To the Editor,
Coronavirus disease 2019 (COVID‐19) presents a major worldwide public health emergency. Many research efforts are ongoing to find effective antiviral treatments via novel drug design or drug repurposing. 1 One drug, remdesivir, has been shown to have activity against the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) RNA dependent RNA polymerase (RdRp), and has been used clinically in severe COVID‐19 disease, but more accessible and readily available treatments are needed for all stages of infection.
Two recent reports discussed both anecdotal positive clinical benefits and the potential for off‐label use of the drug montelukast in certain patients with COVID‐19, 2 , 3 which led us to investigate its potential anti‐SARS‐CoV‐2 properties in silico. Montelukast had previously been shown to have antiviral activity against ZIKA and dengue viruses, 4 as well as immune modulatory properties. We hypothesized that montelukast might have antiviral activity against SARS‐CoV‐2, and act as an anti‐inflammatory agent effective against exuberant immune activation in COVID‐19 disease. Montelukast had been shown to inhibit macrophage M2 related cytokines, acting as a cysteinyl leukotriene receptor antagonist. 5 It can also protect against Influenza A virus induced pneumonia by reducing infection of type‐1 alveolar epithelial cells and modulating other proinflammatory mediators. 6 In a rat model, montelukast lowered TNF‐alpha and interleukin‐6, increased glutathione and superoxide dismutase 7 and lowered mortality related to sepsis. The cytokine storm in COVID‐19 is at least partially caused by mast cell activation, and leukotriene receptor antagonists like montelukast could also be used for their ability to attenuate mast cell activation. 8
We undertook an in silico molecular docking analysis to simulate binding of montelukast to catalytically active sites within the SARS‐CoV‐2 Main protease (Mpro) and RNA dependent RNA polymerase (RdRp). If montelukast could bind to key residues typically required for the enzymatic activity of these proteins, and effectively inhibit the activity of the Mpro, it should be able to disrupt the substrate binding site. We performed docking simulations using the Mpro (Protein Data Bank [PDB] ID: 6Y2E), and the RdRp (PDB ID: 6M71) of SARS‐Cov‐2, using the Protein‐Ligand ANT System (PLANTS) 9 program. The ligand docking sites were specified as the catalytically active sites by Zhang et al. 10 and Gao et al. 11 The resulting protein‐ligand scores (PLANTS scores) were calculated using the empirical scoring algorithm CHEMPLP, 9 and reflect the energy change when ligands and proteins come together, with values more negative than (−91.00) suggesting likely protein‐ligand interactions. 12 , 13 All other docking parameters and forcefields are noted in our previous work. 13 Protein‐ligand structures were visualized using PyMol 2.3.5. The PLANTSchemplp scores reflects the energy change when montelukast binds to the catalytic site of either the Mpro or RdRp with more negative numbers suggesting a more probable drug‐protein interaction (see Figure 1). As the PLANTSchemplp program employs an empirical scoring function and utilizes a semi‐flexible docking method, the scoring function employs some level of molecular dynamics.
Figure 1.
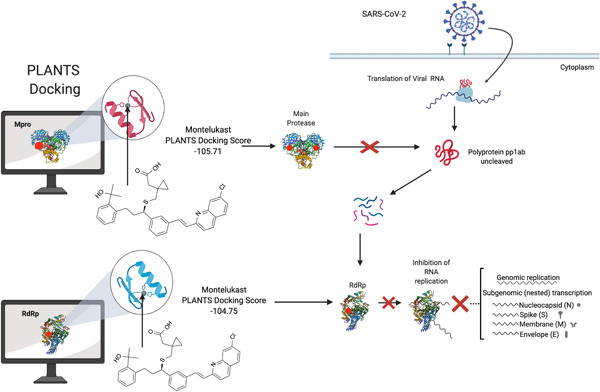
Overall schematic for the methods used in this paper and significant results. Molecules are docked using Protein‐Ligand ANT System (PLANTS) to key residues of the viral enzymes. The red dots indicate the designated catalytic sites for the purpose of this study. The binding and potential inhibition of these enzymes would disrupt the replication machinery of this virus, as shown by the schematic. The image in Figure 1 was created using BioRender
The PLANTSchemplp docking score of montelukast against the Mpro is −105.71, and the RdRp −104.75. These docking scores suggest that montelukast is likely to dock to both the Mpro and the RdRp of SARS‐CoV‐2 (Figure 2). For comparison, the docking score of remdesivir to the SARS‐CoV‐2 RdRp is −102.09. The mechanism of action likely conferred by binding would need to be determined in vitro, but is probably through competitive inhibition at the enzymatic sites. To disrupt the catalytic site of the polymerase it would need to have a lower free energy than the elongating RNA and ribonucleotides at this site. The accumulation of data on the drug montelukast, including the data presented here, it's known antiviral activity 4 and immunomodulation, 5 , 6 , 7 , 8 along with anecdotal evidence in patients with COVID‐19, 2 suggests a repurposing potential for montelukast in the treatment of COVID‐19. We would like to caution readers that, despite the in silico evidence described here, there is no robust evidence yet that montelukast will be an effective treatment for COVID‐19. Montelukast is used for allergic rhinitis and now off‐label for COVID‐19, however physicians are ultimately responsible for prescribing drugs like montelukast. There is a Blackbox warning for the use of montelukast, noting serious mental health side‐effects. However, our studies suggest that further investigation into the role of montelukast in SARS‐CoV‐2 prevention or COVID‐19 amelioration is warranted.
Figure 2.
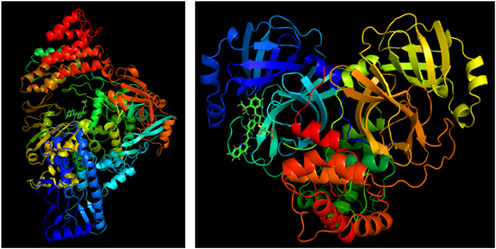
Image of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) RNA dependent RNA polymerase (RdRp) enzyme (left), and Main protease (Mpro) of SARS‐CoV‐2 (right). Montelukast is shown docked to each viral enzyme's catalytic site separately
REFERENCES
- 1. Duarte RRR, Copertino DC Jr., Iñiguez LP, Marston JL, Nixon DF, Powell TR. Repurposing FDA‐approved drugs for COVID‐19 using a data‐driven approach. ChemRxiv. 2020. 10.26434/chemrxiv.12148764.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aiello T. (2020). Though not FDA approved, off‐label singulair showing promise as coronavirus treatment, say doctors. In: CBS New York. https://newyork.cbslocal.com/2020/04/22/coronavirus-covid-19-singulair-montelukast/ [Google Scholar]
- 3. Almerie MQ, Kerrigan DD. The association between obesity and poor outcome after COVID‐19 indicates a potential therapeutic role for montelukast. Med Hypotheses. 2020;143:109883. 10.1016/j.mehy.2020.109883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Y, Li Y, Wang X, Zou P. Montelukast, an anti‐asthmatic drug, inhibits Zika virus infection by disrupting viral integrity. Front Microbiol. 2019;10:3079. 10.3389/fmicb.2019.03079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin YC, Huang MY, Lee MS, et al. Effects of montelukast on M2‐related cytokine and chemokine in M2 macrophages. J Microbiol Immunol Infect. 2018;51(1):18‐26. 10.1016/j.jmii.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 6. Cardani A, Boulton A, Kim TS, Braciale TJ. Alveolar macrophages prevent lethal influenza pneumonia by inhibiting infection of type‐1 alveolar epithelial cells. PLoS Pathog. 2017;13(1):e1006140. 10.1371/journal.ppat.1006140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coskun AK, Yigiter M, Oral A, et al. The effects of montelukast on antioxidant enzymes and proinflammatory cytokines on the heart, liver, lungs, and kidneys in a rat model of cecal ligation and puncture‐induced sepsis. Sci World J. 2011;11:1341‐1356. 10.1100/tsw.2011.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cikler E, Ersoy Y, Cetinel S, Ercan F. The leukotriene d4 receptor antagonist, montelukast, inhibits mast cell degranulation in the dermis induced by water avoidance stress. Acta Histochem. 2009;111(2):112‐118. 10.1016/j.acthis.2008.04.006 [DOI] [PubMed] [Google Scholar]
- 9. Korb O, Stutzle T, Exner TE. Empirical scoring functions for advanced protein‐ligand docking with PLANTS. J Chem Inf Model. 2009;49(1):84‐96. 10.1021/ci800298z [DOI] [PubMed] [Google Scholar]
- 10. Zhang L, Lin D, Sun X, et al. Crystal structure of SARS‐CoV‐2 main protease provides a basis for design of improved alpha‐ketoamide inhibitors. Science. 2020;368:409‐412. 10.1126/science.abb3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao Y, Yan L, Huang Y, et al. Structure of the RNA‐dependent RNA polymerase from COVID‐19 virus. Science. 2020;368:779‐782. 10.1126/science.abb7498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Douguet D, Munier‐Lehmann H, Labesse G, Pochet S. LEA3D: a computer‐aided ligand design for structure‐based drug design. J Med Chem. 2005;48(7):2457‐2468. 10.1021/jm0492296 [DOI] [PubMed] [Google Scholar]
- 13. Copertino DC Jr., Lima B, Duarte R, et al. Antiretroviral drug activity and potential for pre‐exposure prophylaxis against COVID‐19 and HIV infection. ChemRxiv. 2020. 10.26434/chemrxiv.12250199.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]


