Abstract
Introduction
Prognostic factors are needed to aid clinicians in managing Covid‐19, a respiratory illness. Lymphocytopenia has emerged as a simply obtained laboratory value that may correlate with prognosis.
Methods
In this article, we perform a retrospective cohort review study on patients admitted to one academic hospital for Covid‐19 illness. We analyzed basic demographic, clinical, and laboratory data to understand the relationship between lymphocytopenia at the time of hospital admission and clinical outcomes.
Results
We discovered that lymphocyte count is lower (P = .01) and lymphocytopenia more frequent by an odds ratio of 3.40 (95% CI: 1.06‐10.96; P = .04) in patients admitted to the Intensive Care Unit (ICU), a marker of disease severity, relative to those who were not. We additionally find that patients with lymphocytopenia were more likely to develop an acute kidney injury (AKI), a marker of organ failure, during admission by an odds ratio of 4.29 (95% CI: 1.35‐13.57; P = .01).
Conclusion
This evidence supports the hypothesis that lymphocytopenia can be an early, useful, and easily obtained, prognostic factor in determining the clinical course and disease severity of a patient admitted to the hospital for Covid‐19.
Keywords: acute kidney injury, covid‐19, lymphopenia, prognosis, SARS‐CoV‐2 infection
1. INTRODUCTION
Coronavirus disease 2019 (Covid‐19) is a predominantly respiratory illness caused by the SARS‐CoV‐2 virus. Data regarding prognostic factors are currently scarce given the novelty of the disease. Prognostic information would aid clinicians in managing patients, who are often left without data‐driven guidelines to make important clinical decisions. It is known that lymphocytopenia, defined as an absolute lymphocyte count (ALC) < 1000 cells/µL, occurs in Covid‐19 and may correlate with increased disease severity 1 , 2 , 3 , 4 , 5 ; indeed, lymphocytopenia is a common systemic manifestation of many viral illnesses 6 ; in particular, other coronaviruses like Severe Acute Respiratory Syndrome coronavirus (SARS‐CoV) and Middle Eastern Respiratory Syndrome coronavirus (MERS‐CoV) have been demonstrated to cause lymphocytopenia. 2 However, few studies have examined whether lymphocytopenia found at the time of admission to the hospital is helpful in understanding the disease course. Here, we set out to study a cohort of patients admitted to the hospital diagnosed with Covid‐19 to determine whether lymphocytopenia, found at the time of admission to the hospital, was associated with disease severity and other clinical outcomes.
2. MATERIALS AND METHODS
Data were obtained for patients admitted to one local, academic, community‐based hospital in Houston, TX, USA. IRB approval was granted by the University of Texas Health Science Center at Houston, Houston, TX, USA. Patients were included if they had a positive diagnosis of Covid‐19 based on a polymerase chain reaction‐based assay to detect the SARS‐CoV‐2 virus or had been diagnosed in the community, were over the age of 18, and were admitted and discharged from the hospital between 03/01/2020 and 05/07/2020. Data were collected and extracted from an electronic medical record system and included many variables, such as demographic, clinical outcomes, and laboratory data. We define severe disease as those patients who required admission to the ICU; non‐severe disease is classified as those admitted to the hospital, but did not require ICU admission. Admission to the ICU was determined by clinical factors, namely respiratory failure and hemodynamic instability. Lymphocytopenia was not part of these criteria. Acute Kidney Injury (AKI) is defined as a rise in serum creatinine > 0.3 mg/dL from baseline within 48 hours at any time during admission (if baseline data were unavailable, the lowest value during admission was presumed to be the baseline; if only one value was available, the patient was not presumed to have an AKI).
2.1. Laboratory data
All laboratory data were collected within 24 hours of admission. Laboratory data were analyzed by our hospital's hematology laboratory. All laboratory samples are typically processed within hour of receipt. Complete blood counts (CBCs) were measured on automated CBC and differential analyzer (X‐N 3000), if the XN3000 could not classify a WBC an attached module (SP10) automatically made the blood smear slides. This blood smear side was manually loaded onto a cell locator imaging (DM96). Technician classified WBC with differential, if technician had difficulty in interpreting results; the pathologist reviewed the slide. Quality control materials were run every 8 hours. Lymphocytopenia is defined as an absolute lymphocyte count (ALC) < 1.0 × 103 cells/µL. Anemia is defined as hemoglobin < 14 gm/dL for men or < 12 gm/dL for women. Thrombocytopenia is defined as platelet count < 150.0 × 103 cells/µL. Leukopenia is defined as leukocyte count < 4.4 × 103 cells/µL. Leukocytosis is defined as a leukocyte count > 11.0 × 103 cells/µL.
2.2. Data analysis
For statistical analyses, the computer program R and accompanying R studio (version 1.2.5033, Orange Blossom) were used to perform analyses. 7 , 8 R is an open source statistical software program widely used in the academic research community. For continuous variables, a Welch's two‐sided t test was performed, assuming variances were unequal between samples. To correct for multiple comparisons, a Bonferroni test was performed to adjust P‐values. For categorical data, a Fisher Exact test was used to make comparisons. For all analyses, a P‐value < .05 was used to reject the null hypothesis that either there was no difference between two samples tested or that samples were independent. Odds ratios and confidence intervals were calculated using the package epiR 9 in the R‐studio software. Code is available upon request. Figures were prepared using the ggplot2 10 software in the R software platform.
3. RESULTS
3.1. Basic population demographics
We obtained a cohort of 57 patients who were admitted to and discharged from the hospital between 03/01/2020 and 05/01/2020. The cohort was predominantly male (59%), obese (average BMI of 32.3 ± 1.19 kg/m2) with an average age of 58.2 ± 2.08 years. Our cohort consisted mostly of patients with minority backgrounds (86%). Thirty‐one percent of patients (N = 18) were admitted to the ICU and mortality was 16% (N = 9). Of note, two patients had a diagnosis of Human Immunodeficiency Virus (HIV) infection. The median Charlson comorbidity index was 4 (1.5‐6), indicating an median 10‐year survival rate of 53%. 11 In our study, we found that 50% (9/18) of patients admitted to the ICU required intubation and 38% (7/18) required vasopressors (Table 3). Thus, patients admitted to the ICU were classified as having severe disease given the relatively common occurrence of hemodynamic instability and respiratory failure in this population.
TABLE 3.
The relationship between lymphocytopenia and clinical outcomes
| ALC < 1000, Count (%) | ALC > 1000, Count (%) | Odds Ratio, OR (95% CI) | Significance | |
|---|---|---|---|---|
| Need for intubation in the ICU | 6 (54), N = 11 | 3 (43), N = 7 | — | P = 1.0 |
| ICU stay > 7 d | 6 (54), N = 11 | 3 (43), N = 7 | — | P = 1.0 |
| Need for vasopressors in the ICU | 6 (54), N = 11 | 2 (28), N = 7 | — | P = .36 |
| Developed AKI during admission | 15 (68), N = 22 | 11 (33), N = 33 | 4.29 (1.35‐13.57) | *P = .01 |
| Mortality | 6 (26), N = 23 | 3 (9), N = 33 | — | P = .13 |
Variables are reported as counts (percentage of N); N = total sample size, listed adjacent to the data point. Significance was determined with a Fisher's Exact test. An asterisk indicates a statistically significant result.
Abbreviations: AKI, Acute Kidney Injury; ALC, Absolute Lymphocyte Count; CI, confidence interval; ICU, Intensive Care Unit.
3.2. Lymphocytopenia at the time of admission is related to disease severity in Covid‐19
The average ALC count obtained at the time of admission to the hospital in patients requiring ICU admission was lower (0.8 ± 0.11 × 103 cells/µL) relative to those not needing ICU admission (1.4 ± 0.15 × 103 cells/µL; P = .01; Table 1, Figure 1). Additionally, more patients admitted to the ICU had lymphocytopenia (62%) at the time of admission to the hospital compared to those not admitted to the ICU (32%; P = .04; Table 2). Interestingly, the presence of lymphocytopenia conferred an odds ratio of 3.40 (95% CI: 1.06‐10.96) for admission to the ICU.
TABLE 1.
Basic hematological laboratory values collected at the time of admission to the hospital of patients based on the need for ICU admission at any time during hospitalization
| Non‐ICU | ICU | Significance | |
|---|---|---|---|
| Sample size, N | 39 | 18 | — |
| ALC | 1.4 ± 0.15 | 0.8 ± 0.11 | *P = .01 |
| Hemoglobin | 12.9 ± 0.37 | 11.3 ± 0.54 | P = .08 |
| Hematocrit | 38.5 ± 1.38 | 35.4 ± 1.30 | P = .55 |
| Platelet count | 202.8 ± 11.84 | 274.7 ± 20.22 | *P = .02 |
| WBC | 8.8 ± 0.73 | 10.4 ± 1.13 | P = 1.0 |
Data are expressed as mean ± SEM. Welch's two‐sample t test with a Bonferroni post hoc correction for multiple comparisons was used to compare groups. An asterisk indicates a statistically significant result.
Abbreviations: ALC, Absolute Lymphocyte Count; ICU, Intensive Care Unit; WBC, White Blood Cell count.
FIGURE 1.
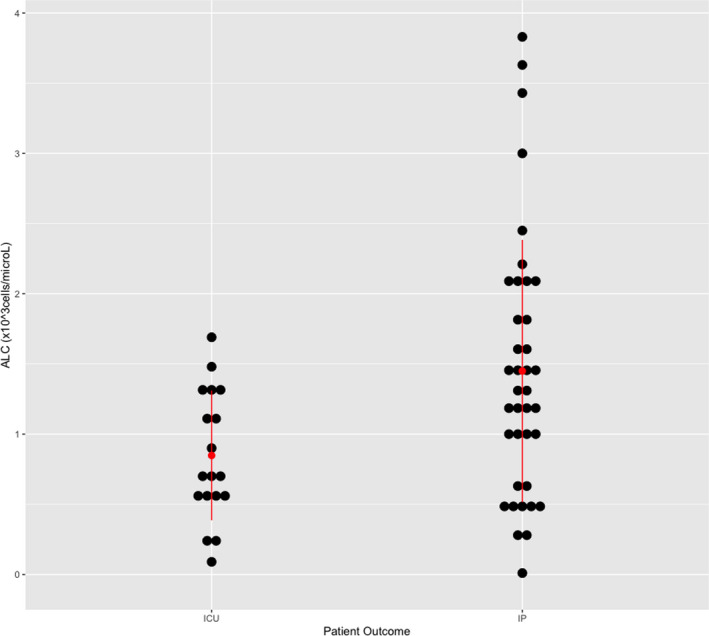
Scatter plot for Absolute Lymphocyte Count (ALC)‐based ICU admission status (patient outcome). Red bars represent standard deviation, and red dot represents the mean. Data points are represented by black dots. Abbreviations: ICU, patients admitted to the Intensive Care Unit; IP, patients admitted to the In‐Patient unit, not the ICU
TABLE 2.
Frequency of laboratory results and corresponding odds ratios based on ICU admission status
|
Non‐ICU No (%) |
ICU No (%) |
Odds ratio, OR (95% CI) | Significance | |
|---|---|---|---|---|
| Sample Size | 39 | 18 | — | |
| Lymphocytopenia Frequency | 12 (32), N = 38 | 11 (61) | 3.40 (1.06‐10.96) | *P = .04 |
| Anemia Frequency | 19 (49) | 14 (78) | 3.68 (1.03‐13.20) | *P = .04 |
| Thrombocytopenia Frequency | 6 (15) | 1 (5) | 0.32 (0.04‐2.91) | P = .41 |
| Leukopenia Frequency | 5 (13) | 0 | — | — |
| Leukocytosis Frequency | 9 (23) | 6 (33) | 1.67 (0.49‐5.71) | P = .5 |
Raw count data are presented as the count (% of total sample size). Anemia, thrombocytopenia, lymphocytopenia, and leukopenia are defined in the methods. Fisher's Exact test was performed to compare groups above. Odds ratio is reported as OR (95% CI). Sample sizes are listed as above, unless otherwise stated, with the value N adjacent to the data point. An asterisk indicates a statistically significant result.
Abbreviation: ICU, Intensive Care Unit.
3.3. Anemia and platelet count at the time of admission are related to disease severity in Covid‐19
We found that patients admitted to the ICU were more likely to have anemia (78% of ICU patients versus 49% of non‐ICU patients, P = .04; Table 2) than those that were not and there was a non‐significant trend toward a lower hemoglobin concentration in ICU patients (Table 1). Additionally, platelet count was found to be higher in patients admitted to the ICU versus those who were not (P = .02, Table 1); however, there was no difference in the number of patients in or outside of the ICU with thrombocytopenia (Table 2). No other significant differences in hematocrit or leukocyte count (leukopenia or leukocytosis) were found.
3.4. Lymphocytopenia is related to the development of AKI
To see if lymphocytopenia would correlate with clinical outcomes, we examined the relationship of lymphocytopenia with multiple clinical factors (Table 3). We found that patients with lymphocytopenia more commonly developed an AKI during hospital admission (68% with ALC < 1000 cells/µL vs 33% with ALC > 1000 cells/µL; P = .01); an odds ratio was computed and found to be 4.29 (95% CI: 1.35‐13.57; P = .01). Other variables analyzed, including need for intubation, need for vasopressors, ICU stay lasting more than (>) 7 days and mortality all showed a trend favoring predominance in patients with lymphocytopenia; however, none reached statistical significance.
4. DISCUSSION
Given the novelty of Covid‐19, prognostic markers are needed to better understand the clinical course for patients admitted to the hospital. Other groups have found evidence that lymphocytopenia may correlate with disease severity, including one recently published meta‐analysis. 1 , 2 , 5 , 12 However, most of these data have been obtained from patients in China and thus, data are lacking regarding trends in other parts of the world. In this community hospital‐based study, we find evidence of a relationship between lymphocytopenia and disease severity. Patients with Covid‐19 admitted to the ICU were more likely to have lymphocytopenia at the time of hospital admission compared to patients that did not get admitted to the ICU. We additionally find that the odds of getting admitted to an ICU for a patient with lymphocytopenia or anemia on admission is approximately 3.4 and 3.6, respectively, similar to what has been found regarding lymphocytopenia in a recent meta‐analysis. 12
In this study, we focused on admission laboratory values, given that these values are often the first to shed light on disease severity. However, other groups have found that patients with persistent lymphocytopenia during the Covid‐19 disease course may be more likely to have severe outcomes. 13 This is interesting, given that persistent lymphocytopenia is associated with poor outcomes after diagnosis with sepsis 14 ; Drewery et al (2014) hypothesized that this could be due to an anti‐inflammatory response in later stages of sepsis. Thus, it is possible that lymphocytopenia causes an immune‐suppressive environment, causing more profound illness in Covid‐19, necessitating ICU admission. Future studies should examine the course of pro‐ and anti‐inflammatory markers and lymphocytopenia in Covid‐19 patients to see whether there are different phenotypes that result. Additionally, prospective trials are needed to evaluate the predictive ability of lymphocyte count in determining disease severity.
Regarding clinical outcomes, it is interesting that patients that developed an AKI at any time during admission seemed to be more common in patients with than those without lymphocytopenia in Covid‐19. Recently, SARS‐CoV‐2 viral load was detected in human kidney samples, 15 thus confirming that this virus can directly infect renal tissue. Additionally, it has been reported that mortality is higher in patients with Covid‐19 and AKI compared to those without AKI. 16 Although lymphocytopenia was not seen to be associated with mortality in this study, the finding of an association with AKI warrants further research. Nevertheless, it is apparent here that lymphocytopenia may serve as a prognostic marker for AKI in Covid‐19 patients.
Finally, although lymphocytopenia was not found to be statistically related to other clinical outcomes, such as mortality and need for intubation, the trend was largely in favor of a correlation; our sample size was likely small to demonstrate this association. Indeed, mortality is higher in patients with lower lymphocyte counts, 16 thus confirming the trends observed here. Future studies should address ICU outcomes such as the need for intubation based on lymphocytopenia with appropriate statistical power to validate these findings.
The limitations of this study include smaller sample size and focus on one community hospital. Additionally, other leukocyte subtypes such as eosinophil and neutrophil counts were not examined in this study. The strengths of this study include the use of an easily obtained laboratory value that is associated with clinical outcomes and a focus on a predominantly minority population of patients who seem to be heavily impacted by Covid‐19. In summary, we find that lymphocytopenia and anemia are more common in patients admitted to the ICU, with an odds ratio of approximately 3.4 and 3.6, respectively. Additionally, patients with lymphocytopenia are more likely to develop an AKI relative to those without lymphocytopenia by an odds ratio of 4.2. Thus, it appears likely that lymphocytopenia is related to disease severity and clinical outcomes in Covid‐19.
CONFLICTS OF INTEREST
None declared for JW, AD, SL, AF, and BC.
AUTHOR CONTRIBUTIONS
JW and AF were involved in planning the study and collected data. JW, AD, SL, BC, and AF performed data analysis and wrote the manuscript. All authors have approved of the final draft.
Wagner J, DuPont A, Larson S, Cash B, Farooq A. Absolute lymphocyte count is a prognostic marker in Covid‐19: A retrospective cohort review. Int J Lab Hematol. 2020;42:761–765. 10.1111/ijlh.13288
REFERENCES
- 1. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan W‐J, Ni Z‐Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020. https://academic.oup.com/cid/article/doi/10.1093/cid/ciaa272/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Longo DLFA, Kasper D, Hauser S, Jameson JL, Loscalzo J. Harrison's principles of internal medicine, 20 ed. New York, NY: McGraw Hill; 2018. [Google Scholar]
- 7. Team RDC . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 8. RStudio: Integrated Development for R. [computer program] Boston, MA: RStudio Inc; 2015. [Google Scholar]
- 9. EpiR: Tools for the Analysis of Epidemiological Data [computer program]. Version 1.0‐142020.
- 10. Wickham H. ggplot2: elegant graphics for data analysis. New York, NY: Springer‐Verlag. ISBN 978‐3‐319‐24277‐4; 2016. [Google Scholar]
- 11. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 12. Zhao Q, Meng M, Kumar R, et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a systemic review and meta‐analysis. Int J Infect Dis. 2020;96:131‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tan LI, Wang QI, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42(5):383‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS‐CoV‐2. N Engl J Med. 2020. https://www.nejm.org/doi/full/10.1056/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]


