Abstract
In coronavirus disease 2019 (COVID‐19), higher morbidity and mortality are associated with age, male gender, and comorbidities, such as chronic lung diseases, cardiovascular pathologies, hypertension, kidney diseases, diabetes mellitus, and obesity. All of the above conditions are characterized by increased sympathetic discharge, which may exert significant detrimental effects on COVID‐19 patients, through actions on the lungs, heart, blood vessels, kidneys, metabolism, and/or immune system. Furthermore, COVID‐19 may also increase sympathetic discharge, through changes in blood gases (chronic intermittent hypoxia, hyperpnea), angiotensin‐converting enzyme (ACE)1/ACE2 imbalance, immune/inflammatory factors, or emotional distress. Nevertheless, the potential role of the sympathetic nervous system has not yet been considered in the pathophysiology of COVID‐19. In our opinion, sympathetic overactivation could represent a so‐far undervalued mechanism for a vicious circle between COVID‐19 and comorbidities.
Keywords: autonomic nervous system, COVID‐19, diabetes, heart failure, hypertension, kidney disease, obesity, SARS‐CoV‐2, smoking, sympathoactivation
Aging and comorbidities (lung, cardiovascular, kidney, and metabolic diseases) are characterized by sympathetic overactivity, which may exert detrimental effects on lungs, heart, vessels, kidney, metabolism, and/or immune system of coronavirus disease 2019 (COVID‐19) patients. COVID‐19 may furtherly increase sympathetic discharge, through hypoxia, angiotensin‐converting enzyme (ACE)1/ACE2 imbalance, immune/inflammatory factors, and emotional distress. Thus, sympathetic activation could represent a so‐far undervalued mechanism for a vicious circle between COVID‐19 and comorbidities.
Abbreviations
- ACE1
angiotensin‐converting enzyme type 1
- ACE2
angiotensin‐converting enzyme type 2
- AngI
angiotensin I
- AngII
angiotensin II
- ARDS
acute respiratory distress syndrome
- AT1‐R
angiotensin II type 1 receptor
- COVID‐19
coronavirus disease 2019
- IL
interleukin
- MasR
Mas receptor
- MSNA
muscle sympathetic nerve activity
- SARS‐CoV
severe acute respiratory syndrome–coronavirus
- TNF‐α
tumor necrosis factor‐α
Introduction
In coronavirus disease 2019 (COVID‐19), higher morbidity and mortality are associated with comorbidities, such as chronic lung disease, cardiovascular pathologies, hypertension, kidney diseases, diabetes mellitus, and obesity [1, 2, 3]. Conversely, COVID‐19 deaths are frequently caused by a final homeostasis dysregulation caused not only by pulmonary damage but also by cardiac, circulatory, renal, and/or metabolic effects. Attention has been focused on the mechanisms involved in the comorbidity‐induced increase in morbidity/mortality but the potential role of the sympathetic nervous system has not yet been considered, despite sympathetic activation represents one of the specific characteristics of most above comorbidities and it could play a detrimental effect on COVID‐19 patients.
All comorbidities associated with increased morbidity/mortality in COVID‐19 are characterized by sympathetic overactivation
It is widely known that increased sympathetic discharge is associated with chronic obstructive pulmonary disease, obstructive sleep apnea syndrome, cardiovascular diseases (hypertension, heart failure), renal pathologies, and metabolic disturbances (diabetes, obesity, metabolic syndrome). Increase in peripheral hypoxic chemosensitivity is a common mechanism stimulating sympathetic activation in the above conditions [4, 5, 6, 7, 8, 9], but other stimulatory mechanisms are present.
Chronic obstructive pulmonary disease and obstructive sleep apnea syndrome increase sympathetic activation mainly through chronic intermittent hypoxia, which acts by increasing the peripheral chemosensory response [10, 11].
In heart failure, the increased sympathetic outflow correlates with disease progression and poor prognosis [12, 13]. It has been ascribed to decreased arterial/cardiopulmonary baroreflex, increased chemosensitivity, increased metabolic reflexes, or progression of correlated renal insufficiency or sleep apnea syndrome [14, 15, 16].
Renal damage (for instance, experimental models of renal ischemia–reperfusion) is also associated with sympathetic activation, due to the activation of the renal afferents and brain renin–angiotensin system. Conversely, in a positive feedback loop, sympathetic overactivity stimulates tubular Na+/H2O reabsorption, decreases renal blood flow, and stimulates renin–angiotensin system. It also aggravates ischemia/reperfusion‐induced renal damage through pro‐inflammatory mechanisms (reviewed in Ref. [17]).
In obesity, diabetes, and metabolic syndrome, sympathetic overactivity has been ascribed to high levels of circulating insulin and leptin, which stimulate the sympathetic outflow both centrally and peripherally, and/or to chronic intermittent hypoxia due to obstructive sleep apnea [18, 19]. Sympathetic overactivation in turn increases insulin resistance, maintaining a positive feedback loop [20].
The effects of smoking on COVID‐19 are still highly controversial, and conflicting data are present in the literature. Epidemiological studies and meta‐analyses report unexpectedly low prevalence of smoking among COVID‐19 hospitalized patients [21]. Conversely, other authors observed significant associations of smoking with clinical progression and mortality of hospitalized COVID‐19 patients, consistently with the well‐known detrimental effects of smoking on lung function [22, 23]. The potential role of autonomic effects could also warrant an evaluation, as cigarette smoking results in increased sympathetic discharge and decreased baroreflex activity [24, 25, 26, 27, 28].
Sympathetic overactivity may exert significant detrimental effects on COVID‐19 patients
In COVID‐19, the comorbidity‐induced increase in sympathetic activity may show negative effects on pulmonary, cardiovascular, renal, metabolic, and immune/inflammatory homeostasis.
In COVID‐19, cardiovascular complications frequently occur, including arrhythmias, myocarditis, heart failure, and myocardial infarction [29]. All these conditions are negatively affected by sympathetic overactivation and could represent a way through which comorbidity‐induced sympathoactivation may increase COVID‐19 morbidity/mortality. In some reports, myocardial injury has been reported in 20–40% of hospitalized cases [30, 31, 32, 33]. Cases of Takotsubo syndrome have also been reported in COVID‐19 [34, 35, 36, 37]. In this kind of stress‐related cardiomyopathy, myocardial injury is probably mediated by catecholamine‐induced vascular spasm and/or direct catecholamine action on myocytes. In particular, catecholamine release in response to cytokine storm, or metabolic and emotional distress has been proposed to play a role in COVID‐19‐related Takotsubo syndromes [34, 35, 36, 37], consistently with our hypothesis.
Acute kidney injury has been reported in > 20% of severe or deceased COVID‐19 patients, and chronic kidney diseases are also significantly associated with severe COVID‐19 [2]. Moreover, the above mechanisms involved in the vicious cycle between sympathetic overactivity and renal function also show detrimental effects on the cardiocirculatory [38, 39] and lung [40] functions. Thus, it appears reasonable that sympathetic activation in comorbidities may exert negative homeostasis effects in COVID‐19 also through renal effects. Moreover, liver injuries have also been reported in COVID‐19 patients [41] and sympathetic activation may also be detrimental for liver function [42].
The autonomic system also exerts a modulatory role on the immune system, and its potential role in the complex immunological situation of COVID‐19 is all to be studied. Sympathetic nerve fibers innervate most lymphoid organs, including bone marrow [43] and adrenergic receptors are present in many different immune cell types [44]. The effects of sympathetic system on immune system are quite complex and depend on the differentiation state of the immune cells. However, the evidence is available about a pro‐inflammatory effect at least in some tissues and experimental or pathological conditions. For instance, in a mouse model of angiotensin (Ang)II‐mediated hypertension, sympathetic stimulation produces noradrenaline‐mediated T‐cell activation and vascular inflammation [45]. Bilateral ablation of renal sympathetic nerves prevents immune activation and renal inflammation in a murine model of AngII‐induced hypertension [46]. Catheter‐based renal denervation has been demonstrated to reduce monocyte activation and inflammation markers in hypertensive patients [47]. In an experimental mouse model of chronic stress, hematopoietic stem cell proliferation and increased output of neutrophils and inflammatory monocytes have been reported, in response to noradrenaline release by sympathetic nerve fibers [48]. Increased sympathetic discharge through the splenic nerve has also been reported to increase cytokine release by splenocytes [49]. Conversely, the vagal nerve has an inhibitory effect on tumor necrosis factor‐α (TNF‐α) release by macrophages [50, 51]. The anti‐inflammatory effects of the parasympathetic vagal system have been observed also with reference to intestinal diseases [52, 53, 54] and arthritis [55]. These potential immune/inflammatory effects of a sympathetic/parasympathetic imbalance seem particularly intriguing in the pathophysiology of COVID‐19, which led to homeostasis derangement also through a ‘cytokine storm’.
Apart from the above effects on systemic homeostasis, increased sympathetic activity may have specific detrimental effects on the respiratory system. Sympathetic overactivation and the correlated renin–angiotensin system overflow play a pivotal role in progression of pulmonary hypertension [56, 57, 58]. It has been pointed out that angiotensin‐converting enzyme (ACE)1/ACE2 imbalance may contribute to progression to acute respiratory distress syndrome (ARDS) in COVID‐19 patients through pulmonary vasoconstriction, inflammation, and oxidative and fibrotic damage [29]. Sympathetic innervation is known to increase pulmonary capillary leakage and favor ARDS [59, 60, 61, 62]. Restrictive lung function has been associated with increased sympathetic nerve activity in heart failure, possibly due to interstitial pulmonary edema or changes in alveolar capillary units [63].
Aging and male gender are also associated with sympathoactivation
Risks of severe COVID‐19 and related mortality increase with advancing age and male gender, as also sympathetic activation. In fact, muscle sympathetic nerve activity (MSNA) has been reported to increase with age in nonobese normotensive men and women, the latter showing lower values for age < 50 years [64]. Conversely, children are known to be protect by severe disease; various mechanisms are probably involved (developmental changes in immunity, lower prevalence of comorbidities, higher lung regenerative potential), but a possible role of the quite complex maturation of the sympathetic/parasympathetic balance may not be excluded. For instance, plasma norepinephrine, which is mainly derived from sympathetic nerve endings, increases with advancing puberty in males [65]. Relevant gender differences are present in obesity‐induced increase in sympathetic activity. For instance, resting MSNA is positively correlated with body mass index in men but not in women [66, 67]. This gender‐based difference is partly explained by correlation of sympathetic overactivation with abdominal fat, more than subcutaneous one [68]. Thus, increased sympathetic activation could (at least partially) contribute to the pathophysiologic association of aging and male gender with COVID‐19 morbidity/mortality.
COVID‐19 may furtherly increase sympathetic output in a vicious circle
The sympathetic nervous system is activated by the hypoxic and hypercapnic stimuli which characterize respiratory dysfunctions. In particular, a large amount of studies has stressed that chronic intermittent hypoxia increases sympathetic output through increased carotid body sensitivity. Thus, COVID‐19‐induced alterations of the respiratory function may furtherly aggravate sympathetic overactivity (Fig. 1).
Fig. 1.
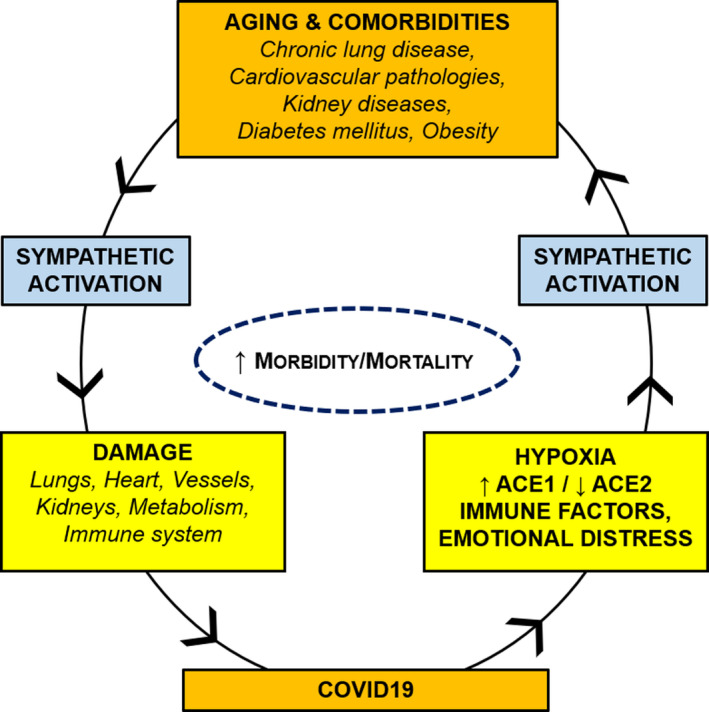
Vicious circle between COVID‐19 and comorbidities. Aging and comorbidities (lung, cardiovascular, kidney, and metabolic diseases) are characterized by sympathetic overactivity, which may exert detrimental effects on lungs, heart, vessels, kidney, metabolism, and/or immune system of COVID‐19 patients. COVID‐19 may furtherly increase sympathetic discharge, through hypoxia, ACE1/ACE2 imbalance, immune/inflammatory factors, and emotional distress.
COVID‐19 may also activate the sympathetic system through increased production and release of AngII. The cellular receptor for severe acute respiratory syndrome–coronavirus (SARS‐CoV) and SARS‐CoV‐2 is ACE2, a usually membrane‐bound homologue of angiotensin‐converting enzyme. ACE2 is widely expressed not only in lungs but also in other organs, such as heart, brain, kidney, and intestine. ACE1 and ACE2 have different enzymatic functions and produce different effects: ACE1 converts AngI in AngII; ACE2 converts AngI in Ang(1–9), which is then converted in Ang(1–7), and may also convert AngII in Ang(1–7). Thus, in the different tissues, a balance between the two pathways [ACE1/AngII/angiotensin II type 1 receptor (AT1‐R) and ACE2/Ang(1–7)/Mas receptor (MasR)] is present, which can be affected in various clinical conditions. As a consequence, ACE2 decreases the production of AngII in favor of Ang(1–7). AngII mediates vasoconstriction, fibrosis, hypertrophy, and inflammation through AT1‐R binding; Ang(1–7) mediates vasodilation, antifibrosis, antigrowth, and anti‐inflammation through MasR binding [4]. Apart from the above effects, AngII mediates sympathoexcitation, whereas Ang(1–7) mediates sympathoinhibition. Internalization of SARS‐CoV‐2 causes inhibition of ACE2 activity and progressive depletion of membrane‐bound ACE2 [69, 70, 71, 72, 73], with ACE1/ACE2 imbalance and increase in AngII.
Circulating AngII may increase the sympathetic output both centrally, at the level of the circumventricular organs (area postrema and subfornical organ) [58], and peripherally, by acting on the carotid body [58, 74]. Thus, COVID‐19‐induced increase in AngII (proportional to the viral load) [75] may represent an additional way to furtherly worsen sympathoactivation in comorbidities.
Moreover, the brainstem, and particularly the solitary tract nucleus, is directly invaded by different types of coronaviruses, so that neuroinvasion by SARS‐CoV‐2 has also been hypothesized [76]. ACE2 is also expressed in the solitary tract nucleus and carotid body so that sympathetic activation may be furtherly increased by local ACE1/ACE2 imbalance and AngII stimulation.
In the COVID‐19 severe patients, the occurrence of a ‘cytokine storm’ [interleukin (IL)‐6, IL‐10, and TNF‐α] has been reported [77]. AngII may also activate macrophages and other immune cells to produce inflammatory cytokines, such as IL‐6, TNF‐α, and others [78, 79, 80]. Circulating cytokines mainly activate the parasympathetic system, through the so‐called inflammatory reflex pathway, but in certain conditions stimulation of the sympathetic output has also been reported [81].
In the discussion of cardiovascular implications of COVID‐19, Guzik et al. [82] have recently recalled that the activation of the sympathetic nervous system is associated with viral infections themselves and even with social isolation [83, 84].
Conclusions
In conclusion, all comorbidities associated with increased morbidity/mortality in COVID‐19 are characterized by sympathetic overactivation, similarly to aging and male gender. Sympathetic overactivity may exert significant detrimental effect on COVID‐19 patients through its actions on lungs, heart, vessels, kidney, metabolism, and/or immune system. In turn, COVID‐19 may also furtherly increase sympathetic discharge through change in blood gases (chronic intermittent hypoxia, hyperpnea), ACE1/ACE2 imbalance, or cytokine release. Thus, sympathetic overactivation could represent a so‐far undervalued mechanism at the basis of the vicious circle between COVID‐19 and comorbidities. Finally, it must be kept in mind that the clinical course of COVID‐19 is characterized by different evolutionary phases and heterogeneous individual responses, so that the potential role of the sympathetic nervous system will have to be investigated consistently with this pathophysiological and clinical complexity.
Conflicts of interest
The authors declare no conflict of interest.
Author contributions
AP, AE, and RDC conceptualized the data; AP, AE, SB, RB‐B, CS, ES, VM, and RDC curated the data; AP wrote the original draft preparation; AP, AE, SB, RB‐B, CS, ES, VM, and RDC wrote, reviewed, and edited the manuscript; and AP and RDC supervised the data.
Acknowledgement
No funding was received for this work.
References
- 1. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sardu C, Gambardella J, Morelli MB, Wang X, Marfella R & Santulli G (2020) Hypertension, thrombosis, kidney failure, and diabetes: is COVID‐19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J Clin Med 9, E1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, Prill M, Chai SJ, Kirley PD, Alden NB et al. (2020) Hospitalization rates and characteristics of patients hospitalized with laboratory‐confirmed coronavirus disease 2019 ‐ COVID‐NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep 69, 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel KP & Schultz HD (2013) Angiotensin peptides and nitric oxide in cardiovascular disease. Antioxid Redox Signal 19, 1121–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Conde SV, Sacramento JF, Guarino MP, Gonzalez C, Obeso A, Diogo LN, Monteiro EC & Ribeiro MJ (2014) Carotid body, insulin, and metabolic diseases: unraveling the links. Front Physiol 5, 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conde SV, Sacramento JF & Guarino MP (2018) Carotid body: a metabolic sensor implicated in insulin resistance. Physiol Genomics 50, 208–214. [DOI] [PubMed] [Google Scholar]
- 7. Paton JF, Sobotka PA, Fudim M, Engelman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M et al. (2013) The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension 61, 5–13. [DOI] [PubMed] [Google Scholar]
- 8. Porzionato A, Macchi V & De Caro R (2013) Role of the carotid body in obesity‐related sympathoactivation. Hypertension 61, e57. [DOI] [PubMed] [Google Scholar]
- 9. Cunha‐Guimaraes JP, Guarino MP, Timóteo AT, Caires I, Sacramento JF, Ribeiro MJ, Selas M, Santiago JCP, Mota‐Carmo M & Conde SV (2020) Carotid body chemosensitivity: early biomarker of dysmetabolism in humans. Eur J Endocrinol 182, 549–557. [DOI] [PubMed] [Google Scholar]
- 10. Rey S, Del Rio R, Alcayaga J & Iturriaga R (2004) Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol 560 (Pt2), 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prabhakar NR, Peng YJ, Jacono FJ, Kumar GK & Dick TE (2005) Cardiovascular alterations by chronic intermittent hypoxia: importance of carotid body chemoreflexes. Clin Exp Pharmacol Physiol 32, 447–449. [DOI] [PubMed] [Google Scholar]
- 12. Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB & Rector T (1984) Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311, 819–823. [DOI] [PubMed] [Google Scholar]
- 13. Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, Roveda F, de Matos LN, Braga AM, Middlekauff HR et al. (2009) Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol 135, 302–307. [DOI] [PubMed] [Google Scholar]
- 14. Ueno H, Asanoi H, Yamada K, Oda Y, Takagawa J, Kameyama T, Hirai T, Nozawa T, Takashima S & Inoue H (2004) Attenuated respiratory modulation of chemoreflex‐mediated sympathoexcitation in patients with chronic heart failure. J Card Fail 10, 236–243. [DOI] [PubMed] [Google Scholar]
- 15. Floras JS (2009) Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol 54, 375–385. [DOI] [PubMed] [Google Scholar]
- 16. Oda Y, Joho S, Harada D, Hirai T, Asanoi H & Inoue H (2010) Renal insufficiency coexisting with heart failure is related to elevated sympathetic nerve activity. Auton Neurosci 155, 104–108. [DOI] [PubMed] [Google Scholar]
- 17. Grisk O (2020) The sympathetic nervous system in acute kidney injury. Acta Physiol 228, e13404. [DOI] [PubMed] [Google Scholar]
- 18. Fu Q (2019) Sex differences in sympathetic activity in obesity and its related hypertension. Ann N Y Acad Sci 1454, 31–41. [DOI] [PubMed] [Google Scholar]
- 19. Shi Z, Wong J & Brooks VL (2020) Obesity: sex and sympathetics. Biol Sex Differ 11, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC & Conde SV (2013) Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes 62, 2905–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farsalinos K, Barbouni A & Niaura R (2020) Systematic review of the prevalence of current smoking among hospitalized COVID‐19 patients in China: could nicotine be a therapeutic option? Intern Emerg Med 1–8. doi: 10.1007/s11739-020-02355-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patanavanich R & Glantz SA (2020) Smoking is associated with COVID‐19 progression: a meta‐analysis. Nicotine Tob Res. doi: 10.1093/ntr/ntaa082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karanasos A, Aznaouridis K, Latsios G, Synetos A, Plitaria S, Tousoulis D & Toutouzas K (2020) Impact of smoking status on disease severity and mortality of hospitalized patients with COVID‐19 infection: a systematic review and meta‐analysis. Nicotine Tob Res ntaa107. doi: 10.1093/ntr/ntaa107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kotamäki M (1995) Smoking induced differences in autonomic responses in military pilot candidates. Clin Auton Res 5, 31–36. [DOI] [PubMed] [Google Scholar]
- 25. Narkiewicz K, van de Borne PJ, Hausberg M, Cooley RL, Winniford MD, Davison DE & Somers VK (1998) Cigarette smoking increases sympathetic outflow in humans. Circulation 98, 528–534. [DOI] [PubMed] [Google Scholar]
- 26. Gerhardt U, Vorneweg P, Riedasch M & Hohage H (1999) Acute and persistant effects of smoking on the baroreceptor function. J Auton Pharmacol 19, 105–108. [DOI] [PubMed] [Google Scholar]
- 27. Arosio E, De Marchi S, Rigoni A, Prior M & Lechi A (2006) Effects of smoking on cardiopulmonary baroreceptor activation and peripheral vascular resistance. Eur J Clin Invest 36, 320–325. [DOI] [PubMed] [Google Scholar]
- 28. Manzano BM, Vanderlei LC, Ramos EM & Ramos D (2011) Acute effects of smoking on autonomic modulation: analysis by Poincaré plot. Arq Bras Cardiol 96, 154–160. [DOI] [PubMed] [Google Scholar]
- 29. Matsushita K, Marchandot B, Jesel L, Ohlmann P & Morel O (2020) Impact of COVID‐19 on the cardiovascular system: a review. J Clin Med 9, E1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruan Q, Yang K, Wang W, Jiang L & Song J (2020) Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 46, 846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L et al. (2020) Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med 8, 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q et al. (2020) Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol 5, 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang I, Lim MA & Pranata R (2020) Diabetes mellitus is associated with increased mortality and severity of disease in COVID‐19 pneumonia ‐ a systematic review, meta‐analysis, and meta‐regression. Diabetes Metab Syndr 14, 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Minhas AS, Scheel P, Garibaldi B, Liu G, Horton M, Jennings M, Jones SR, Michos ED & Hays AG (2020) Takotsubo syndrome in the setting of COVID‐19 infection. JACC Case Rep 2, 1321–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pasqualetto MC, Secco E, Nizzetto M, Scevola M, Altafini L, Cester A & Rigo F (2020) Stress cardiomyopathy in COVID‐19 Disease. Eur J Case Rep Intern Med 7, 001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giustino G, Croft LB, Oates CP, Rahman K, Lerakis S, Reddy VY & Goldman M (2020) Takotsubo cardiomyopathy in males with Covid‐19. J Am Coll Cardiol. doi: 10.1016/j.jacc.2020.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taza F, Zulty M, Kanwal A & Grove D (2020) Takotsubo cardiomyopathy triggered by SARS‐CoV‐2 infection in a critically ill patient. BMJ Case Rep 13, e236561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schiller AM, Pellegrino PR & Zucker IH (2015) The renal nerves in chronic heart failure: efferent and afferent mechanisms. Front Physiol 6, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kiuchi MG, Ho JK, Nolde JM, Gavidia LML, Carnagarin R, Matthews VB & Schlaich MP (2020) Sympathetic activation in hypertensive chronic kidney disease ‐ a stimulus for cardiac arrhythmias and sudden cardiac death? Front Physiol 10, 1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sorino C, Scichilone N, Pedone C, Negri S, Visca D & Spanevello A (2019) When kidneys and lungs suffer together. J Nephrol 32, 699–707. [DOI] [PubMed] [Google Scholar]
- 41. Kukla M, Skonieczna‐Żydecka K, Kotfis K, Maciejewska D, Łoniewski I, Lara LF, Pazgan‐Simon M, Stachowska E, Kaczmarczyk M, Koulaouzidis A et al. (2020) COVID‐19, MERS and SARS with concomitant liver injury‐systematic review of the existing literature. J Clin Med 9, E1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Joung JY, Cho JH, Kim YH, Choi SH & Son CG (2019) A literature review for the mechanisms of stress‐induced liver injury. Brain Behav 9, e01235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Felten DL, Felten SY, Carlson SL, Olschowka JA & Livnat S (1985) Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol 135 (2 Suppl), 755s–765s. [PubMed] [Google Scholar]
- 44. Brodde OE, Engel G, Hoyer D, Bock KD & Weber F (1981) The beta‐adrenergic receptor in human lymphocytes: subclassification by the use of a new radio‐ligand, (+/‐)‐125 Iodocyanopindolol. Life Sci 29, 2189–2198. [DOI] [PubMed] [Google Scholar]
- 45. Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ & Harrison DG (2010) Central and peripheral mechanisms of T‐lymphocyte activation and vascular inflammation produced by angiotensin II‐induced hypertension. Circ Res 107, 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, Takahashi T, Loperena R, Foss JD, Mernaugh RL et al. (2015) Renal denervation prevents immune cell activation and renal inflammation in angiotensin II‐induced hypertension. Circ Res 117, 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zaldivia MT, Rivera J, Hering D, Marusic P, Sata Y, Lim B, Eikelis N, Lee R, Lambert GW, Esler MD et al. (2017) Renal denervation reduces monocyte activation and monocyte‐platelet aggregate formation: an anti‐inflammatory effect relevant for cardiovascular risk. Hypertension 69, 323–331. [DOI] [PubMed] [Google Scholar]
- 48. Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J et al. (2014) Chronic variable stress activates hematopoietic stem cells. Nat Med 20, 754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ganta CK, Lu N, Helwig BG, Blecha F, Ganta RR, Zheng L, Ross CR, Musch TI, Fels RJ & Kenney MJ (2005) Central angiotensin II‐enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Am J Physiol Heart Circ Physiol 289, H1683–H1691. [DOI] [PubMed] [Google Scholar]
- 50. Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L et al. (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421, 384–388. [DOI] [PubMed] [Google Scholar]
- 51. Huston JM, Ochani M, Rosas‐Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch‐Puerta M, Ashok M, Czura CJ, Foxwell B et al. (2006) Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med 203, 1623–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ghia JE, Blennerhassett P, Kumar‐Ondiveeran H, Verdu EF & Collins SM (2006) The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology 131, 1122–1130. [DOI] [PubMed] [Google Scholar]
- 53. Sun P, Zhou K, Wang S, Li P, Chen S, Lin G, Zhao Y & Wang T (2013) Involvement of MAPK/NF‐κB signaling in the activation of the cholinergic anti‐inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS One 8, e69424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Goverse G, Stakenborg M & Matteoli G (2016) The intestinal cholinergic anti‐inflammatory pathway. J Physiol 594, 5771–5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McAllen RM, Cook AD, Khiew HW, Martelli D & Hamilton JA (2015) The interface between cholinergic pathways and the immune system and its relevance to arthritis. Arthritis Res Ther 17, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shirai M, Tsuchimochi H, Nagai H, Gray E, Pearson JT, Sonobe T, Yoshimoto M, Inagaki T, Fujii Y, Umetani K et al. (2014) Pulmonary vascular tone is dependent on the central modulation of sympathetic nerve activity following chronic intermittent hypoxia. Basic Res Cardiol 109, 432. [DOI] [PubMed] [Google Scholar]
- 57. Vaillancourt M, Chia P, Sarji S, Nguyen J, Hoftman N, Ruffenach G, Eghbali M, Mahajan A & Umar S (2017) Autonomic nervous system involvement in pulmonary arterial hypertension. Respir Res 18, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Iturriaga R & Castillo‐Galán S (2019) Potential contribution of carotid body‐induced sympathetic and renin‐angiotensin system overflow to pulmonary hypertension in intermittent hypoxia. Curr Hypertens Rep 21, 89. [DOI] [PubMed] [Google Scholar]
- 59. Bilodeau MS & Leiter JC (2018) Angiotensin 1–7 in the rostro‐ventrolateral medulla increases blood pressure and splanchnic sympathetic nerve activity in anesthetized rats. Respir Physiol Neurobiol 247, 103–111. [DOI] [PubMed] [Google Scholar]
- 60. Cure E & Cumhur Cure M (2020) Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers may be harmful in patients with diabetes during COVID‐19 pandemic. Diabetes Metab Syndr 14, 349–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cure E & Cumhur Cure M (2020) Comment on "Should COVID‐19 concern nephrologists? Why and to what extent? The emerging impasse of angiotensin blockade". Nephron 144, 251–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Al‐Dhahir MA, Das J M & Sharma S (2020) Neurogenic Pulmonary Edema. StatPearls. StatPearls Publishing, Treasure Island, FL. [PubMed] [Google Scholar]
- 63. Joho S, Ushijima R, Akabane T, Hirai T & Inoue H (2017) Restrictive lung function is related to sympathetic hyperactivity in patients with heart failure. J Card Fail 23, 96–103. [DOI] [PubMed] [Google Scholar]
- 64. Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F & Mano T (1998) Gender difference in age‐related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol 275, R1600–R1604. [DOI] [PubMed] [Google Scholar]
- 65. Weise M, Eisenhofer G & Merke DP (2002) Pubertal and gender‐related changes in the sympathoadrenal system in healthy children. J Clin Endocrinol Metab 87, 5038–5043. [DOI] [PubMed] [Google Scholar]
- 66. Lambert E, Straznicky N, Eikelis N, Esler M, Dawood T, Masuo K, Schlaich M & Lambert G (2007) Gender differences in sympathetic nervous activity: influence of body mass and blood pressure. J Hypertens 25, 1411–1419. [DOI] [PubMed] [Google Scholar]
- 67. Tank J, Heusser K, Diedrich A, Hering D, Luft FC, Busjahn A, Narkiewicz K & Jordan J (2008) Influences of gender on the interaction between sympathetic nerve traffic and central adiposity. J Clin Endocrinol Metab 93, 4974–4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Alvarez GE, Beske SD, Ballard TP & Davy KP (2002) Sympathetic neural activation in visceral obesity. Circulation 106, 2533–2536. [DOI] [PubMed] [Google Scholar]
- 69. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W et al. (2005) A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med 11, 875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Haga S, Yamamoto N, Nakai‐Murakami C, Osawa Y, Tokunaga K, Sata T, Yamamoto N, Sasazuki T & Ishizaka Y (2008) Modulation of TNF‐alpha‐converting enzyme by the spike protein of SARS‐CoV and ACE2 induces TNF‐alpha production and facilitates viral entry. Proc Natl Acad Sci USA 105, 7809–7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO, Simmons G, Hofmann H, Kuri T, Weber F et al. (2010) Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol 84, 1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang H, Penninger JM, Li Y, Zhong N & Slutsky AS (2020) Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46, 586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gurwitz D (2020) Angiotensin receptor blockers as tentative SARS‐CoV‐2 therapeutics. Drug Dev Res. doi: 10.1002/ddr.21656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fung ML (2014) The role of local renin‐angiotensin system in arterial chemoreceptors in sleep‐breathing disorders. Front Physiol 5, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Li J, Feng C et al. (2020) Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 63, 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li YC, Bai WZ & Hashikawa T (2020) The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol 92, 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pedersen SF & Ho YC (2020) SARS‐CoV‐2: a storm is raging. J Clin Invest 130, 2202–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lee YB, Nagai A & Kim SU (2002) Cytokines, chemokines, and cytokine receptors in human microglia. J Neurosci Res 69, 94–103. [DOI] [PubMed] [Google Scholar]
- 79. Recinos A 3rd, LeJeune WS, Sun H, Lee CY, Tieu BC, Lu M, Hou T, Boldogh I, Tilton RG & Brasier AR (2007) Angiotensin II induces IL‐6 expression and the Jak‐STAT3 pathway in aortic adventitia of LDL receptor‐deficient mice. Atherosclerosis 194, 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yamamoto S, Yancey PG, Zuo Y, Ma LJ, Kaseda R, Fogo AB, Ichikawa I, Linton MF, Fazio S & Kon V (2011) Macrophage polarization by angiotensin II‐type 1 receptor aggravates renal injury‐acceleration of atherosclerosis. Arterioscler Thromb Vasc Biol 31, 2856–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lu P, Liang LW, Xu AL, Sun YY, Jiang SJ & Shi Z (2020) Pro‐inflammatory cytokines in the paraventricular nucleus mediate the adipose afferent reflex in rats. Pflugers Arch 472, 343–354. [DOI] [PubMed] [Google Scholar]
- 82. Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli‐Berg FM, Madhur MS, Tomaszewski M, Maffia P, D’Acquisto F et al. (2020) COVID‐19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res 116, 1666–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Custaud MA, Belin de Chantemele E, Larina IM, Nichiporuk IA, Grigoriev A, Duvareille M, Gharib C & Gauquelin‐Koch G (2004) Hormonal changes during long‐term isolation. Eur J Appl Physiol 91, 508–515. [DOI] [PubMed] [Google Scholar]
- 84. Xia N & Li H (2018) Loneliness, social isolation, and cardiovascular health. Antioxid Redox Signal 28, 837–851. [DOI] [PMC free article] [PubMed] [Google Scholar]


