Abstract
We aimed to examine independent predictive factors for the severity and survival of COVID‐19 disease, from routine blood parameters, especially the blood urea nitrogen (BUN)/creatinine (Cr) ratio. A total of 139 patients with COVID‐19 were investigated at Siirt State Hospital. According to the disease severity, the patients were categorized as three groups (moderate: 85, severe: 54, and critical: 20). Then, patients were divided into two groups: nonsevere (moderate) and severe (severe and critical). Demographic, clinical data, and routine blood parameters were analyzed. In multivariate model adjusted for potential confounders BUN/Cr ratio (odds ratio [OR] = 1.70; 95% confidence interval [CI]: 1.20‐2.40; P = .002) and neutrophil to lymphocyte ratio (NLR) (OR = 2.21; 95% CI: 1.20‐4.30; P < .001) were independent predictive factors for disease severity. In multivariate Cox proportional hazard model BUN/Cr ratio (hazard ratio [HR] = 1.02; 95% CI: 1.01‐1.05; P = .030), and NLR (HR = 1.17; 95% CI: 1.06‐1.30; P = .020) were independent predictors for survival of COVID‐19 disease. The optimal thresholds of the BUN/Cr ratio at 33.5 and 51.7 had the superior possibility for severe disease and mortality, area under the curve (AUC) were 0.98 and 0.95, respectively. The optimal thresholds of NLR at 3.27 and 5.72 had a superior possibility for severe disease and mortality, AUC were 0.87 and 0.85, respectively. BUN/Cr and NLR are independent predictors for COVID‐19 patient severity and survival. Routine evaluation of BUN/Cr and NLR can help identify high‐risk cases with COVID‐19.
Keywords: blood urea nitrogen, coronavirus, COVID‐19, creatinine, neutrophil to lymphocyte ratio
Highlights
Since the severity of COVID‐19 disease can change rapidly, it is very valuable to predict this at admission. BUN/Cr ratio and NLR among routine blood parameters were determined as independent predictive factors in predicting disease severity and survival.
1. INTRODUCTION
In the latter part of 2019, unknown pneumonia cases began to appear in Wuhan, China (Hubei province). 1 , 2 In January 2020, a new beta‐coronavirus subtype in China was found through high‐throughput sequencing along with the use of throat swab samples. 3 The World Health Organization (WHO) named this coronavirus disease as “COVID‐19” and declared it to be a serious international pandemic and public health problem. 4 According to the WHO data, as of early May 2020, over 3 million global cases had been confirmed, more than 20 000 deaths. 5 While this virus affects many systems in animals, it usually occurs in the form of pneumonia in humans. 6 , 7 However, mildly‐ and moderately‐affected patients have a better prognosis, and milder symptoms. Yet, the prognosis for severe and critical patients is poor with high mortality rates. 8 In these critical groups, mortality from acute respiratory distress syndrome and multiple organ failure can be rapidly observed. Therefore, it is vital to predicting the factors that can determine this group of patients at the time of diagnosis.
Blood urea nitrogen (BUN) and creatinine (Cr) are the end products of nitrogen metabolism in humans. Since they are small molecules, they can be easily filtered from the nephrons. Usually, about 30% to 40% of BUN is reabsorbed from tubules, while Cr is not reabsorbed very well. 9 , 10 Studies show that the affected neurohormonal system is responsible for the reabsorption process in patients with acute heart failure (AHF). 9 Other studies have similarly demonstrated that the BUN/Cr ratio is more valuable than BUN or Cr alone in predicting the progression of patients with AHF. 11 , 12 , 13 We believe that multisystem inflammation, including a cytokine storm, can occur in severe and critical groups of COVID‐19 patients; this can in turn increase BUN reabsorption and the BUN/Cr ratio by similar mechanisms. As such, this ratio would be beneficial in assessing the severity and survival of those with COVID‐19 disease. In addition, there are parameters in peripheral blood whose predictive properties for COVID‐19 have been demonstrated in previous studies. The neutrophil‐lymphocyte ratio (NLR) and C‐reactive protein (CRP) make up some of these parameters. 5 , 14 In our study, we aimed to evaluate the role of applicable and cost‐effective BUN/Cr ratios, as well as other routine blood parameters, to predict both the severity and survival of those with COVID‐19 disease.
2. MATERIALS AND METHODS
2.1. Study design and patients
This study was approved by the Republic of Turkey Ministry of Health and Institutional Ethics Board of Siirt University (Approval Number 2020/08.01). A total of 139 patients with COVID‐19 included in this study were diagnosed with COVID‐19 disease in Siirt State Hospital (Siirt, Turkey) between April and May 2020. The New Coronavirus Pneumonia Prevention and Control Program (7th edition) of the Chinese National Health Commission was used for the diagnosis of COVID‐19 disease. 15 According to this program, patients divided into three groups as moderate, severe, and critical. Then, patients were classified as nonsevere (moderate) and severe (severe and critical). Nonsevere patients met all of the features such as the history of contact, respiratory symptoms or fever, typical viral pneumonia involvement in computed tomography, and positive test result of RT‐PCR for SARS‐CoV‐2 RNA. Severe patients also had at least one of the following features; PaO2/FiO2 ≤300 mm Hg, or oxygen saturation (at rest) less than 93%, or shortness of breath, respiratory rate ≥30 times/minute. Respiratory samples were taken and assessed for SARS‐CoV‐2, according to WHO recommendations for quantitative reverse transcriptase‐polymerase chain reaction. A positive test result confirmed the diagnosis of COVID‐19 disease from samples. Patients with chronic kidney disease, patients received chemotherapy in the last 6 months, hematological disorders or blood transfusion during hospitalization, age under 18 years old, died on admission, being pregnant, and having missing baseline data were excluded. Those with asthma and chronic obstructive pulmonary disease ere also excluded because they affect the risk groups.
2.2. Data collection
The electronic hospital information system was used for epidemiological data, including demographic, clinical, and laboratory findings. Complete blood count, CRP, and blood chemistry on admission were recorded as laboratory tests. Peripheral venous blood samples were evaluated by standard procedures in the central laboratory of Siirt State Hospital. The routine blood tests (including white blood cell count [WBC], leukocyte subtypes, and platelet count) were measured with Mindray BC‐6800 automatic hematology analyzer (Mindray Bio‐Medical Electronics Co, Ltd, Shenzhen, China). Siemens ADVIA 1800 automated biochemistry analyzer (Siemens Healthcare Diagnostics, Germany) was used to measure the biochemical parameters.
2.3. Statistical analysis
SPSS Statistics software version 26.0 (IBM, Armonk, NY) was used for all statistical analyses. Categorical variables were expressed as counts and percentages in each category. Continuous variables were specified as appropriate means and standard deviations or medians and interquartile ranges. The Student t test and Mann‐Whitney U tests were used for continuous variables. The χ 2 and Fisher's exact tests were applied to categorical variables. Spearman correlation coefficient was used to describe the association between blood parameters and disease severity. Univariate and multivariate binary logistic regression analysis was used to determine the predictive factors for COVID‐19 disease severity. The Cox proportional hazard models analyzed predictors of mortality. In the multivariate‐adjusted models, age, gender, hypertension (HT), and heart disease were included. Receiver operator characteristics (ROC) curve analysis was used to determine optimal thresholds via area under the curve (AUC). Youden's Index in ROC curves was used to determine an optimum cut‐off value of associated parameters for predicting COVID‐19 disease severity and survival. The Kaplan‐Meier analysis was used to evaluate the survival rates of predictive factors' cut‐off groups. P value of less than .05 was defined as statistical significance.
3. RESULTS
3.1. Results of BUN/Cr ratio, routine blood tests, CRP, and clinical characteristics of patients
The primary demographic factors and laboratory parameters of severe and nonsevere patients are shown in Table 1. There were 85 (61.2%) patients in the nonsevere group and 54 (38.8%, 34 severe, and 20 critical) patients in the severe group. The mean age was 47.2 ± 15.7 years in the nonsevere group and 68.3 ± 14.9 years in the severe group (P < .001). There was no significant difference between the two groups in terms of gender (P = .976). The rates of total comorbidities, HT, and heart disease were significantly higher in the severe group (all P < .001). There was no significant difference in terms of diabetes mellitus (DM) between the two groups (P = .218). The overall number of in‐hospital death was 13 (9.4%), and all of them was in severe group (P < .001).
Table 1.
The comparison of demographic and blood parameters between patient groups
| Characteristics | Nonsevere (n = 85) | Severe (n = 54) | Total (n = 139) | Test statistic | P value | |
|---|---|---|---|---|---|---|
| Moderate (n = 85) | Severe (n = 34) | Critical (n = 20) | ||||
| Age, mean ± SD | 47.2 ± 15.7 | 68.3 ± 14.9 | 55.5 ± 18.5 | F = 0.139 | <.001 | |
| Gender, n (%) | ||||||
| Male | 38 (44.7) | 24 (44.4) | 62 (44.6) | χ 2 = 0.001 | .976 | |
| Female | 47 (55.3) | 30 (55.6) | 77 (55.4) | |||
| Comorbidities, n (%) | 19 (22.4) | 33 (61.1) | 52 (37.4) | χ 2 = 21.18 | <.001 | |
| DM, n (%) | 12 (14.1) | 12 (22.2) | 24 (17.3) | χ 2 = 1.518 | .218 | |
| HT, n (%) | 9 (10.6) | 24 (44.4) | 33 (23.7) | χ 2 = 20.90 | <.001 | |
| Heart disease, n (%) | 2 (2.4) | 17 (31.5) | 19 (13.7) | χ 2 = 23.74 | <.001 | |
| All‐cause death | 0 | 13 (24.1) | 13 (9.4) | χ 2 = 22.57 | <.001 | |
| BUN, median (IQR), mg/dL | 23.8 (6.9) | 46 (33.7) | 28.2 (16.9) | Z = −9.279 | <.001 | |
| Cr, median (IQR), mg/dL | 0.95 (0.18) | 1 (0.41) | 0.97 (0.25) | Z = −0.340 | .734 | |
| BUN/Cr ratio, median (IQR) | 24.2 (5.8) | 50.3 (19.1) | 27.6 (23.9) | Z = −9.516 | <.001 | |
| WBC, median (IQR), 10^9/L | 6 (3.1) | 8.1 (4.4) | 6.7 (3.6) | Z = −3.204 | .001 | |
| NEU, median (IQR), 10^9/L | 3.99 (2.3) | 6.33 (3.4) | 4.6 (3.38) | Z = −4.181 | <.001 | |
| LYM, median (IQR), 10^9/L | 1.48 (0.87) | 1.16 (0.7) | 1.37 (0.82) | Z = −2.971 | .001 | |
| MON, median (IQR), 10^9/L | 0.44 (0.21 | 0.47 (0.45) | 0.45 (0.26) | Z = −1.267 | .205 | |
| EOS, median (IQR), 10^9/L | 0.06 (0.08) | 0.06 (0.12) | 0.06 (0.09) | Z = −0.795 | .426 | |
| PLT, median (IQR), 10^9/L | 224 (98.5) | 212 (98.5) | 219 (95) | Z = −0.975 | .330 | |
| NLR, median (IQR) | 2.46 (2.3) | 6.1 (5.1) | 3.2 (2.99) | Z = −4.386 | <.001 | |
| MLR, median (IQR) | 0.27 (0.17) | 0.4 (0.42) | 0.29 (0.25) | Z = −2.999 | <.001 | |
| PLR, median (IQR) | 143.6 (94.2) | 197.8 (178.2) | 160.3 (114.7) | Z = −1.802 | .072 | |
| CRP, median (IQR), mg/dL | 14.2 (25.5) | 53.6 (92.8) | 21.8 (45.9) | Z = −5.653 | <.001 | |
Note: The bold values provide statistically significancy. Abbreviations: BUN, blood urea nitrogen; Cr, creatinine; CRP, C‐reactive protein; DM, diabetes mellitus; EOS, eosinophil; HT, hypertension; IQR, interquartile range; LYM, lymphocyte; MLR, monocyte to lymphocyte ratio; MON, monocyte; NEU, neutrophil; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; PLT, platelet; SD, standard deviation; WBC, white blood cell.
There were many differences in the routine blood parameters between patients in nonsevere and severe groups on admission. Severe cases had higher BUN count (46.0 vs 23.8 mg/dL; P < .001), higher BUN/Cr ratio (50.3 vs 24.2; P < .001), higher WBC count (8.1 vs 6.0 × 10^9/L; P = .001), higher neutrophil count (6.33 vs 3.99 × 10^9/L; P < .001), lower lymphocyte count (1.16 vs 1.48 × 10^9/L; P = .001), higher NLR (6.1 vs 2.46; P < .001), higher monocyte to lymphocyte ratio (MLR) (0.4 vs 0.27; P < .001), and higher CRP count (53.6 vs 14.2; P < .001). There were no significant differences in Cr count, monocyte count, eosinophil count, platelet count, and platelet to lymphocyte ratio.
3.2. Analysis of BUN/Cr ratio and other routine blood parameters for predicting COVID‐19 disease severity and optimum cut‐off values
Table 2 shows the relationships between blood parameters and disease severity, using the Spearman correlation coefficient. The parameters which demonstrated a significant correlation with disease severity were included in a binary logistic regression analysis. BUN/Cr ratio was included in the regression analysis because it showed a stronger correlation than BUN alone. Similarly, NLR was used instead of neutrophil and lymphocyte. The results of univariate and multivariate logistic regression models for predictive factors for disease severity were shown in Table 3. The univariate analysis indicated that BUN/Cr ratio (odds ratio [OR] = 1.48; 95% confidence interval [CI]: 1.27‐1.73; P < .001), WBC (OR = 1.18; 95% CI: 1.06‐1.33; P = .003), NLR (OR = 2.37; 95% CI: 1.73‐3.24; P < .001), MLR (OR = 1.03; 95% CI: 1.02‐1.51; P = .001), and CRP (OR = 1.02; 95% CI: 1.012‐1.032; P < .001) values were positively correlated with disease severity. In the multivariate model adjusted for age, gender, and comorbidities BUN/Cr ratio (OR = 1.70; 95% CI: 1.20‐2.40; P = .002) and NLR (OR = 2.21; 95% CI: 1.20‐4.30; P < .001) were independent predictors for disease severity.
Table 2.
Spearman correlation analysis between disease severity and blood parameters
| Characteristics | Severe disease | |
|---|---|---|
| ρ | P value | |
| BUN | 0.790 | <.001 |
| BUN/Cr ratio | 0.810 | <.001 |
| WBC | 0.273 | .001 |
| NEU | 0.356 | <.001 |
| LYM | −0.253 | .003 |
| NLR | 0.373 | <.001 |
| MLR | 0.256 | .002 |
| CRP | 0.481 | <.001 |
Note: The bold values provide statistically significancy. Abbreviations: BUN, blood urea nitrogen; Cr, creatinine; CRP, C‐reactive protein; LYM, lymphocyte; MLR, monocyte to lymphocyte ratio; NEU, neutrophil; NLR, neutrophil to lymphocyte ratio; WBC: white blood cell.
Table 3.
Univariate and multivariate logistic regression analysis of BUN/Cr ratio and other routine blood parameters for predicting COVID‐19 disease severity
| Characteristics | Univariate model | Multivariate model a | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| BUN/Cr ratio | 1.48 (1.27‐1.73) | <.001 | 1.70 (1.2‐2.4) | .002 |
| WBC | 1.18 (1.06‐1.33) | .003 | 0.94 (0.55‐1.63) | .837 |
| NLR | 2.37 (1.73‐3.24) | <.001 | 2.21 (1.2‐4.3) | .030 |
| MLR | 1.03 (1.02‐1.51) | .001 | 1.02 (0.88‐1.08) | .362 |
| CRP | 1.02 (1.012‐1.032) | <.001 | 1.02 (0.99‐1.05) | .136 |
Note: The bold values provide statistically significancy. Abbreviations: BUN, blood urea nitrogen; CI, confidence interval; Cr, creatinine; CRP, c‐reactive protein; MLR, monocyte to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; OR, odds ratio; WBC, white blood cell.
Multivariate model was adjusted age, gender, history of hypertension, history of heart disease.
ROC curve analysis was performed to determine optimum cut‐off value by Youden Index in Figure 1. The optimum cut‐off value for the BUN/Cr ratio, which can predict COVID‐19 disease severity, was assigned to be 33.5, with AUC of 0.98 (95% CI: 0.96‐1.00). The highest sensitivity and specificity were 0.93 and 0.98. We determined the optimal cut‐off value for NLR above 3.27, which estimated disease severity an AUC of 0.87 (95% CI: 0.81‐0.93). The highest sensitivity and specificity were 0.79 and 0.71.
Figure 1.
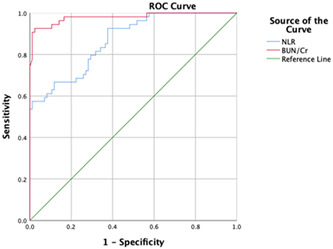
The receiver operating characteristic (ROC) curve analysis of the BUN/Cr ratio and NLR for COVID‐19 disease severity. BUN, blood urea nitrogen; Cr, creatinine; NLR, neutrophil to lymphocyte ratio
3.3. Analysis of BUN/Cr ratio and NLR for predicting survival of COVID‐19 disease and optimum cut‐off values
The results of univariate and multivariate Cox proportional hazard models of BUN/Cr ratio and NLR for predicting survival of COVID‐19 disease are shown in Table 4. In the multivariate model adjusted for age, gender, and comorbidities BUN/Cr ratio (hazard ratio [HR] = 1.02; 95% CI: 1.01‐1.05; P = .030), and NLR (HR = 1.17; 95% CI: 1.06‐1.30; P = .020) were independent predictors for the survival of COVID‐19 disease.
Table 4.
Cox proportional hazard models of BUN/Cr ratio and NLR for predicting survival of COVID‐19 disease
| Characteristics | Univariate model | Multivariate model a | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| BUN/Cr ratio | 1.035 (1.02‐1.53) | <.001 | 1.02 (1.01‐1.05) | .030 |
| NLR | 1.170 (1.09‐1.25) | <.001 | 1.173 (1.06‐1.30) | .020 |
Note: The bold values provide statistically significancy. Abbreviations: BUN, blood urea nitrogen; CI, confidence interval; Cr, creatinine; HR, hazard ratio; NLR, neutrophil to lymphocyte ratio.
Multivariate model was adjusted age, gender, history of hypertension, history of heart disease.
The ROC analysis calculated the optimal cut‐off values of the BUN/Cr ratio and NLR for predicting disease survival, and the ROC curves are presented in Figure 2. The AUC of the BUN/Cr ratio and NLR were 0.95 and 0.85. The optimal cut‐off values were 51.7 and 5.72 for the BUN/Cr ratio and NLR, respectively. The highest specificity and sensitivity were 0.92 and 0.90, 0.85, and 0.83 for the BUN/Cr ratio and NLR, respectively. In Figures 3 and 4, the Kaplan‐Meier survival curves present, BUN/Cr ratio more than 51.7 and NLR more than 5.72 had a significantly higher in‐hospital mortality rate (log‐rank test, both P < .001).
Figure 2.
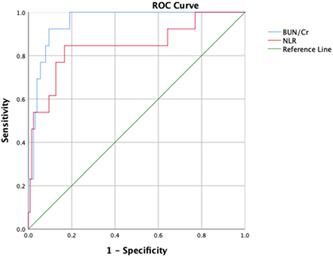
The receiver operating characteristic (ROC) curve analysis of the BUN/Cr ratio and NLR for the survival of COVID‐19 disease. BUN, blood urea nitrogen; Cr, creatinine; NLR, neutrophil to lymphocyte ratio
Figure 3.
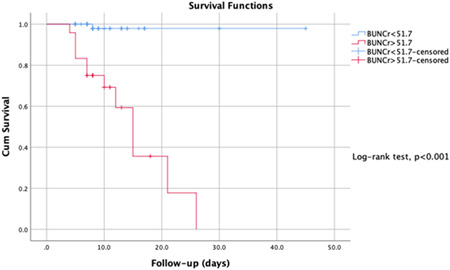
Kaplan‐Meier survival curves of the BUN/Cr ratio for the survival of COVİD‐19 disease. BUN, blood urea nitrogen; Cr, creatinine
Figure 4.
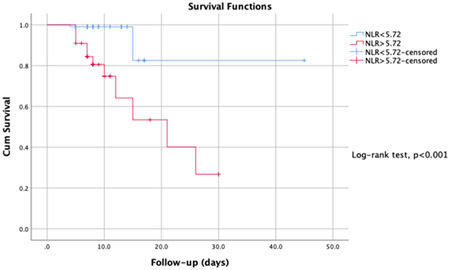
Kaplan‐Meier survival curves of NLR for the survival of COVİD‐19 disease. NLR, neutrophil to lymphocyte ratio
4. DISCUSSION
COVID‐19, which has an average incubation period of 3 days, is easily transmitted from person to person, leading rapidly to a pandemic situation. 16 Patients with mild or moderate COVID‐19 have milder symptoms and better prognoses than severe or critical patients who typically experience more complex symptoms and have a high mortality rate. 8 Determining the predictive variables for severe disease using routine blood tests can assist in the treatment and management of COVID‐19. The demographic, comorbidity, and usual blood parameters of 139 patients with COVID‐19 in Siirt, Turkey, were analyzed to determine potential biomarkers for disease severity and survival.
The age distribution of COVID‐19 is variable. In our data, we showed that severe patients had an older age. The female‐male proportions were similar in both groups. Total comorbidities, HT, and heart disease rates were higher in severe patients. Although the DM rate of severe patients was higher, the difference was not significant. The clinical features of our patient groups are compatible with other studies. 3 , 17 Yuwei et al reported that their total mortality rate was 13.47%. 18 In our cohort, the overall mortality rate was 9.4%.
The body's immune status and severe inflammatory response are the main factors that determine the progression and prognosis of COVID‐19. 19 Neutrophils (NEUs) are an essential component of the leukocyte family and play a critical role in the immune response. NEUs release reactive oxygen species, causing DNA damage in virus cells. 20 NEUs also interact with different compartments to produce a large number of cytokines and mediators, especially vascular endothelial growth factor. 21 However, the human immune response induced by a viral infection is primarily associated with lymphocytes. Systematic inflammation significantly reduces CD4+ T lymphocytes and increases suppressive CD8+ T lymphocytes by significantly reducing cellular immunity. 22 For this reason, inflammation caused by the virus increases the NLR. Some studies have examined the basal leukocyte counts in COVID‐19 patients at different clinical stages. Qin et al 23 reported that the neutrophil count was higher and the lymphocyte count lower among the COVID‐19 patients in the severe group compared to the nonsevere group in their study. Accordingly, higher NLR levels were seen in patients with severe infection.
In this study, the NLR similarly correlated with disease severity. Using the multivariate logistic regression model, which adjusted age, gender, and comorbidities to minimize the potential impact of a confounding NLR, it was determined that the NLR was an independent predictor of disease severity (OR = 2.21). In the adjusted Cox proportional hazard analysis, the NLR (HR = 1.17) was associated with mortality.
BUN and Cr levels and the BUN/Cr ratio are the main parameters showing kidney function. 24 The BUN/Cr ratio plays a vital role in the treatment and clinical follow‐up of patients with acute myocardial infarction (AMI), with a strong correlation between a high BUN/Cr ratio and the long‐term mortality of AMI patients. 25 Yoichi et al reported that the BUN/Cr ratio is associated with an increased risk of death in AHF patients. 11 Gotsman et al 26 indicated that in patients with AHF in their study, the BUN/Cr ratio on admission was associated with increased 1 year and long‐term (mean: 6.5‐year follow‐up) mortality. Brisco et al 27 also found a significant relationship between a high admission BUN/Cr ratio and increased mortality. The new coronavirus enters the cells using angiotensin‐converting enzyme 2 (ACE2) as a receptor. 28 According to the latest human tissue RNA sequencing data, ACE2 is expressed approximately 100 times higher in the kidneys than in the lungs. 29 Coronavirus can affect kidney function by entering kidney cells in a direct ACE2‐dependent way 30 and activating the renin‐angiotensin‐aldosterone system (RAAS) with the systemic effects it generates. The RAAS increases the absorption of water and sodium in the kidney tubules, causing passive reabsorption of BUN. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 These systemic effects cause renal vasoconstriction, and accordingly, glomerular filtration and BUN excretion are reduced. 25 Notwithstanding, the BUN/Cr ratio increases as Cr is filtered through the glomeruli and not reabsorbed. 10 In light of this information, it is the authors’ opinion that the BUN/Cr ratio may function as a predictive factor for the severity and survival of COVID‐19.
This is the first study to investigate the value of the admission BUN/Cr ratio in predicting the severity and survival of COVID‐19. In this cohort, the Cr counts were similar in the severe and nonsevere patients. The BUN/Cr ratio was higher in the severe patient group and strongly correlated with disease severity (ρ = 0.810). In the adjusted multivariate logistic and Cox regression models, the BUN/Cr ratio was an independent predictor of disease severity (OR = 1.70) and mortality (HR = 1.02). Finally, the study findings indicated that an elevated BUN/Cr ratio and NLR were independent predictors of COVID‐19 disease severity and survival. The BUN/Cr ratio and NLR can provide important prospective information in the evaluation of COVID‐19 patients on admission. The BUN/Cr ratio and NLR are, therefore, recommended for use in predicting the severity of COVID‐19 because they are cost‐effective and easy to apply.
This study had some limitations. First, this was a retrospective, single‐center clinical trial and, therefore, had a small sample size. Second, even though attempts were made to adjust for many confounders, other unknown features may have played a role. Third, there were no data from the group of mild patients who were sent home at the time of admission. Fourth, there was insufficient data regarding factors such as a high protein diet, which may have influenced the baseline BUN/Cr ratio. Due to these limitations, multicenter comprehensive investigations are needed.
5. CONCLUSION
The severity of COVID‐19 varies from patient to patient. Advanced age, comorbidity, and immune status are the main risk factors affecting disease severity. Severe illness causes poor survival results. The BUN/Cr ratio and NLR may be associated with disease severity, and routine use of these parameters may be beneficial in the evaluation of the disease. This study supports the authors’ hypothesis that a high BUN/Cr ratio and NLR are independent predictors of COVID‐19 patient severity and survival.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
FO and OE developed the idea for and designed the study and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. ED and SC contributed to the writing of the report. FO contributed to the critical revision of the report. FO, AC, OE, and SC contributed to the statistical analysis. All authors contributed to data acquisition, data analysis, or data interpretation, and reviewed and approved the final version.
ETHICS STATEMENT
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Ethics Board of Siirt University (No. 2020/05.02).
ACKNOWLEDGMENTS
We would like to thank all healthcare professionals and hospital management who worked intensively during the pandemic process.
Ok F, Erdogan O, Durmus E, Carkci S, Canik A. Predictive values of blood urea nitrogen/creatinine ratio and other routine blood parameters on disease severity and survival of COVID‐19 patients. J Med Virol. 2021;93:786–793. 10.1002/jmv.26300
REFERENCES
- 1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382(13):1199‐1207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92(4):401‐402. 10.1002/jmv.25678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Rolling Updates on Coronavirus Disease (COVID‐19) (Updated 18 April 2020). https://Www.Who.Int/Emergencies/Diseases/Novel-Coronavirus-2019/Events-as-They-Happen. Accessed Apr 21, 2020.
- 5. Zheng Y, Wang L, Ben S. Meta‐analysis of chest CT features of patients with COVID‐19 pneumonia. J Med Virol. 2020;1–9. 10.1002/jmv.26218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Organization WH. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID‐19 Disease Is Suspected: Interim Guidance, 13 March 2020.; 2020. Accessed May 27, 2020. https://www.who.int/
- 7. Yin Y, Wunderink RGMERS. SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130‐137. 10.1111/resp.13196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun S, Cai X, Wang H, et al. Abnormalities of peripheral blood system in patients with COVID‐19 in Wenzhou, China. Clin Chim Acta. 2020;507:174‐180. 10.1016/j.cca.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsue Y, Van Der Meer P, Damman K, et al. Blood urea nitrogen‐to‐creatinine ratio in the general population and in patients with acute heart failure. Heart. 2017;103(6):407‐413. 10.1136/heartjnl-2016-310112 [DOI] [PubMed] [Google Scholar]
- 10. Qian H, Tang C, Yan G. Predictive value of blood urea nitrogen/creatinine ratio in the long‐term prognosis of patients with acute myocardial infarction complicated with acute heart failure. Medicine (Baltimore). 2019;98(11):e14845. 10.1097/MD.0000000000014845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takaya Y, Yoshihara F, Yokoyama H, et al. Risk stratification of acute kidney injury using the blood urea nitrogen/creatinine ratio in patients with acute decompensated heart failure. Circ J. 2015;79(7):1520‐1525. 10.1253/circj.CJ-14-1360 [DOI] [PubMed] [Google Scholar]
- 12. Tung YC, Chang CH, Chen YC, Chu PH. Combined biomarker analysis for risk of acute kidney injury in patients with ST‐segment elevation myocardial infarction. PLoS One. 2015;10(4):0125282. 10.1371/journal.pone.0125282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Otto CM. Heartbeat:Blood urea nitrogen to creatinine ratio predicts outcome in acute heart failure. Heart. 2017;103(6):399‐401. 10.1136/heartjnl-2017-311339 [DOI] [PubMed] [Google Scholar]
- 14. Sun Y, Dong Y, Wang L, et al. Characteristics and prognostic factors of disease severity in patients with COVID‐19: the Beijing experience. J Autoimmun. 2020:102473. 10.1016/j.jaut.2020.102473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. China. NHC o . New Coronavirus Pneumonia Prevention and Control Program ( 7th Edn ). 2020.
- 16. Yang AP, Liu J‐P, Tao W‐Q, Li H‐M. The diagnostic and predictive role of NLR, d‐NLR, and PLR in COVID‐19 patients. Int Immunopharmacol. 2020;84:84. 10.1016/j.intimp.2020.106504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hui DS, I Azhar E, Madani TA, et al. The continuing 2019‐nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264‐266. 10.1016/j.ijid.2020.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Y, Du X, Chen J, et al. Neutrophil‐to‐lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID‐19. J Infect. 2020;81:6. 10.1016/j.jinf.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kusumanto YH, Dam WA, Hospers GAP, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6(4):283‐287. 10.1023/B:AGEN.0000029415.62384.ba [DOI] [PubMed] [Google Scholar]
- 21. Hanrahan V, Currie MJ, Gunningham SP, et al. The angiogenic switch for vascular endothelial growth factor (VEGF)‐A, VEGF‐B, VEGF‐C, and VEGF‐D in the adenoma‐carcinoma sequence during colorectal cancer progression. J Pathol. 2003;200(2):183‐194. 10.1002/path.1339 [DOI] [PubMed] [Google Scholar]
- 22. Menges T, Engel J, Welters I, et al. Changes in blood lymphocyte populations after multiple trauma: association with posttraumatic complications. Crit Care Med. 1999;27(4):733‐740. 10.1097/00003246-199904000-00026 [DOI] [PubMed] [Google Scholar]
- 23. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. academic.oup.com. doi: 10.1093/cid/ciaa248/5803306 [DOI] [PMC free article] [PubMed]
- 24. Schefold JC, Lainscak M, Hodoscek LM, Blöchlinger S, Doehner W, von Haehling S. Single baseline serum creatinine measurements predict mortality in critically ill patients hospitalized for acute heart failure. ESC Hear Fail. 2015;2(4):122‐128. 10.1002/ehf2.12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murata A, Kasai T, Matsue Y, et al. Relationship between blood urea nitrogen‐to‐creatinine ratio at hospital admission and long‐term mortality in patients with acute decompensated heart failure. Heart Vessels. 2018;33(8):877‐885. 10.1007/s00380-018-1135-3 [DOI] [PubMed] [Google Scholar]
- 26. Gotsman I, Zwas D, Planer D, Admon D, Lotan C, Keren A. The significance of serum urea and renal function in patients with heart failure. Medicine (Baltimore). 2010;89(4):197‐203. 10.1097/MD.0b013e3181e893ee [DOI] [PubMed] [Google Scholar]
- 27. Brisco MA, Zile MR, Ter Maaten JM, et al. The risk of death associated with proteinuria in heart failure is restricted to patients with an elevated blood urea nitrogen to creatinine ratio. Int J Cardiol. 2016;215:521‐526. 10.1016/j.ijcard.2016.04.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Z, Wu M, Guo J, et al. Caution on kidney dysfunctions of 2019‐nCoV patients 2020. MedRxiv preprint. Available at: 10.1101/2020.02.08.20021212. Accessed March 14, 2020. [DOI]
- 30. Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in‐hospital death of patients with COVID‐19. Kidney Int. 2020;97(5):829‐838. 10.1016/j.kint.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]


