abnormalities in confirmed COVID‐19 patients.
Preexisting gastrointestinal and liver diseases were present in 5.9% and 4.2% of COVID‐19 patients. After adjusting for these illnesses, diarrhea (8.7%) became the most prevalent gastrointestinal finding.
The prevalence of gastrointestinal and hepatic abnormalities were markedly higher in patients with severe COVID‐19 infection, compared to non‐severe cases.
1. INTRODUCTION
Coronavirus disease‐2019 (COVID‐19) emerged in December 2019 in Wuhan, China. 1 , 2 , 3 As of 27 June 2020, it has infected near 10 million individuals from over 200 countries around the world with around 500 000 deaths, causing a major pandemic. The WHO considered the outbreak of COVID‐19 infection as a health emergency. 4 , 5 , 6 , 7 , 8
Respiratory symptoms of COVID‐19 including fever, dry cough, and dyspnea are the most common manifestations of this novel infectious disease, similar to severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). 9 Although other manifestations such as gastrointestinal symptoms are less common, the initial presentation of the disease in some patients was gastrointestinal symptoms. 9 , 10 Different studies have reported various prevalence rates for gastrointestinal symptoms such as diarrhea in patients with COVID‐19. 11
In the SARS epidemic, 16% to 73% of patients had diarrhea during the period of the disease usually in the first week of sickness. In the initial MERS outbreak in 2012, a quarter of patients presented gastrointestinal symptoms such as diarrhea or abdominal pain. Besides, patients with SARS and MERS have showed different degrees of liver injury. 1 Due to the phylogenetic similarities between COVID‐19 and previous SARS‐like coronaviruses, it is not unlikely that this novel coronavirus infection present with gastrointestinal symptoms in some patients. 10
A growing body of evidence indicates the possibility of gastrointestinal tract and liver being target organs for COVID‐19, which can be potentially linked to the fact that the main receptor‐mediated entry for the novel coronavirus, angiotensin‐converting enzyme 2 (ACE2), is highly expressed in the gastrointestinal tract and the liver. This potential involvement of the gastrointestinal tract can possibly justify the presence of viral RNA in the stool exams of patients, indicating a possibility for fecal‐oral transmission of COVID‐19. 12 , 13 , 14 , 15 , 16
Recognition of clinical characteristics of COVID‐19 is not only important for clinicians but also it can be helpful for health policy makers to make proper decisions. 17 Previous systematic reviews on the gastrointestinal and hepatic manifestations of COVID‐19 have indicated that gastrointestinal symptoms are relatively common among the patients, with nausea or vomiting, loss of appetite, and diarrhea being the most common symptoms in this regard. However, these studies mostly included merely symptomatic patients and fail to address the effect of comorbid gastrointestinal and liver conditions on the prevalence of these symptoms. 16 , 18 In the present study, a systematic review and meta‐analysis of the available data on the gastrointestinal and hepatic manifestations of patients with COVID‐19 was performed. We also investigated the effect of comorbid gastrohepatic disorders on the rate of gastrointestinal and liver manifestations of COVID‐19.
2. METHODS
2.1. Search strategy and data sources
Two independent inspectors (MZB and AA) searched PubMed and Scopus databases and Google Scholar search engine for published and unpublished preprint articles from 1 January 2020 to 10 April 2020. Reference list of all selected articles were searched to look for possible missing articles. This search was manually expanded to recognize additional related articles. No language limitations were imposed. Different combinations of the following search terms were used: “Gastrointestinal” OR “Digestive” AND “Liver” OR “Hepatic”, AND “Coronavirus Disease 2019.” For each term, all synonyms and subjects with the same heading were also searched.
2.2. Study selection
After removing the duplicates, five investigators (MZB, MR, HG, AA, and MSG) independently screened the remaining studies for the inclusion criteria. Original studies providing information on clinical digestive symptoms or biomarkers of liver function in patients with reverse‐transcriptase polymerase chain reaction confirmed diagnosis of COVID‐19 were included in the review, according to current diagnostic guidelines. 19 , 20 , 21 Letters, comments, review articles, communications, and original articles that did not provide any reliable confirmation of COVID‐19 and studies with insufficient data were excluded. To minimize the risk of bias, studies that were conducted exclusively on a specific population and were not representative of the whole range of manifestations (eg, studies only on children, severe and critical cases, fatal cases, etc) were also excluded. The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses checklist (Figure 1) was followed.
Figure 1.
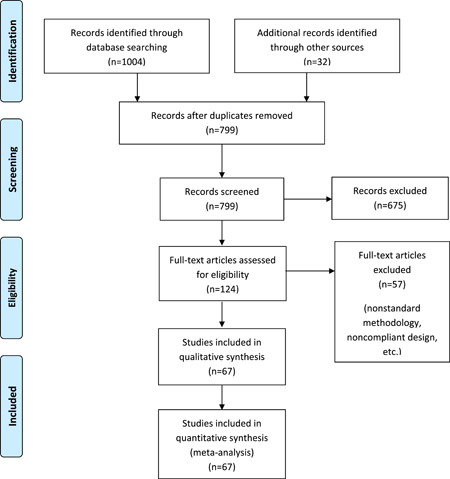
PRISMA flow diagram of the study. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
2.3. Critical appraisal
The Strengthening the Reporting of Observational studies in Epidemiology checklist was used for critical appraisal to assess the quality of studies. The checklist comprises 22 items, of which six have subitems. Twenty items can be scored a maximum of 1 point while a maximum of 2 points can be allocated to 14 other items where all the criteria regarding a certain item were reported. For each study, the final summed score out of a maximum of 48 points was converted into percentage with a maximum of 100%, to provide a clear estimation. 22 Five investigators independently appraised the papers (MZB, MR, HG, AA, and MSG) and consensus resolved the possible inconsistences.
2.4. Data extraction
Five investigators (MZB, MR, HG, AA, and MSG) extracted the data from the included studies and possible disagreements were resolved by consensus. Extracted study characteristics were title, journal, first author, publication date, sample size, study type, and origin (country and city). Demographics of the patients including age and gender were extracted and population type (inpatient, outpatient, or both) was recorded. Main gastrointestinal symptoms including diarrhea, vomiting, nausea, anorexia, abdominal pain, and abdominal distension as well as gastrointestinal comorbidities (chronic preexisting gastrointestinal or liver diseases) were extracted as main outcomes. Other main outcomes were markers of liver function, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, total bilirubin, alkaline phosphatase (ALP), and prothrombin time. Regarding the serum levels of these biomarkers, only data from studies with standard reference values and measurement methods were included.
2.5. Publication bias
Publication bias was kept as minimum as possible by applying no language restrictions and searching different databases. However, in the existence of heterogeneity between studies, potential publication bias was evaluated using funnel plots, Egger's test, and Begg's test. 23 , 24
2.6. Statistical analysis
All data were pooled and the meta‐analysis was performed using a random‐effects model in Comprehensive Meta‐analysis version 3.3.070. Point estimates with 95% confidence interval (95% CI) were used to present the results of meta‐analysis. The heterogeneity between studies was reported with relevant indicators including I 2. 25 P < .05 was considered as statistically significant.
We repeated the meta‐analysis, adjusting for the preexisting gastrointestinal and liver diseases, to investigate the effect of these comorbidities on the prevalence of COVID‐19 findings. A subgroup analysis was performed only looking at the published studies (excluding preprint studies). Moreover, we conducted another subgroup analysis according to the severity of disease, to see whether the severity of COVID‐19 affects the estimated prevalence of findings.
3. RESULTS
3.1. Literature selection and study characteristics
Initial search retrieved 1036 records from different sources. After removing duplicates, 675 records were screened via title and abstract review. Having excluded the irrelevant studies, the full text articles of 124 records were reviewed, of which 57 were excluded due to noncompliant design, insufficient data, or nonstandard diagnostic methods. Finally, 67 studies with a pooled population of 13 251 confirmed patients with COVID‐19 were included in this systematic review and meta‐analysis (Figure 1). Overall, 5079 patients (53.3%) were female and there was a relatively balanced sex distribution in the pooled population.
Characteristics of the included studies, including first author, study design, publication date, origin, sample size, and quality assessment score, as well as population type and patient demographics including sex and age are illustrated in Table 1.
Table 1.
Characteristics of the studies included in the systematic review and meta‐analysis
| First author | Study design | Date (MM/DD) | City, country | Quality score (%) | COVID‐19 patients | |||
|---|---|---|---|---|---|---|---|---|
| N | Age mean ± SD/median (1st quartile‐3rd quartile) | Sex (male/female) | Population type | |||||
| Bai T 26 | Cross‐sectional | (Preprint) 03/05 | Wuhan, China | 62.5 | 127 | 55.0 (44.0‐67.0) | 80/47 | Inpatient |
| Cai Q 27 | Cross‐sectional | 04/02 | Shenzhen, China | 66.7 | 298 | 47.0 (33.0‐61.0) | 149/149 | Inpatient |
| Chen D 28 | Cohort | 06/11 | Wenzhou, China | 72.9 | 175 | 46 (34.0‐54.0) | 88/87 | Inpatient |
| Chen G 29 | Cross‐sectional | 03/27 | Wuhan, China | 75.0 | 21 | 56.3 ± 14.3 | 17/4 | Inpatient |
| Chen J 30 | Cohort | 03/19 | Shanghai, China | 70.8 | 249 | 51.0 (36.0‐64.0) | 126/123 | Inpatient |
| Chen L 31 | Cross‐sectional | 03/14 | Wuhan, China | 60.4 | 29 | 56.0 (26.0‐79.0) | 21/8 | Inpatient |
| Chen N 32 | Case series | 02/03 | Wuhan, China | 62.6 | 99 | 55·5 ± 13·1 | 67/32 | Inpatient |
| Chen Z 33 | Cross‐sectional | (Preprint) 03/02 | Wuhan, China | 64.6 | 89 | 33·3 ± 6·6 | 30/59 | Inpatient |
| Cheng JL 34 | Cross‐sectional | 03/02 | Henan province, China | 50.0 | 1079 | 46.0 (IQR = 24.0) | 573/505 | Inpatient |
| Fan Z 35 | Cohort | 04/10 | Shanghai, China | 68.7 | 148 | 50.0 (36.0‐64.0) | 73/75 | Inpatient |
| Feng Z 36 | Cohort | (Preprint) 02/23 | Changsha, China | 75.0 | 141 | 44.0 (34.0‐55.0) | 72/69 | Inpatient |
| Fu H 37 | Cross‐sectional | (Preprint) 03/01 | Kunming, China | 47.9 | 36 | Median: 45.0 | 16/20 | Inpatient |
| Range, 3.0‐79.0 | ||||||||
| Fu L 38 | Cohort | (Preprint) 03/16 | Wuhan, China | 64.6 | 200 | ‐ | 99/101 | Inpatient |
| Gong J 39 | Cohort | 04/16 | Guangzhou, Wuhan, China | 79.2 | 189 | 49.0 (35.0‐63.0) | 88/101 | Inpatient |
| Guan WJ 40 | Cohort | 02/28 | Guangzhou, Wuhan, China | 58.3 | 1099 | 47.0 (35.0–58.0) | 637/459 | Inpatient and outpatient |
| Han R 41 | Cross‐sectional | 03/18 | Wuhan, China | 58.3 | 108 | Mean: 45.0 | 38/70 | Inpatient |
| Huang C 42 | Cohort | 01/24 | Wuhan, China | 68.7 | 41 | 49.0 (41.0‐58.0) | 30/11 | Inpatient |
| Huang M 43 | Cohort | (Preprint) 02/19 | Chongqing, China | 64.6 | 197 | 49.0 (41·0‐58·0) | 109/88 | Inpatient |
| Jin JM 44 | Case series | 04/29 | Wuhan, China | 54.2 | 43 | 62.0 (51.0‐70.0) | 22/21 | Inpatient |
| Jin X 45 | Case‐control | 03/24 | Zhejiang province, China | 77.1 | 651 | 45.2 ± 14.4 | 331/320 | Inpatient |
| Jin X 46 | Cross‐sectional | 03/17 | Zhejiang province, China | 68.7 | 788 | 45.8 ± 14.9 | 407/381 | Inpatient |
| Kong I 47 | Case series | 02/14 | South Korea | 41.7 | 28 | 42.6 (20.0‐73.0) | 15/13 | Inpatient |
| Kuang Y 48 | Cross‐sectional | (Preprint) 02/28 | Zhejiang province, China | 62.5 | 944 | 47.4 ± 22.9 | 476/468 | N/A |
| Lei Z 49 | Cross‐sectional | 04/09 | Guangzhou, China | 64.6 | 20 | 43.2 ± 14·0 | 10/10 | Inpatient |
| Li J 50 | Case series | (Preprint) 02/12 | Dazhou, China | 54.2 | 17 | 45.0 (22.0‐65.0) | 9/8 | Inpatient |
| Li L 51 | Cross‐sectional | (Preprint) 03/10 | Beijing, China | 75.0 | 85 | 49.0 (36.0‐64.0) | 47/38 | Inpatient |
| Li YY 52 | Cross‐sectional | 02/14 | Wuhan, China | 64.6 | 31 | 54.0 ± 13.0 | 15/16 | Inpatient |
| Liang Y 53 | Cross‐sectional | (Preprint) 02/28 | Beijing, China | 75.0 | 21 | 42.0 (34.5‐66.0) | 11/10 | Outpatient |
| Liu C 54 | Cross‐sectional | 02/20 | Lanzhou, Shenyang, Ankang, Lishui, Zhenjiang, Baoding, Linxiazhou, China | 70.1 | 32 | 38.6 (26.3‐45.8) | 20/12 | Inpatient |
| Liu F 55 | Case series | 03/12 | Hangzhou, China | 47.8 | 10 | 42.0 (34.0‐50.0) | 4/6 | Inpatient |
| Liu K 56 | Cohort | 02/07 | Hubei province, China | 52.1 | 137 | 55.0 ± 16.0 | 61/76 | Inpatient |
| Liu W 57 | Case series | (Preprint) 02/20 | Wuhan, China | 72.9 | 936 | 53.0 ± 14·8 | 296/332 | Outpatient |
| Liu Y 58 | Case series | 02/09 | Shenzhen, China | 58.3 | 12 | 54.0 (10.0‐72.0) | 8/4 | Inpatient |
| Lo IL 15 | Case series | 03/15 | Macau, China | 64.6 | 10 | 54.0 (27.0–64.0) | 3/7 | Inpatient |
| Luo S 59 | Case series | 03/18 | Wuhan, China | 41.7 | 1141 | Mean: 53.8 | 102/81 | Inpatient |
| Miao C 60 | Cross‐sectional | (Preprint) 03/24 | Shanghai, Nanchang, Yichun, China | 68.7 | 62 | 43.8 ± 13.9 | 32/30 | Inpatient |
| Mo P 61 | Case series | 03/16 | Wuhan, China | 83.3 | 155 | 54.0 (42.0‐66.0) | 86/69 | Inpatient |
| Pan L 62 | Cross‐sectional | 04/14 | Wuhan, Huanggang, China | 81.2 | 204 | 52.9 ± 16.0 | 107/97 | Inpatient |
| Shi H 63 | Case series | 02/24 | Wuhan, China | 70.1 | 81 | 49·5 ± 11·0 | 42/39 | Inpatient |
| Song F 64 | Cross‐sectional | 02/06 | Shanghai, China | 72.9 | 51 | 49.0 ± 16.0 | 25/26 | Inpatient |
| Sun W 65 | Cross‐sectional | 03/15 | Zhejiang province, China | 54.2 | 148 | 48.0 (37.0‐56.0) | 73/75 | N/A |
| Sun Y 66 | Case‐control | 03/25 | Singapore | 91.7 | 54 | 42.0 (34.0‐54.0) | 29/25 | Inpatient and outpatient |
| Tang X 67 | Case‐sontrol | 03/26 | Wuhan, China | 72.9 | 73 | 67.0 (57.0‐72.0) | 45/28 | Inpatient |
| Wan S 68 | Cohort | 03/21 | Chongqing, China | 77.1 | 135 | 47.0 (36.0‐55.0) | 72/63 | Inpatient |
| Wang D 69 | Case series | 02/07 | Wuhan, China | 75.0 | 138 | 56.0 (42.0‐68.0) | 75/63 | Inpatient |
| Wei XS 70 | Case series | 04/18 | Wuhan, China | 68.7 | 84 | 37.0 (24.0‐74.0) | 28/56 | Inpatient |
| Wen Y 71 | Cross‐sectional | (Preprint) 03/23 | Shenzhen, China | 75.0 | 417 | 45.4 ± 17.7 | 197/220 | Inpatient and outpatient |
| Wu J 72 | Cross‐sectional | 02/29 | Jiangsu, China | 64.6 | 80 | 46.1 ± 15.4 | 39/41 | Inpatient |
| Xu T 73 | Cohort | 04/14 | Jiangsu, China | 68.7 | 51 | ‐ | 25/26 | Inpatient |
| Xu W 74 | Cross‐sectional | (Preprint) 03/18 | Suzhou, China | 70.1 | 87 | 44.6 ± 14.7 | 46/41 | Inpatient |
| Fang Y 75 | Case series | 02/19 | Zhejiang province, China | 68.7 | 62 | 41.0 (32.0‐52.0) | 35/27 | Inpatient |
| Xu YH 76 | Cross‐sectional | 02/25 | Beijing, China | 54.1 | 50 | 43.9 ± 16.8 | 29/21 | Inpatient |
| Yang W 77 | Case series | 02/26 | Wenzhou, China | 79.2 | 149 | 45.1 ± 13.4 | 81/68 | Inpatient |
| Yao N 78 | Cross‐sectional | 03/10 | Xi'an, China | 56.3 | 40 | 53.9 ± 15.8 | 25/15 | Inpatient |
| Young BE 79 | Case series | 03/03 | Singapore | 62.5 | 18 | 47.0 (31.0‐73.0) | 9/9 | Inpatient |
| Yu F 80 | Cohort | 03/28 | Beijing, China | 70.1 | 76 | 40.0 (32.0‐63.0) | 38/38 | Inpatient |
| Zhang G 81 | Case series | 04/09 | Wuhan, China | 77.1 | 221 | 55.0 (39.0‐66.5) | 108/113 | Inpatient |
| Zhang JJ 82 | Cross‐sectional | 02/19 | Wuhan, China | 68.7 | 140 | 57.0 (25.0‐87.0) | 71/69 | Inpatient |
| Zhang MQ 83 | Case series | 03/01 | Beijing, China | 41.7 | 9 | 36.0 (15.0‐49.0) | 5/4 | Inpatient |
| Zhang X 84 | Cross‐sectional | 03/20 | Zhejiang province, China | 60.4 | 645 | 45.3 ± 13.9 | 295/278 | Inpatient |
| Zhang Y 85 | Cohort | 04/02 | Wuhan, China | 66.7 | 115 | 49.5 ± 17.1 | 49/66 | Inpatient |
| Zhang Y 86 | Cross‐sectional | (Preprint) 03/27 | Wuhan, China | 72.9 | 212 | 48.5 ± 13.2 | 119/93 | Inpatient |
| Zhao D 87 | Case‐control | 03/12 | Anhui province, China | 62.5 | 19 | 48.0 (27.0‐56.0) | 11/8 | Inpatient |
| Zhao W 88 | Cohort | 03/03 | Changsha, Yueyang, Changde, Xiangtan, China | 60.4 | 101 | 44.4 ± 12.3 | 56/45 | Inpatient |
| Zhao W 89 | Cohort | (Preprint) 03/17 | Beijing, China | 62.5 | 77 | 52.0 ± 20.0 | 34/43 | Inpatient |
| Zhao Z 90 | Case series | (Preprint) 03/06 | Hefei, China | 54.1 | 75 | 47.0 (34.0‐55.0) | 42/33 | Inpatient |
| Zhou F 91 | Cohort | 03/11 | Wuhan, China | 85.4 | 191 | 56·0 (46.0–67.0) | 119/72 | Inpatient |
Abbreviations: COVID‐19, coronavirus disease‐2019; IQR, interquartile range; N/A, not available; SD, standard deviation.
3.2. Publication bias
Regarding publication bias, funnel plots for two of the common findings are shown in Figures 2 and 3. Although funnel plots showed asymmetry for some findings, Eggers's test confirmed publication bias only for vomiting (t = 3.47, P < .001). However, the results of the Begg's test showed no notable evidence of publication bias for any of these findings (Table S1).
Figure 2.
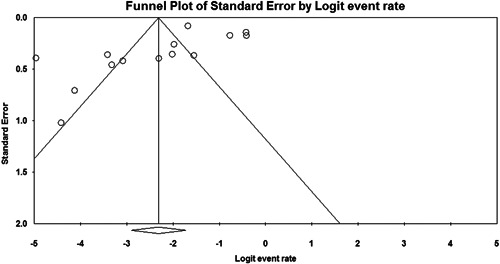
Funnel plot of studies reporting anorexia in the primary unadjusted meta‐analysis
Figure 3.
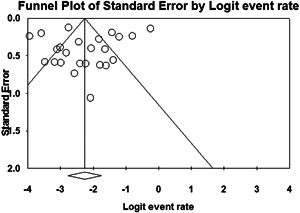
Funnel plot of studies reporting diarrhea, after adjusting for preexisting diseases
3.3. Meta‐analysis of gastrointestinal and liver symptoms
This meta‐analysis showed that the three most prevalent gastrointestinal symptoms among patients with confirmed COVID‐19 were anorexia (10.2%, 95% CI = 6.2%‐16.4%), diarrhea (8.4%, 95% CI = 6.2%‐11.2%), and nausea (5.7%, 95% CI = 3.7%‐8.6%), respectively (Table 2; Figure 4). Common liver function abnormalities were mild decrease in albumin level (39.8%, 95% CI = 15.3%‐70.8%), and mild increase in AST (22.8%, 95% CI = 18.1%‐28.4%), ALT (20.6%, 95% CI = 16.7%‐25.1%) (Table 2; Figure 5). Moreover, 18.0% (95% CI = 3.0%‐60.8%) showed elevated prothrombin time. Total bilirubin and ALP levels were slightly elevated in 7.8% (95% CI = 5.0%‐12.0%) and 4.6% (95% CI = 2.6%‐7.9%), respectively. Chronic gastrointestinal and liver diseases were present in 5.9% (95% CI = 4.1%‐8.5%) and 4.2% (95% CI = 3.3%‐5.3%) of the patients, respectively.
Table 2.
Pooled prevalence of gastrointestinal symptoms, preexisting diseases, and liver function abnormalities in all studies reporting patients with COVID‐19
| Findings | All studies (published and preprint) | Published studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N patients | N studies | Point estimate (%) | 95% CI (%) | I 2 | N patients | N studies | Point estimate (%) | 95% CI (%) | I 2 | |
| Gastrointestinal symptoms | ||||||||||
| Anorexia | 3871 | 15 | 10.2 | 6.2‐16.4 | 95.65 | 2590 | 11 | 16.2 | 10.3‐24.5 | 94.83 |
| Diarrhea | 10 652 | 56 | 8.4 | 6.2‐11.2 | 93.80 | 7101 | 41 | 8.6 | 6.8‐10.8 | 83.07 |
| Nausea | 5089 | 23 | 5.7 | 3.7‐8.6 | 88.08 | 3740 | 19 | 7.2 | 4.7‐10.9 | 87.31 |
| Vomiting | 4567 | 20 | 3.8 | 2.5‐5.9 | 81.80 | 3434 | 18 | 4.6 | 3.0‐6.8 | 78.57 |
| Abdominal pain | 2342 | 10 | 3.2 | 2.1‐4.7 | 44.17 | 2267 | 9 | 3.3 | 2.2‐4.9 | 46.97 |
| Abdominal distension | 1217 | 3 | 1.1 | 0.2‐5.6 | 78.76 | 84 | 1 | 3.6 | 1.2‐10.5 | 0.00 |
| Liver function abnormalities | ||||||||||
| Decreased albumin | 505 | 7 | 39.8 | 15.3‐70.8 | 94.77 | 505 | 7 | 39.8 | 15.3‐70.8 | 94.77 |
| Elevated AST | 2062 | 16 | 22.8 | 18.1‐28.4 | 83.05 | 1910 | 14 | 22.4 | 17.2‐28.5 | 84.31 |
| Elevated ALT | 1496 | 8 | 20.6 | 16.7‐25.1 | 65.63 | 1282 | 5 | 20.3 | 15.2‐26.6 | 78.12 |
| Elevated PT | 323 | 3 | 18.0 | 3.0‐60.8 | 97.03 | 248 | 2 | 8.3 | 3.7‐17.3 | 63.90 |
| Elevated TBIL | 1429 | 9 | 7.8 | 5.0‐12.0 | 72.02 | 1354 | 7 | 6.8 | 4.1‐11.0 | 72.64 |
| Elevated ALP | 263 | 2 | 4.6 | 2.6‐7.9 | <0.01 | 263 | 2 | 4.6 | 2.6‐7.9 | <0.01 |
| Preexisting diseases | ||||||||||
| Digestive disease | 1152 | 9 | 5.9 | 4.1‐8.5 | 51.73 | 861 | 6 | 5.9 | 3.7‐9.2 | 58.31 |
| Liver disease | 5891 | 30 | 4.2 | 3.3‐5.3 | 56.74 | 5207 | 24 | 4.3 | 3.3‐5.6 | 61.84 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; COVID‐19, coronavirus disease‐2019; PT, prothrombin time; TBIL, total bilirubin.
Figure 4.
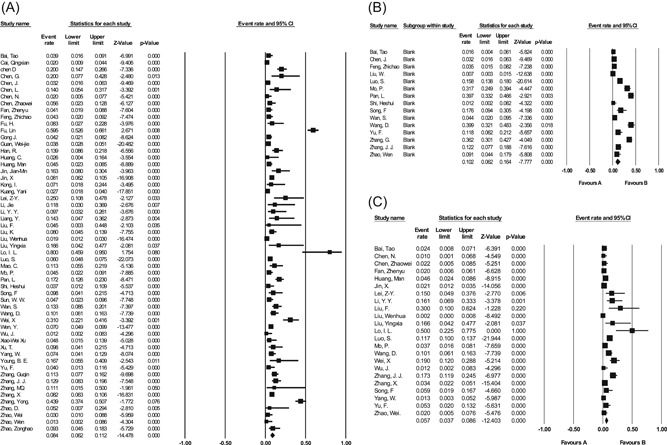
Forest plots for the prevalence of major gastrointestinal findings in meta‐analysis of all studies. A, Diarrhea. B, Anorexia. C, Nausea. CI, confidence interval
Figure 5.
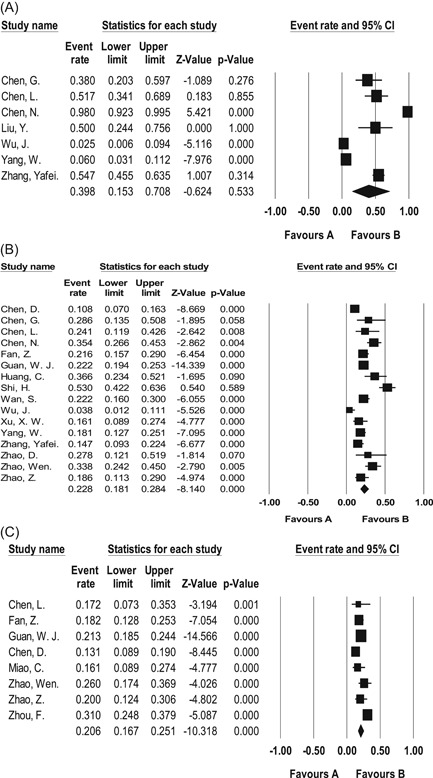
Forest plots for the prevalence of major hepatic findings in meta‐analysis of all studies. A, Decreased albumin. B, Increased AST. C, Increased ALT. ALT, aminotransferase; AST, aspartate aminotransferase; CI, confidence interval
3.4. Subgroup analysis: published studies
When assessing only the published studies, we saw a change in the estimated prevalence of nearly all findings in patients with COVID‐19, although the order of the most common findings remained unchanged (Table 2). Anorexia (16.2%, 95% CI = 10.3%‐24.5%) and decreased albumin (39.8%, 95% CI = 15.3%‐70.8%) were the most common digestive and hepatic findings, respectively. The prevalence of preexisting gastrointestinal and hepatic findings remained almost the same after exclusion of preprint articles (Table 2).
3.5. Meta‐analysis of gastrointestinal and liver symptoms after adjustment for preexisting disease
To assess the effect of comorbid gastrointestinal and hepatic conditions on the prevalence of COVID‐19 findings, the meta‐analysis was repeated, adjusting for these comorbid conditions. The respective results are reported in Table 3.
Table 3.
Pooled prevalence of gastrointestinal and liver symptoms in patients with COVID‐19, after adjustment for preexisting diseases
| Findings | All studies (published and preprint) | Published studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N patients | N studies | Point estimate (%) | 95% CI (%) | I 2 | N patients | N studies | Point estimate (%) | 95% CI (%) | I 2 | |
| Gastrointestinal symptoms | ||||||||||
| Diarrhea | 5104 | 23 | 8.7 | 5.4‐13.9 | 94.62 | 2308 | 15 | 9.9 | 6.5‐14.9 | 85.90 |
| Anorexia | 2515 | 5 | 8.0 | 3.0‐19.8 | 97.21 | 1438 | 3 | 20.0 | 9.5‐37.2 | 95.97 |
| Nausea | 2458 | 7 | 5.1 | 2.3‐11.0 | 88.63 | 1349 | 4 | 8.1 | 4.0‐15.6 | 70.30 |
| Vomiting | 2513 | 6 | 3.7 | 1.6‐8.3 | 85.29 | 1577 | 5 | 5.8 | 3.3‐10.2 | 71.67 |
| Abdominal pain | 1400 | 3 | 3.7 | 2.8‐4.8 | <0.01 | 1400 | 3 | 3.7 | 2.8‐4.8 | <0.01 |
| Abdominal distension | 1020 | 2 | 0.7 | 0.0‐18.9 | 89.22 | 84 | 1 | 3.6 | 1.2‐10.5 | 0.00 |
| Liver function abnormalities | ||||||||||
| Decreased albumin | 136 | 2 | 49.3 | 34.4‐64.4 | 48.41 | 136 | 2 | 49.3 | 34.4‐64.4 | 48.41 |
| Elevated ALT | 426 | 3 | 19.4 | 9.9‐34.3 | 88.68 | 364 | 2 | 20.8 | 8.3‐43.4 | 93.71 |
| Elevated AST | 311 | 3 | 15.2 | 9.3‐23.8 | 59.98 | 311 | 3 | 15.2 | 9.3‐23.8 | 59.98 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; COVID‐19, coronavirus disease‐2019.
Overall, after adjusting for chronic preexisting illnesses, the three most common gastrointestinal findings were diarrhea (8.7%, 95% CI = 5.4%‐13.9%), anorexia (8.0%, 95% CI = 3.0%‐19.8%), and nausea (5.1%, 95% CI = 2.3%‐11.0%). Common laboratory abnormalities in liver function were mild reduction in albumin (49.3%, 95% CI = 34.4%‐64.4%) and elevations in ALT (19.4%, 95% CI = 9.9%‐34.3%) and AST (15.2%, 95% CI = 9.3%‐23.8%).
3.6. Subgroup analysis: Published studies
As reported by published studies, after adjusting for preexisting illnesses, the most common gastrointestinal feature of COVID‐19 was anorexia with an estimated prevalence of 20.0% (95% CI = 9.5%‐37.2%), followed by diarrhea (9.9%, 95% CI = 6.5%‐14.9%). Table 3 elaborates the details in this regard.
3.7. Gastrointestinal and liver symptoms and disease severity
Results of the subgroup analysis according to disease severity are illustrated in Table 4. As is evident from the table, although anorexia was the most common gastrointestinal finding in both subgroups, its prevalence was two times higher in severe patients compared with nonsevere ones (31.4% vs 14.9%). Besides, the prevalence of diarrhea, vomiting, and abdominal pain were also markedly higher in patients with severe disease, while abdominal distension was more frequent in nonsevere cases (Table 4). The severe subgroup showed significantly higher prevalence of liver function abnormalities and preexisting illnesses, as well (Table 4).
Table 4.
Pooled prevalence of gastrointestinal symptoms, preexisting diseases, and liver function abnormalities in patients with different severities of COVID‐19
| Findings | Nonsevere | Severe | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N patients | N studies | Point estimate (%) | 95% CI (%) | I 2 | N patients | N studies | Point estimate (%) | 95% CI (%) | I 2 | |
| Gastrointestinal symptoms | ||||||||||
| Anorexia | 502 | 5 | 14.9 | 7.6‐27.2 | 84.92 | 208 | 5 | 31.4 | 12.4‐59.7 | 91.49 |
| Diarrhea | 2006 | 11 | 5.5 | 3.5‐8.4 | 71.67 | 535 | 10 | 11.1 | 6.7‐18.0 | 69.02 |
| Nausea | 326 | 3 | 9.5 | 3.0‐26.2 | 89.24 | 148 | 3 | 9.5 | 5.7‐15.4 | <0.01 |
| Vomiting | 326 | 3 | 2.5 | 0.7‐9.2 | 59.59 | 148 | 3 | 5.1 | 2.4‐10.3 | <0.01 |
| Abdominal pain | 350 | 3 | 1.8 | 0.8‐4.0 | <0.01 | 148 | 3 | 8.1 | 4.5‐14.0 | <0.01 |
| Abdominal distension | 142 | 1 | 2.1 | 0.7‐6.3 | <0.01 | 55 | 1 | 1.8 | 0.3‐11.8 | <0.01 |
| Liver function abnormalities | ||||||||||
| Decreased albumin | 94 | 2 | 27.2 | 6.2‐67.9 | 66.39 | 42 | 2 | 80.2 | 44.1‐95.4 | 72.81 |
| Elevated AST | 1265 | 8 | 11.6 | 7.2‐18.0 | 81.77 | 355 | 8 | 36.7 | 30.0‐43.9 | 34.97 |
| Elevated ALT | 1039 | 4 | 15.0 | 8.5‐25.2 | 89.27 | 253 | 4 | 30.8 | 25.0‐37.3 | 8.81 |
| Elevated TBIL | 918 | 3 | 7.5 | 4.7‐11.7 | 59.33 | 217 | 3 | 17.3 | 11.4‐25.4 | 39.20 |
| Elevated ALP | 324 | 2 | 0.1 | 0.01‐8.7 | 56.97 | 89 | 2 | 5.7 | 0.7‐32.4 | 71.40 |
| Preexisting diseases | ||||||||||
| Digestive disease | 281 | 3 | 5.2 | 3.1‐8.6 | <0.01 | 133 | 3 | 6.9 | 1.9‐22.3 | 69.18 |
| Liver disease | 1781 | 8 | 3.2 | 1.8‐5.7 | 69.94 | 488 | 8 | 4.9 | 2.4‐9.5 | 50.32 |
4. DISCUSSION
The emergence of the COVID‐19 outbreak has infected millions of people worldwide and caused around 500 000 mortalities. The WHO has characterize the infection as a pandemic and called for research on all clinical aspects of COVID‐19. Facing this pandemic requires a critical response and preparedness from all communities, especially health care professionals. 6 , 7 , 8 , 92 , 93
A variety of symptoms has been reported in patients with COVID‐19, from mild pulmonary involvement to severe bilateral pneumonia that can rapidly progress to acute respiratory distress syndrome and respiratory failure. 94 The vast majority of the symptoms associated with COVID‐19 are related to respiratory tract, except for general manifestations such as fever. This is in line with the symptoms previously reported in patients with SARS and MERS, which are caused by viruses from the same phylogenic family. 95
The focus of researchers has been on respiratory symptoms, which are thought to be the main cause of fatality in this disease, and little is known about the extrapulmonary manifestations that might accompany COVID‐19, especially gastrointestinal and liver abnormalities. It has been reported that presenting with gastrointestinal symptoms can delay the diagnosis and subsequently lead to worse outcomes in patients with COVID‐19. 18 Therefore, this systematic review and meta‐analysis was performed to provide more insight into the frequency and characteristics of gastrointestinal and liver involvements in patients with COVID‐19.
The most common gastrointestinal symptoms among all patients with confirmed COVID‐19 were anorexia (10.2%), diarrhea (8.4%), and nausea (5.7%). Other less common presentations were vomiting, abdominal pain, and abdominal distension. Common liver function abnormalities were mild decrease in serum albumin (39.8%) and mild increase in AST and ALT levels, which was found in 22.8% and 20.6% of the patients. Total bilirubin was mildly elevated in 7.8%, 4.6% had elevated ALP, and so was prothrombin time in 18% of patients.
The elevation of ALP can possibly be related to the pathophysiology of COVID‐19 and its main entry mechanism, which is related to ACE2 receptors. It has been reported that the biliary tree abundantly expresses ACE2, and thus it can be a potential target for the virus. 14
Chronic gastrointestinal and liver comorbidities were found to be present in 5.9% and 4.2% of the patients, respectively. Since the prevalence of digestive findings in this study was relatively low (10% and lower), these comorbidities might have affected the actual prevalence of gastrointestinal and hepatic findings of COVID‐19 in the pooled population. As reported previously, preexisting digestive disease has been reported with poorer outcomes in patients with COVID‐19. 96 Therefore, in an attempt to remove the effects of these chronic preexisting conditions, the studies in which patients had preexisting diseases were excluded. The adjusted estimates revealed that common gastrointestinal findings were diarrhea (8.7%), anorexia (8.0%), and nausea (5.1%). This change in the prevalence rates after adjustment indicates the possibility of some gastrointestinal symptoms, which have been reported in several studies as common gastrointestinal features of COVID‐19, being a result of comorbid preexisting conditions. In particular, anorexia and to some extent nausea were found to have a marked decrease in their frequency, after excluding preexisting illnesses.
When considering only published studies, we observed that anorexia was the most common gastrointestinal symptom with an estimated prevalence of 16.2%, which rose to 20% after adjusting for preexisting comorbidities and remained the most common gastrointestinal findings in patients with COVID‐19.
It seems that diarrhea is the main gastrointestinal feature of patients with COVID‐19, as was the case in patients infected with the similar pathologies of SARS and MERS. 95 , 97 , 98 However, this higher estimated prevalence of diarrhea in this study might be because some of the included studies did not assess other gastrointestinal symptoms. It is also worth noting that although rarely reported in previous studies, COVID‐19 can present with constipation or other uncommon gastrointestinal symptoms. 99 For instance, few sparse studies reported gastrointestinal bleeding and this finding was not incorporated into meta‐analysis. 26 , 68 It should be studied further by large‐scale studies.
The exact pathologic mechanisms behind the gastrointestinal involvement of COVID‐19 is yet to be known. However, as the virus targets ACE2 to infect human cells, it seems likely that intestinal cells, which largely express the ACE2 cell receptors, are infected in the same way. Moreover, viral particles have been detected in stool samples of a large number of patients with COVID‐19, even longer than what detected in respiratory samples. Interestingly, patients with diarrhea reportedly have higher frequency of virus being detected in their feces. 16
In a subgroup analysis, we found that anorexia was the most common gastrointestinal finding in both severe and nonsevere groups. However, the prevalence of almost all gastrointestinal and liver symptoms were markedly higher in severe patients compared with nonsevere ones, with the exception of nausea and abdominal distension. The severe subgroup showed significantly higher prevalence of liver function abnormalities, as well. Decreased albumin was seen in about 80% of the severe cases. As expected, the prevalence of digestive and hepatic comorbidities were also higher in severe patients, which is in line with the previous reports indicating the higher risk of severe COVID‐19 in patients with comorbidities. 96
The higher prevalence of gastrointestinal and liver symptoms among severe cases has been reported in two previous meta‐analysis study. 16 , 18 Several studies have suggested a relationship between gastrointestinal and hepatic manifestations of COVID‐19 and the disease severity in these patients. 32 , 100 , 101
The results from the meta‐analysis by Mao et al 18 are relatively similar to the findings of our main meta‐analysis in this study. They reported diarrhea, nausea, or vomiting, and abdominal pain to be present in about 9%, 6%, and 3% of patients, respectively, which is in line with our findings. They also found the prevalence of digestive and hepatic comorbidities to be 4% and 3%, respectively, which is slightly lower the rates we found. Although our results regarding the frequency of elevated AST, ALT, and bilirubin were consistent with those reported by Mao et al, 18 they reported a far lower rate for decreased albumin (6%). These inconsistencies might be attributed to different eligibility criteria, lower number of included studies, and different analytic methodology.
A recent meta‐analysis on the gastrointestinal symptoms of COVID‐19 by Cheung et al 16 indicated relatively higher prevalence for virtually every symptom, compared with these findings. They found anorexia to be the most prevalent gastrointestinal presentation of COVID‐19, which is in line with these findings in patients with any preexisting conditions. However, they found it in about 26.8% of patients, which is by far higher than what were found in this study. Besides, after comorbid chronic conditions were adjusted, diarrhea overtook anorexia to become the most prevalent symptom in patients with COVID‐19. Nevertheless, it did not happen when assessing only the published studies, where anorexia remained the most prevalent symptom after adjustment. Also in contrast with these findings, Cheung et al 16 found diarrhea and nausea/vomiting in 12.5% and 10.2% of patients, respectively, which is also relatively higher than these findings. This inconsistency can be ascribed to different reasons. First, there are several differences between the eligibility criteria and those used in the study by Cheung et al. 16 They excluded the asymptomatic cases of COVID‐19, while the patients with a variety of manifestations from asymptomatic to severely symptomatic were included in this study, to reduce the risk of bias. Moreover, to reduce the chance of bias, the studies that were conducted exclusively on specific populations, for example, studies that only included critically ill patients or fatal cases were excluded. As reported by Cheung et al, 16 patients with severe COVID‐19 are more likely to present with gastrointestinal symptoms and show higher prevalence of these symptoms compared with nonsevere cases. Therefore, excluding the studies that only reported severe cases might be the reason behind these lower prevalence estimations. Second, the data regarding preexisting conditions were reported and calculated the prevalence of different findings in absence of these comorbidities, while Cheung et al 16 did not consider preexisting diseases that can affect the prevalence of gastrointestinal symptoms. Finally, it could be due to the difference in the methodology of studies; for instance, nausea and vomiting separately were reported, while Cheung et al 16 merged them into one single symptom. In contrast with the study by Cheung et al, 16 a recent review of the literature on the prevalence of diarrhea in about 2500 patients with COVID‐19 showed an overall prevalence of 5.8%, which is more comparable with the findings of the present study. 102
Different studies have reported varying rates regarding the prevalence of liver injury in COVID‐19, between 15% and 78%. However, similar to these findings, most studies have reported slight elevation of AST, ALT, and bilirubin levels as common findings in COVID‐19. 103 The inconsistency in the prevalence of hepatic findings between this review and the previous ones can be attributed to different inclusion and exclusion criteria, as stated before regarding the gastrointestinal findings. The studies that reported high frequency of abnormal liver function are mainly conducted only on severe or fatal cases of COVID‐19. 104 As reported in a recent meta‐analysis, liver injury is associated with high severity of disease in patients with COVID‐19, 105 which was the case in the results of our subgroup analysis according to disease severity. The results regarding the prevalence of abnormal liver function are comparable to a previous meta‐analysis in terms of elevations in ALT, AST, and bilirubin levels. However, Rodriguez‐Morales et al 106 reported a prevalence of 75.8% for decreased albumin that is in contrast with the findings of current review. The reason behind this inconsistency probably lies in the limited number of studies that reported this finding in patients with COVID‐19. Seven studies reporting decreased albumin were included in this meta‐analysis, only two of which were reported in the above mentioned study. Of course, further studies with large sample sizes would provide more accurate estimates. Furthermore, other hepatic biomarkers including gamma glutamyl transferase, international normalized ratio, and direct bilirubin levels were reported in few studies and thus they were not incorporated in the analyses. Further studies are recommended to assess these markers as well, to provide more insight into the extent of liver injury in COVID‐19.
The present study provides several important insights into gastrointestinal and hepatic manifestations of COVID‐19, which can be of interest especially for clinicians and epidemiologists to obtain a clear overview of the prevalence and characteristics of these findings. As reported previously, gastrointestinal symptoms can precede other commonly reported symptoms of fever and respiratory abnormalities, 107 which can delay the diagnosis. 18 Therefore, every clinician should be vigilant when facing a patient presenting these symptoms, particularly in the highly infected regions. Precautionary measures and possible evaluation of such patients for COVID‐19 is recommended.
This study had some limitations. For one thing, there are currently limited large‐scale studies available on patients with COVID‐19 outside of China and most of the included studies are from a limited geographical area. Moreover, gastrointestinal symptoms might have been underreported in some of the included studies, which can affect the prevalence of some findings. Although disease severity was incorporated into this meta‐analysis, the results can be subject to bias as the literature is too heterogeneous in this regard and studies have used different criteria for severity.
In conclusion, gastrointestinal and hepatic presentations are not rare in COVID‐19. The prevalence of these manifestations in patients with COVID‐19 might be affected by preexisting comorbidities. However, diarrhea and mild elevation of liver enzymes are ostensibly the most common gastrointestinal and hepatic features of COVID‐19, in the absence of preexisting diseases.
AUTHOR CONTRIBUTIONS
MZB, AZ, MK, MA, and AG contributed to the design and conceptualization of the study. MZB, AA, AZ, SA, and AG contributed in designing the search strategy and literature search. Data extraction was conducted by MZB, MR, HG, AA, and MSG. Data entry and statistical analysis were performed by SA and MZB. Critical appraisal was done by MZB, MR, HG, AA, MK, and MSG. AZ and MR contributed to drafting and writing the manuscript. All the authors reviewed and contributed in editing the manuscript.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Supporting information
Supporting information
ACKNOWLEDGMENTS
We authors would like to thank Dr Sajjad Sahab Negah and the Student Research Committee of Mashhad University of Medical Sciences for their cooperation and support.
Zarifian A, Zamiri Bidary M, Arekhi S, et al. Gastrointestinal and hepatic abnormalities in patients with confirmed COVID‐19: A systematic review and meta‐analysis. J Med Virol. 2021;93:336–350. 10.1002/jmv.26314
DATA AVAILABILITY STATEMENT
Data available on request from the authors
REFERENCES
- 1. Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS‐CoV‐2 possible? Lancet Gastroenterol Hepatol. 2020;5(4):335‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adhikari SP, Meng S, Wu YJ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID‐19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong SH, Lui RN, Sung JJ. Covid‐19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744‐748. [DOI] [PubMed] [Google Scholar]
- 5. WHO. World Health Organization Coronavirus disease (COVID‐19) Situation Dashboard. https://who.sprinklr.com/. Accessed April 14, 2020.
- 6. WHO . Rolling updates on coronavirus disease (COVID‐19). 2020; https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Accessed March 20, 2020.
- 7. WHO . Coronavirus disease 2019 (COVID‐19) Situation Report – 159. 2020; https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200627-covid-19-sitrep-159.pdf?sfvrsn=93e027f6_2. Accessed June 27, 2020.
- 8.World Health Organization. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019‐nCoV); 2020.
- 9. Gu J, Han B, Wang J. COVID‐19: Gastrointestinal manifestations and potential fecal‐oral transmission. Gastroenterology. 2020;158:1518‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao QY, Chen YX, Fang JY. 2019 Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21:125‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liang W, Feng Z, Rao S, et al. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69(6):1141‐1143. [DOI] [PubMed] [Google Scholar]
- 12. Wong SH, Lui RN, Sung JJ. Covid‐19 and the digestive system. J Gastroenterol Hepatol. 2020;35(5):744‐748. [DOI] [PubMed] [Google Scholar]
- 13. Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;133:9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Front Med. 2020;14:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lo IL, Lio CF, Cheong HH, et al. Evaluation of SARS‐CoV‐2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID‐19 in Macau. Int J Biol Sci. 2020;16(10):1698‐1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheung KS, Hung IF, Chan PP, et al. Gastrointestinal manifestations of SARS‐CoV‐2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta‐analysis [published online ahead of print April 03, 2020]. Gastroenterology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS‐CoV‐2 infection: a single arm meta‐analysis. J Med Virol. 2020:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mao R, Qiu Y, He J‐S, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID‐19: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected (2020). URL https://www/who/int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed 5 May, 2020.
- 20.Centers for Disease Control and Prevention. Chinese Center for Disease Control and Prevention Technical Guidance for Prevention and Control of COVID‐19 Audio and Video Training Courseware www.chinacdc.cn/en/COVID19/202003/P020200323390496137554.pdf. Accessed April 11, 2020.
- 21.Centers for Disease Control and Prevention. Interim Guidance on Management of Coronavirus Disease 2019 (COVID‐19) in Correctional and Detention Facilities. https://www.cdc.gov/coronavirus/2019-ncov/community/correction-detention/guidance-correctional-detention.html. Accessed April 11, 2020.
- 22. Weierink L, Vermeulen RJ, Boyd RN. Brain structure and executive functions in children with cerebral palsy: a systematic review. Res Dev Disabil. 2013;34(5):1678‐1688. [DOI] [PubMed] [Google Scholar]
- 23. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sterne JA, Egger M. Regression methods to detect publication and other bias in meta‐analysis. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication bias in meta‐analysis: Prevention, assessment and adjustments. 1st ed. Chichester, England: Wiley; 2005:99‐110. [Google Scholar]
- 25. Higgins J, Thompson S, Deeks J, Altman D. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy. 2002;7(1):51‐61. [DOI] [PubMed] [Google Scholar]
- 26. Bai T, Tu S, Wei Y, et al. Clinical and laboratory factors predicting the prognosis of patients with COVID‐19: an analysis of 127 patients in Wuhan, China [published online ahead of print March 05, 2020]. SSRN. 2020. [Google Scholar]
- 27. Cai Q, Huang D, Ou P. COVID‐19 in a designated infectious diseases hospital outside Hubei Province, China [published online ahead of print April 02, 2020]. Allergy. 2020. [DOI] [PubMed] [Google Scholar]
- 28. Chen D, Li X, Song Q, et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Netw Open. 2020;3(6):e2011122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen J, Qi T, Liu L, et al. Clinical progression of patients with COVID‐19 in Shanghai, China. J Infect. 2020;80(5):e1‐e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen L, Liu HG, Liu W, et al. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):203‐208. [DOI] [PubMed] [Google Scholar]
- 32. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen S, Prettner K, Kuhn M, et al. Caution: clinical characteristics of COVID‐19 patients are changing at admission [published online ahead of print March 06, 2020]. medRxiv. 2020. [Google Scholar]
- 34. Cheng JL, Huang C, Zhang GJ, et al. [Epidemiological characteristics of novel coronavirus pneumonia in Henan]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(0):E027. [DOI] [PubMed] [Google Scholar]
- 35. Fan Z, Chen L, Li J, et al. Clinical features of COVID‐19‐related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18(7):1561‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Feng Z, Yu Q, Yao S, et al. Early prediction of disease progression in 2019 novel coronavirus pneumonia patients outside Wuhan with CT and clinical characteristics [published online ahead of print February 23, 2020]. medRxiv. 2020. [Google Scholar]
- 37. Han L, Dinga R, Hahn T, et al. Analysis on the clinical characteristics of 36 cases of novel coronavirus pneumonia in Kunming [published online ahead of print March 01, 2020]. medRxiv. 2020. [Google Scholar]
- 38. Fu L, Fei J, Xiang H, et al. Influence factors of death risk among COVID‐19 patients in Wuhan, China: a hospital‐based case‐cohort study [published online ahead of print March 16, 2020]. medRxiv. 2020. [Google Scholar]
- 39. Gong J, Ou J, Qiu X, et al. A tool for early prediction of severe coronavirus disease 2019 (COVID‐19): a multicenter study using the risk Nomogram in Wuhan and Guangdong, China [published online ahead of print April 16, 2020]. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Han R, Huang L, Jiang H, Dong J, Peng H, Zhang D. Early clinical and CT manifestations of coronavirus disease 2019 (COVID‐19) pneumonia. AJR Am J Roentgenol. 2020:1‐6. [DOI] [PubMed] [Google Scholar]
- 42. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang M, Zhan F, Wang J, et al. Epidemiological and clinical features of 197 patients infected with 2019 novel coronavirus in Chongqing.China: A single center descriptive study [published online ahead of print February 19, 2020]. SSRN. 2020. [Google Scholar]
- 44. Jin J‐M, Bai P, He W, et al. Gender differences in patients with COVID‐19: focus on severity and mortality. Front Public Health. 2020;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jin X, Lian JS, Hu JH. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus‐infected disease 2019 (COVID‐19) with gastrointestinal symptoms [published online ahead of print February 19, 2020]. Gut. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jin X, Xu K, Jiang P, et al. Virus strain from a mild COVID‐19 patient in Hangzhou represents a new trend in SARS‐CoV‐2 evolution potentially related to Furin cleavage site. Emerg Microb Infect. 2020:1‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kong I, Park Y, Woo Y, et al. Early epidemiological and clinical characteristics of 28 cases of coronavirus disease in South Korea. Osong Public Health Res Perspect. 2020;1:8‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kuang Y, Zhang H, Zhou R, et al. Epidemiological and clinical characteristics of 944 cases of 2019 novel Coronavirus infection of non‐COVID‐19 exporting city, Zhejiang, China [published online ahead of print April 18, 2020]. SSRN. 2020. [Google Scholar]
- 49. Lei Z, Cao H, Jie Y, et al. A cross‐sectional comparison of epidemiological and clinical features of patients with coronavirus disease (COVID‐19) in Wuhan and outside Wuhan, China. Travel Med Infect Dis. 2020;35:101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li J, Li S, Cai Y, et al. Epidemiological and clinical characteristics of 17 hospitalized patients with 2019 novel coronavirus infections outside Wuhan, China. medRxiv. 2020;201:540‐554. [Google Scholar]
- 51. Li L, Li S, Xu M, et al. Risk factors related to hepatic injury in patients with corona virus disease 2019 [published online ahead of print March 10, 2020]. medRxiv. 2020. [Google Scholar]
- 52. Li YY, Wang WN, Lei Y, et al. [Comparison of the clinical characteristics between RNA positive and negative patients clinically diagnosed with 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(0):E023. [DOI] [PubMed] [Google Scholar]
- 53. Liang D, Shi L, Zhao J, et al. Prevalence and clinical features of 2019 novel coronavirus disease (COVID‐19) in the Fever Clinic of a teaching hospital in Beijing: a single‐center, retrospective study [published online ahead of print February 28, 2020]. medRxiv. 2020. [Google Scholar]
- 54. Liu C, Jiang ZC, Shao CX, et al. [Preliminary study of the relationship between novel coronavirus pneumonia and liver function damage: a multicenter study]. Zhonghua gan zang bing za zhi = Chin J Hepatol. 2020;28(2):148‐152. [DOI] [PubMed] [Google Scholar]
- 55. Liu F, Xu A, Zhang Y, et al. Patients of COVID‐19 may benefit from sustained lopinavir‐combined regimen and the increase of eosinophil may predict the outcome of COVID‐19 progression. Int J Infect Dis. 2020;95:183‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133:1025‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu W, Wang F, Li G, et al. Analysis of 2019 novel coronavirus infection and clinical characteristics of outpatients: an epidemiological study from the fever clinic in Wuhan, China [published online ahead of print February 20, 2020]. SSRN. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Luo S, Zhang X, Xu H. Don't overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID‐19). Clin Gastroenterol Hepatol. 2020;18(7):1637‐1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Miao C, Zhuang J, Jin M, et al. A comparative multi‐centre study on the clinical and imaging features of comfirmed and uncomfirmed patients with COVID‐19 [published online ahead of print March 24, 2020]. medRxiv. 2020. [Google Scholar]
- 61. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China [published online ahead of print March 16, 2020]. Clin Infect Dis. 2020. [Google Scholar]
- 62. Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: a descriptive, cross‐sectional, multicenter study [published online ahead of print April 14, 2020]. Am J Gastroenterol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Song F, Shi N, Shan F, et al. Emerging 2019 novel coronavirus (2019‐nCoV) pneumonia. Radiology. 2020;295(1):210‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sun WW, Ling F, Pan JR, et al. Epidemiological characteristics of 2019 novel coronavirus family clustering in Zhejiang Province. Zhonghua yu fang yi xue za zhi [Chin J Prevent Med]. 2020;54:E027. [DOI] [PubMed] [Google Scholar]
- 66. Sun Y, Koh V, Marimuthu K, et al. Epidemiological and clinical predictors of COVID‐19 [published online ahead of print March 25, 2020]. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tang X, Du R, Wang R, et al. Comparison of hospitalized patients with acute respiratory distress syndrome caused by COVID‐19 and H1N1. Chest. 2020;158(1):195‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wan S, Xiang Y, Fang W. Clinical features and treatment of COVID‐19 patients in Northeast Chongqing. J Med Virol. 2020;92(7):796‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wei X‐S, Wang X, Niu Y‐R, et al. Diarrhea is associated with prolonged symptoms and viral carriage in corona virus disease 2019. Clin Gastroenterol Hepatol. 2020;18(8):1753‐1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wen Y, Wei L, Li Y, et al. Epidemiological and clinical characteristics of COVID‐19 in Shenzhen, the largest migrant city of China [published online ahead of print March 23, 2020]. medRxiv. 2020. [Google Scholar]
- 72. Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of COVID‐19 in Jiangsu Province: a multicenter descriptive study [published online ahead of print February 29, 2020]. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Xu T, Chen C, Zhu Z, et al. Clinical features and dynamics of viral load in imported and non‐imported patients with COVID‐19. Int J Infect Dis. 2020;94:68‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xu W, Qu S, Xing M, et al. Epidemiologic features and clinical findings of COVID‐19‐infected patients in Suzhou [published online ahead of print March 18, 2020]. SSRN. 2020. [Google Scholar]
- 75. Fang Y, Zhang H, Xu Y, Xie J, Pang P, Ji WCT. Manifestations of two cases of 2019 novel coronavirus (2019‐nCoV) pneumonia. Radiology. 2020;295(1):208‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Xu YH, Dong JH, An WM, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS‐CoV‐2. J Infect. 2020;80(4):394‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yang W, Cao Q, Qin L, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID‐19): a multi‐center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80(4):388‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yao N, Wang SN, Lian JQ, et al. [Clinical characteristics and influencing factors of patients with novel coronavirus pneumonia combined with liver injury in Shaanxi region]. Zhonghua gan zang bing za zhi = Chin J Hepatol. 2020;28(0):E003. [DOI] [PubMed] [Google Scholar]
- 79. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS‐CoV‐2 in Singapore. JAMA. 2020;323(15):1488‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yu F, Yan L, Wang N. Quantitative detection and viral load analysis of SARS‐CoV‐2 in infected patients [published online ahead of print March 28, 2020]. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang G, Hu C, Luo L, et al. Clinical features and short‐term outcomes of 221 patients with COVID‐19 in Wuhan, China. J Clin Virol. 2020;127:104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75(7):1730‐1741. [DOI] [PubMed] [Google Scholar]
- 83. Zhang MQ, Wang XH, Chen YL, et al. Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing. Zhonghua jie he he hu xi za zhi= Chin J Tuberculosis Respir Dis. 2020;43(3):215‐218. [DOI] [PubMed] [Google Scholar]
- 84. Zhang X, Cai H, Hu J, et al. Epidemiological, clinical characteristics of cases of SARS‐CoV‐2 infection with abnormal imaging findings [published online ahead of print March 20, 2020]. J Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID‐19 patients: a retrospective analysis of 115 cases from a single center in Wuhan city, China. Liver Int. 2020;9:283‐289. [DOI] [PubMed] [Google Scholar]
- 86. Zhang Y. Gastrointestinal tract symptoms in coronavirus disease 2019: analysis of clinical symptoms in adult patients [published online ahead of print March 27, 2020]. medRxiv. 2020. [Google Scholar]
- 87. Zhao D, Yao F, Wang L. A comparative study on the clinical features of COVID‐19 pneumonia to other pneumonias [published online ahead of print March 12, 2020]. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID‐19) pneumonia: a multicenter study. Am J Roentgenol. 2020;214:1‐6. [DOI] [PubMed] [Google Scholar]
- 89. Zhao W, Yu S, Zha X, et al. Clinical characteristics and durations of hospitalized patients with COVID‐19 in Beijing: a retrospective cohort study [published online ahead of print March 30, 2020]. medRxiv. 2020. [Google Scholar]
- 90. Zhao Z, Xie J, Yin M, et al. Clinical and laboratory profiles of 75 hospitalized patients with novel coronavirus disease 2019 in Hefei, China [published online ahead of print March 06, 2020]. medRxiv. 2020. [Google Scholar]
- 91. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study [published online ahead of print March 11, 2020]. Lancet. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.World Health Organization. Responding to community spread of COVID‐19: interim guidance, March 7, 2020.
- 93.World Health Organization. Critical preparedness, readiness and response actions for COVID‐19: interim guidance, March 22, 2020.
- 94. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention [published online ahead of print Febriary 24, 2020]. JAMA. 2020. [DOI] [PubMed] [Google Scholar]
- 95. Meo SA, Alhowikan AM, Al‐Khlaiwi T, et al. Novel coronavirus 2019‐nCoV: prevalence, biological and clinical characteristics comparison with SARS‐CoV and MERS‐CoV. Eur Rev Med Pharmacol Sci. 2020;24(4):2012‐2019. [DOI] [PubMed] [Google Scholar]
- 96. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID‐19) infection: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Assiri A, Al‐Tawfiq JA, Al‐Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. World Health Organization . WHO issues consensus document on the epidemiology of SARS. Wkly Epidemiol Rec. 2003;78(43):373‐375. [PubMed] [Google Scholar]
- 99. Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64‐68. [DOI] [PubMed] [Google Scholar]
- 100. Cai J, Xu J, Lin D, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features [published online ahead of print February 28, 2020]. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li X‐Y, Dai W‐J, Wu S‐N, Yang X‐Z, Wang H‐G. The occurrence of diarrhea in COVID‐19 patients. Clin Res Hepatol Gastroenterol. 2020;44:284‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Musa S. Hepatic and gastrointestinal involvement in coronavirus disease 2019 (COVID‐19): what do we know till now? Arab J Gastroenterol. 2020;21:3‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhang B, Zhou X, Qiu Y, et al. Clinical characteristics of 82 death cases with COVID‐19 [published online ahead of print February 27, 2020]. medRxiv. 2020. [Google Scholar]
- 105. Parohan M, Yaghoubi S, Seraj A. Liver injury is associated with severe Coronavirus disease 2019 (COVID‐19) infection: a systematic review and meta‐analysis of retrospective studies [published online ahead of print May 09, 2020]. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutiérrez‐Ocampo E, et al. Clinical, laboratory and imaging features of COVID‐19: A systematic review and meta‐analysis. Travel Med Infect Dis. 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. An P, Chen H, Jiang X. Clinical features of 2019 novel coronavirus pneumonia presented gastrointestinal symptoms but without fever onset [published online ahead of print January 24, 2020]. SSRN. 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
Data available on request from the authors


