Abstract
Objectives
The aim of the study was to systematically review current studies reporting on clinical outcomes in people living with HIV (PLHIV) infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2).
Methods
We conducted a systematic review using the Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) guidelines. A comprehensive literature search was conducted in Global Health, SCOPUS, Medline and EMBASE using pertinent key words and Medical Subject Headings (MeSH) terms relating to coronavirus disease 2019 (COVID‐19) and HIV. A narrative synthesis was undertaken. Articles are summarized in relevant sections.
Results
Two hundred and eighty‐five articles were identified after duplicates had been removed. After screening, eight studies were analysed, totalling 70 HIV‐infected patients (57 without AIDS and 13 with AIDS). Three themes were identified: (1) controlled HIV infection does not appear to result in poorer COVID‐19 outcomes, (2) more data are needed to determine COVID‐19 outcomes in patients with AIDS and (3) HIV‐infected patients presenting with COVID‐19 symptoms should be investigated for superinfections.
Conclusions
Our findings suggest that PLHIV with well‐controlled disease are not at risk of poorer COVID‐19 disease outcomes than the general population. It is not clear whether those with poorly controlled HIV disease and AIDS have poorer outcomes. Superimposed bacterial pneumonia may be a risk factor for more severe COVID‐19 but further research is urgently needed to elucidate whether PLHIV are more at risk than the general population.
Keywords: AIDS, coronavirus disease 2019, HIV, severe acute respiratory syndrome coronavirus
Introduction
In December 2019, a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), emerged in the city of Wuhan, China. SARS‐CoV‐2 causes coronavirus disease 2019 (COVID‐19), which has resulted in the most catastrophic pandemic in modern history [1]. Presentation can be asymptomatic or consist of mild symptoms, from cough and fever to severe and life‐threatening acute respiratory distress syndrome (ARDS), sepsis, multi‐organ failure and death [2]. There is no current specific treatment for COVID‐19 but rather organ support is provided, and severe cases require admission to hospital for supportive management including mechanical ventilation.
Evidence is emerging that suggests that increasing age, hypertension and diabetes are risk factors that correlate with worse outcomes [3, 4]. However, it is not clear if people living with HIV (PLHIV) are at greater risk than the general population [5]. Left untreated, HIV infection results in a reduced number of CD4 T cells, leading to AIDS. AIDS is defined as a CD4 T‐cell count < 200 cells/μL [3] or the presence of an AIDS‐defining illness [6]. In 2018, it was estimated 37.9 million people worldwide have HIV infection, 23.3 million of whom are on treatment with antiretroviral therapy (ART) [7]. Eighty‐six per cent of those on treatment have successful viral suppression, resulting in undetectable viral load and untransmissible disease, known as U = U [8, 9, 10]. If ART is maintained and adhered to, PLHIV are not immunocompromised. [11]. Despite this, PLHIV may be at risk of severe COVID‐19, especially in areas where HIV infection is poorly controlled.
Limited evidence is available on the impact of HIV on SARS‐CoV‐2 infection and on whether it has any effect on COVID‐19 outcomes [12]. There is a need to understand whether PLHIV are at greater risk of severe illness so that adequate preventative measures can be put in place.
The aim of this systematic review was to identify studies that discuss PLHIV who have been infected with SARS‐CoV‐2 and that report whether coinfection results in a greater risk of adverse outcomes and, furthermore, whether controlled HIV infection vs. uncontrolled HIV infection or AIDS results in different COVID‐19 disease outcomes. We define controlled HIV infection as an undetectable viral load and a CD4 count ≥ 200 cells/μL.
Materials and methods
Search strategy
A comprehensive literature search was carried out in Global Health, SCOPUS, Medline and EMBASE to identify articles that discussed HIV‐positive patients and the clinical implications of HIV infection in COVID‐19 in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) guidelines [13]. Keywords were deconstructed into two categories (Table 1). Pertinent keywords and Medical Suject Headings (MeSH) terms related to these categories were used to maximize the output from the literature search. All relevant articles were identified and screened by two authors; the results are summarized in a narrative manner in each relevant section within the text of this review.
Table 1.
Search terms divided into two categories, coronavirus disease 2019 (COVID‐19) and HIV
| Category | Search terms |
|---|---|
| COVID‐19 | “coronavirus” OR “nCoV*” OR “2019‐nCoV” OR “COVID*” OR “SARS‐CoV*” |
| HIV | “HIV” OR “human immunodeficiency virus*” OR “AIDS” OR “acquired immunodeficiency syndrome” |
Inclusion and exclusion criteria
Inclusion and exclusion criteria are outlined in Table 2. Studies were included if they discussed the correlation between confirmed HIV infection and the diagnosis or prediction of severity of COVID‐19.
Table 2.
Inclusion and exclusion criteria for the search
| Inclusion criteria | Exclusion criteria |
|---|---|
| Exposure: confirmed diagnosis of COVID‐19 | Editorials, letters to the editor, consensus documents, commentaries, and studies discussing the psychosocial impacts of COVID‐19 on HIV‐infected patients |
| Population/outcome: HIV‐positive patients and COVID‐19 disease outcomes | Published before January 2019 |
| Date range: papers published from 2019 to present | Does not contain primary data |
| In the English language | In languages other than English |
COVID‐19, coronavirus disease 2019.
Data extraction
All articles were screened by two authors and any disagreement was resolved by consensus or the involvement of a third author. Data were extracted by two authors and validated by a third author.
Quality assessment
The quality of each publication was evaluated by two independent reviewers according to a predefined scoring system, using the National Heart Lung and Blood Institute (NIH) quality assessment tool for case series and case–control studies, as appropriate (Table 3) [14].
Table 3.
Summary of data extracted from studies included in review
| First author and pub. year | Article title | Location | Study design (quality score) | Population | Population and outcomes |
|---|---|---|---|---|---|
| Blanco et al., 2020 | COVID‐19 in patients with HIV: clinical case series | Spain, Barcelona | Case series (12/18) |
|
Outcomes
|
| Härter et al., 2020 | COVID‐19 in people living with human immunodeficiency virus: a case series of 33 patients | Germany | Case series, Retrospective (13/18) |
|
Main symptoms did not differ from general population Outcomes
|
| Guo et al., 2020 | A survey for COVID‐19 among HIV/AIDS patients in two districts of Wuhan, China | China, Wuhan | Case series (8/18) |
|
|
| Wang et al., 2020 | Case report: one case of Coronavirus disease 2019 (COVID‐19) in patient co‐infected by HIV with a low CD4 T cell count | China, Wuhan | Case report (7/18) |
|
|
| Zhao et al., 2020 | Early virus clearance and delayed antibody response in a case of COVID‐19 with a history of co‐infection with HIV‐1 and HCV | China, Shenzhen | Case report (12/18) |
|
|
| Yang et al., 2020 | The reflection on an AIDS patient with asymptomatic COVID‐19 | China, Wuhan | Case report (10/18) |
|
|
| Haddad et al., 2020 | Encephalopathy and seizure activity in a COVID‐19 well controlled HIV patient | USA | Case report (14/18) |
|
|
| Karmen‐Tuohy et al., 2020 | Outcomes among HIV‐positive patients hospitalized with COVID‐19 | USA, New York | Case control (22/24) |
|
|
ART, antiretroviral therapy; COPD, chronic obstructive pulmonary disease; COVID‐19, coronavirus disease 2019; CRP, C‐reactive protein; CT, computed tomography; ICU, intensive care unit; Ig, immunoglobulin; PLHIV, people living with HIV; RT‐PCR, reverse transcriptase–polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Statistical analysis
It was not possible to conduct an appropriate meta‐analysis because there were not enough research data in the studies on this subject.
Results
A PRISMA flow chart for the literature search is shown in Figure 1. A total of 445 articles were found. After removal of duplicates, a total of 285 articles were used for full‐text screening and, finally, only eight studies were included in our analysis. Table 3 summarizes the study characteristics and the data extracted. A narrative synthesis was conducted as a consequence of the qualitative nature of the studies analysed. The themes that emerged were (1) controlled HIV infection does not appear to result in poorer COVID‐19 outcomes than those found in the general population, (2) more data are needed for COVID‐19 outcomes in AIDS patients and (3) HIV‐infected patients presenting with COVID‐19 symptoms should be investigated for superinfections. These themes are explored in a narrative manner in the sections below.
Fig. 1.
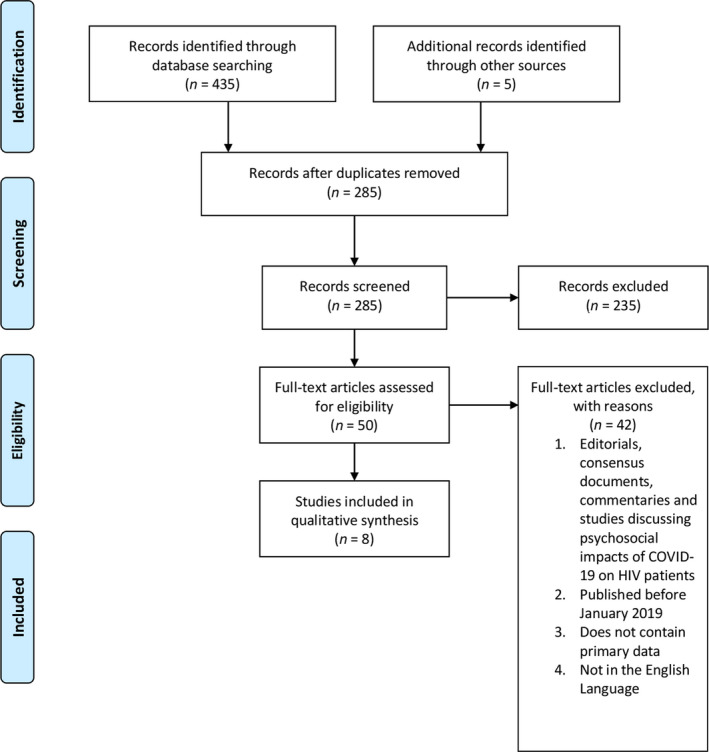
Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) chart for the literature search.
Discussion
Controlled HIV infection (undetectable viral load and normal CD4 T‐cell count) and COVID‐19 outcomes
Of the studies analysed, one case report reported a difference between the symptoms of COVID‐19 in PLHIV and the symptoms of COVID‐19 as defined by the WHO [15]. Haddad et al., reported encephalopathy and seizure activity in a patient with controlled HIV on day 8 of their symptoms with COVID‐19. This was not seen in any other studies, and the patient made a full recovery [16]. The other studies included in our review all reported that symptoms of COVID‐19, such as cough, fever, malaise and breathlessness, in PLHIV were not dissimilar to the normal population [15]. This indicates that co‐infection HIV and SAR‐CoV‐2 does not appear to cause a different presentation. Two studies reported that one patient in Wuhan was asymptomatic despite a positive RT‐PCR test for SAR‐CoV‐2 RNA from a nasopharyngeal swab [17, 18]. No other study tested asymptomatic PLHIV and therefore it is not clear what proportion of PLHIV experience asymptomatic infection and how this compares to the general population. The rate of asymptomatic infection in PLHIV is likely to be underestimated.
Blanco et al. [19], studied five patients in Barcelona; they report four patients with controlled HIV, three recovered from COVID‐19, one patient did not need treatment and recovered well, whilst another patient had developed severe COVID‐19 and required mechanical ventilation in ICU. This patient was 49 with hypothyroidism. All patients were on ART before they were admitted.
Härter et al. [20], investigated the outcomes of 32 patients coinfected with HIV and SARS‐CoV‐2 in a retrospective case series in Germany. Ninety percent of the cohort made a full recovery with 76% experiencing mild symptoms, while the reported mortality was 9%. Mortality, hospitalisation and critical case rate was higher than the control population. The study only looked at symptomatic patients, so this is likely an overestimation. Of the three patients that died, one patient was 82 years old, another had a CD4 T‐Cell count of 69/mm3 and the other patient had multiple comorbidities including hypertension, type 2 diabetes and chronic obstructive pulmonary disease (COPD). The mean age of those included in the study was 48 years old and 90% (30) of the patients were male, therefore this is not representative of the older population or females with HIV. Confounding factors were not accounted for in the methodology.
The findings are supported by a similar study in Wuhan, China by Guo et al. [17] of 1178 patients with HIV. Eight patients with symptoms were diagnosed with COVID‐19 (six via Nasopharyngeal swab and two via CT Chest). This represented 0.68% of patients with HIV who were surveyed, slight but not significantly higher than the percentage of people diagnosed with COVID‐19 in Wuhan (0.5%). Outcomes for were that six recovered fully after mild illness, one experienced severe illness but recovered and one patient died. All patients had HIV controlled with ART. Neither study was controlled or matched for HIV negative patients, however the mortality rates do not appear to be different to those in the general population in Wuhan [21]. Zhao et al. [21], also highlighted a case study of a HIV patient with SARS‐CoV2 co‐infection, diagnosis of viral pneumonia was made on clinical examination and chest CT findings. Three nasopharyngeal swabs SARS‐CoV‐2 were negative. SARS‐CoV‐2 infection was confirmed with antibody testing at a later date. The patient made a full recovery without the need for admission to ICU or ventilation.
Furthermore, Karmen‐Tuohy et al. [22] showed no statistical difference in outcomes of COVID‐19 between PLHIV and the general population. Twenty‐one patients with HIV and SARS‐CoV‐2 co‐infection were matched to 42 HIV negative patients with SARS‐CoV‐2, with no difference in demographics or comorbidities. Twenty of the 21 patients with HIV had normal CD4 T‐Cell counts and were virally suppressed. These patients are not representative of AIDS patients.
COVID‐19 outcomes in patients with AIDS (CD4 T‐Cell Count < 200 cells/mm3)
Blanco et al. [19], reported one case of SARS‐CoV‐2 in a patient who was ART naïve and a late diagnosis of HIV. The patient presented with a CD4 T‐Cell count of 13 cells/mm3 and had superimposed Pneumocystis jirovecii pneumonia (an AIDS defining organism) but no other comorbidities [23]. The patient was admitted to ICU and required non‐invasive ventilation (NIV), responded well to treatment with oxygen, antibiotics, corticosteroids and hydroxychloroquine and was discharged after 12 days. Härter et al. [20], reported that one patient out of three who died had a low CD4 T‐Cell count of 69 cells/mm3 with no other stated comorbidities. The study also included three other patients with low CD4 T‐Cell counts who recovered from COVID‐19, but no further information regarding treatment, disease was given.
Yang et al. [18], presented a case of a patient with a low CD4 T‐Cell Count (21 cells/mm3) and Kaposi's Sarcoma; this patient was also discussed by Guo et al. [17] The patient tested positive for SARS‐CoV‐2 and remained asymptomatic until two negative tests, with no abnormal findings on CT chest. In contrast, Wang et al., reported a patient with 34 CD4 T Cells/mm3 who experienced a 2‐month disease course, far exceeding the typical natural history of COVID‐19 [24]. Viral pneumonia was confirmed via CT chest but SARS‐CoV‐2 RT‐PCR was negative on four occasions. One positive test for ORF1ab (but not the N‐gene) confirmed SARS‐CoV‐2. The patient tested negative twice for SARS‐CoV‐2 antibodies, but later tested positive for IgM antibodies, indicating a delayed immune response which may explain the prolonged disease course.
It is not clear if there is an increased risk of worse outcomes of COVID‐19 for AIDS patients. Outcomes in included studies have ranged from asymptomatic infection to death. Until more good quality, case‐controlled studies are produced, conclusions cannot be drawn on this point.
The risk of superimposed bacterial pneumonia
We found reports of superimposed bacterial pneumonia with COVID‐19. These patients generally had poorer outcomes irrespective of controlled HIV or AIDS. Blanco et al. [19], described a case of Pneumocystis jirovecii in a patient with AIDS and COVID‐19 which responded well to antibiotics, as discussed earlier in this review. They raised the issue of ensuring that pulmonary opportunistic infections are considered in the differential diagnoses of SARS‐CoV‐2 and HIV co‐infection, particularly in AIDS patients. This was supported by Karmen‐Tuohy et al. [22], who found three patients with HIV and COVID‐19 developed superimposed non‐AIDS related bacterial pneumonia, compared to only one HIV negative patient with COVID‐19. All of the patients who developed a superimposed bacterial pneumonia died in this study, despite receiving antibiotic treatment. Whilst this is a small cohort of patients, it highlights potentially worse outcomes for patients who have a superimposed bacterial pneumonia with COVID‐19. Patients with HIV show a higher incidence of bacterial pneumonia, which is inversely proportional to CD4 T‐Cell count, when compared to the general population. Thus, superimposed bacterial pneumonia with COVID‐19 is a significant consideration in PLHIV [25, 26, 27, 28, 29].
Discussion
Recommendations
Current guidelines for PLHIV
At present, the COVID‐19 guidelines for well‐controlled HIV infection state that it is unlikely that PLHIV are at any greater risk of contracting COVID‐19 or experiencing more severe disease than the general population [12, 13]. Our findings support this conclusion, but the data are currently very limited. The recommendations in both the UK and USA are in line with the infection control measures provided to the general public [30]. Specific recommendations include ensuring at least a 30‐day supply of ART and advising up‐to‐date vaccinations including pneumococcal and influenza vaccines [12, 13, 14]. Clinicians should reassure PLHIV that, if the HIV infection is controlled, their risk of serious complications of COVID‐19 and therefore poor outcomes is likely to be low. However, the presence of known COVID‐19 risk factors may put them at greater risk of worse outcomes. The same cannot be said for poorly controlled HIV infection or AIDS.
SARS, Middle East respiratory syndrome (MERS) and H1N1
In previous outbreaks of SARS, H1N1 and Middle East respiratory syndrome (MERS), HIV infection was not associated with increased disease severity. In the SARS and MERS outbreaks, there were only a few reports of mild disease among PLHIV [31, 32]. The risk factors for these outbreaks were similar to those being observed for SARS‐CoV‐2. H1N1 outcomes were similar to outcomes for SARS‐CoV‐2, whereby clinical outcomes of those with well‐controlled HIV infection were similar to those of the general population [33, 34]. A literature review by Cooper [35] further highlighted that HIV‐infected patients and patients who were immunocompromised for other reasons recovered from H1N1 infection without complications.
SARS‐CoV‐2 cannot be presumed to be the same as these viruses. We do not know enough about its mechanisms of action to be sure of the impact of HIV infection in those coinfected with SARS‐CoV‐2 and HIV. However, data from previous outbreaks are reassuring at least in that well‐controlled HIV infection did not pose a greater risk of more severe disease compared with the general population.
PLHIV with undiagnosed HIV infection and those with a confirmed diagnosis not being treated with ART
We identified a small number of studies specifically looking at PLHIV and COVID‐19. We identified two patients who were diagnosed with HIV infection at the same time as SARS‐CoV‐2 infection who were ART naïve [19, 24] . Both patients had low CD4 T‐cell counts. In 2018, an estimated 8.1 million cases of HIV infection globally were undiagnosed and 28% of patients known to be infected were not on treatment [7]. We recommend that, if a clinical picture suggests viral pneumonia, such as symptoms of COVID‐19 or typical viral pneumonitis on a computed tomography (CT) scan (including mild and severe cases of COVID‐19), patients should be offered testing for HIV to rule out undetected HIV infection.
Confirmation of COVID‐19, differential diagnosis and coinfection
We found a disparity in methods of COVID‐19 diagnosis between studies. In six studies, SARS‐CoV‐2 was confirmed by reverse transcriptase–polymerase chain reaction (RT‐PCR) [16, 17, 18, 19, 20, 22], and in two studies COVID‐19 was diagnosed using CT thorax findings [17, 21].In order to ensure that the diagnosis of COVID‐19 is correct and other differential diagnosis are ruled out , we suggest that future studies reporting detection of the virus should use a consistent method of diagnosising COVID‐19. Also, other causes of pneumonia should be screened for and ruled out to ensure that data are accurate both to confirm the causative agent and to identify any coinfection that may exacerbate symptoms and severity of COVID‐19.
Viral pneumonia is generally more severe if a concurrent bacterial pneumonia is present, in both the normal population and PLHIV. This is a plausible reason why some patients included in this review had more severe COVID‐19 [25, 26, 27, 28, 29]. We recommend that sputum and blood cultures should be taken early for detection of superimposed bacterial pneumonia and the presence of other causative agents.
Wider considerations for PLHIV during the COVID‐19 pandemic
Telemedicine
The continuation of out‐patient care for PLHIV presents unique challenges as hospitals deal with COVID‐19 patients. PLHIV could be deterred from accessing HIV care as a consequence of the perceived risks of attending hospitals, and also the risk that out‐patient services may be affected by the re‐deployment of health care professionals to other areas of the hospital. This presents an opportunity for the advancement of telemedicine, the remote diagnosis and treatment of a patient using technology. Young et al. [36] and Ohl et al. [37] both found that, where telemedicine was available to patients, greater viral suppression was achieved. Rogers et al. [38] have described an effective implementation of telemedicine in a clinic in the USA, to ensure continuity of care during the COVID‐19 pandemic. Clinics who implemented telemedicine also reported fewer missed appointments and higher patient engagement. Furthermore, studies showed that text message services resulted in significant improvements in ART adherence and rates of viral suppression in sub‐Saharan Africa [39, 40].
Telemedicine can therefore be effectively used to manage patients with HIV infection, without the need for them to attend clinic, allowing patients to avoid unnecessary journeys to hospitals and clinics and to maintain social distancing. Having said this. as stressed by Mgbako et al. [41], this must be led by a patient‐centred approach, ensuring good communication as well as a good professional–patient relationship.
The psychological impact of COVID‐19 on PLHIV
The disruption in the continuity of care for PLHIV, increased social isolation and the psychological stress of living through a pandemic are all factors that could worsen the mental health problems that PLHIV are at a higher risk of experiencing [42]. PLHIV are more likely to experience social isolation; however, as a consequence of measures to prevent the spread of COVID‐19, this experience may be amplified, which may have a negative effect on mental health [43]. Furthermore, COVID‐19 will potentially exacerbate other psychological stressors such as food insecurity and increased societal stigma [42, 44, 45]. These psychosocial stressors further may increase the risk of adverse health outcomes among PLHIV as a consequence of reduced medication adherence, leading to failure to achieve adequate HIV management [42].
Future research
Whilst further high‐quality studies would be beneficial to confirm that there is no difference in outcomes of COVID‐19 between PLHIV who have controlled HIV infection and the general population, as a consequence of the gap in knowledge about outcomes in AIDS patients with COVID‐19, there needs to be an urgent focus on high‐quality research looking at outcomes for patients with AIDS and COVID‐19. Furthermore, more research into the impact and incidence of superimposed bacterial pneumonia is needed in both the general population and PLHIV. This would help to influence management and provide a good basis for developing treatment pathways that may reduce the trend towards higher mortality.
Limitations
The limitations of this review were the small number of studies included in our analysis that were relevant to our outcomes. The studies included were all case series or case reports with small sample sizes, and confounding variables were not accounted for in the reporting of such data. We acknowledge that, in view of the nature of the study designs included in our analysis, our interpretations and conclusions should be treated with caution.
Conclusions
Our findings indicate that currently PLHIV are not likely to be at increased risk of poorer outcomes of COVID‐19 disease than the general population if they have an undetectable viral load and an adequate CD4 count. This suggests that well‐managed HIV infection is not a risk factor for more severe COVID‐19. However, it is unknown if poorly controlled HIV infection and AIDS put people at a greater risk of severe COVID‐19. Bacterial superinfection appears to be a potential risk factor for poorer outcomes in PLHIV. Therefore, our findings highlight the need for further investigation to elucidate the impact of HIV infection in COVID‐19.
Conflicts of interest: None to be declared.
Financial disclosure: None to be declared.
References
- 1. European Centre for Disease Prevention and Control . Event background COVID‐19. Available at https://www.ecdc.europa.eu/en/novel‐coronavirus/event‐background‐2019 (accessed 18 May 2020).
- 2. Zaim S, Chong J, Sankaranarayanan V, Harky A. COVID‐19 and Multiorgan Response. Current Problems in Cardiology. 2020;45: 8: 100618 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jordan RE, Adab P, Cheng KK. Covid‐19: risk factors for severe disease and death. BMJ 2020; 368: m1198. [DOI] [PubMed] [Google Scholar]
- 4. Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Li Q, Jiang C, Zhou Y, Liu S, Ye C, Zhang P, Xing Y, Guo H, Tang W. Risk factors of critical & mortal COVID‐19 cases: A systematic literature review and meta‐analysis. Journal of Infection. 2020; 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vishnevetsky A, Levy Mi. Rethinking high‐risk groups in COVID‐19. Mult Scler Relat Disord 2020; 42: 102139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siedner MJ, Triant V. Undetectable = untransmittable and your health: the personal benefits of early and continuous therapy for HIV infection. J Infect Dis 2019; 219: 173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. HIV.gov . Global statistics. 2019. Available at https://www.hiv.gov/hiv‐basics/overview/data‐and‐trends/global‐statistics (accessed 18 May 2020).
- 8. Alison R, Tina B, Matthew W et al. Partners of people on ART ‐ a new evaluation of the risks (The PARTNER Study): design and methods. BMC Public Health 2012; 12: 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodger AJ, Cambiano V, Bruun T et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV‐positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet 2019; 393: 2428–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Irini S, Gulick RM, Sonya K et al. ART in HIV‐positive persons with low pretreatment viremia: results from the START trial. J Acquir Immune Defic Syndr 1999; 81: 456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joob B, Wiwanitkit V. SARS‐CoV‐2 and HIV. Journal of Medical Virology. 2020; 10.1002/jmv.25782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. AIDSinfo . Interim guidance for COVID‐19 and persons with HIV COVID‐19 and persons with HIV (Interim Guidance). Available at https://aidsinfo.nih.gov/guidelines/html/8/covid‐19‐and‐persons‐with‐hiv–interim‐guidance‐/554/interim‐guidance‐for‐covid‐19‐and‐persons‐with‐hiv (accessed 18 May 2020).
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Heart, Lung, and Blood Institute (NHLBI) . Study Quality Assessment Tools. Available at https://www.nhlbi.nih.gov/health‐topics/study‐quality‐assessment‐tools (accessed 19 May 2020).
- 15. WHO . Coronavirus [online]. Available at https://www.who.int/westernpacific/health‐topics/coronavirus (accessed 18 May 2020).
- 16. Haddad S, Tayyar R, Risch L et al. Encephalopathy and seizure activity in a COVID‐19 well controlled HIV patient. IDCases 2020; 21: e00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo W, Weng HL, Bai H et al. [Quick community survey on the impact of COVID‐19 outbreak for the healthcare of people living with HIV]. Zhonghua Liu Xing Bing Xue Za Zhi 2020; 41: 663–667. [DOI] [PubMed] [Google Scholar]
- 18. Yang R, Gui X, Gao S, Mo P, Ke H, Zhang Y, Xiong Y.The reflection on an AIDS patient with asymptomatic COVID‐19 ‐ abstract ‐ Europe PMC. Available at https://europepmc.org/article/ppr/ppr129367 (accessed 18 May 2020).
- 19. Blanco JL, Ambrosioni J, Garcia F et al. COVID‐19 in patients with HIV: clinical case series. The Lancet HIV 2020; 7: e314–e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Härter G, Spinner C, Roider J, Bickel M, Krznaric I, Grunwald S, Schabaz F, Gillor D, Postel N, Mueller M, Müller M, Römer K, Schewe K, Hoffmann C. COVID‐19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection. 2020; 10.1007/s15010-020-01438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao J, Liao X, Wang H, Wei L, Xing M, Liu L, Zhang Z. Early Virus Clearance and Delayed Antibody Response in a Case of Coronavirus Disease 2019 (COVID‐19) With a History of Coinfection With Human Immunodeficiency Virus Type 1 and Hepatitis C Virus. Clinical Infectious Diseases. 2020; 10.1093/cid/ciaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karmen‐Tuohy S, Carlucci PM, Zacharioudakis IM et al. Outcomes among HIV‐positive patients hospitalized with COVID‐19. MedRxiv. Available at https://www.medrxiv.org/content/10.1101/2020.05.07.20094797v1 (accessed 18 May 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang L, Cattamanchi A, Davis JL et al. HIV‐associated pneumocystis pneumonia. Proc Am Thorac Soc 2011; 8: 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang M, Luo L, Haiji B, Xia H. Case report: one case of coronavirus desease 2019(COVID‐19) in patient co‐Nfected by HIV with a low CD4+ T cell count. Int J Infect Dis 2020; 96: 148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cillóniz C, García‐Vidal C, Moreno A, Miro JM, Torres A. Community‐acquired bacterial pneumonia in adult HIV‐infected patients. Expert Rev Anti Infect Ther 2018; 16: 579–88. [DOI] [PubMed] [Google Scholar]
- 26. Feikin DR, Feldman C, Schuchat A, Janoff EN. Global strategies to prevent bacterial pneumonia in adults with HIV disease. Lancet Infect Dis 2004; 4: 445–455. [DOI] [PubMed] [Google Scholar]
- 27. Søgaard OS, Reekie J, Ristola M et al. Severe bacterial non‐aids infections in HIV‐positive persons: incidence rates and risk factors. J Infect 2013; 66: 439–446. [DOI] [PubMed] [Google Scholar]
- 28. Kohli R, Lo Y, Homel P et al. Bacterial pneumonia, HIV therapy, and disease progression among HIV‐infected women in the HIV epidemiologic research (HER) study. Clin Infect Dis 2006; 43: 90–98. [DOI] [PubMed] [Google Scholar]
- 29. Croxford S, Kitching A, Desai S et al. Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: an analysis of a national observational cohort. Lancet Public Health 2017; 2: e35–e46. [DOI] [PubMed] [Google Scholar]
- 30. Coronavirus (COVID‐19) and HIV – update from the British HIV Association (BHIVA). Available at https://www.bhiva.org/BHIVA‐statement‐on‐COVID‐19 (accessed 13 March 2020).
- 31. Al‐Omari A, Rabaan AA, Salih S, Al‐Tawfiq JA, Memish ZA. MERS coronavirus outbreak: implications for emerging viral infections. Diagn Microbiol Infect Dis 2019; 93: 265–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moni MA, Liò P. Network‐based analysis of comorbidities risk during an infection: SARS and HIV case studies. BMC Bioinformatics 2014; 15: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feldman C. Potential Impact of SARS‐CoV‐2 infection in HIV‐positive patients in South Africa. Wits J Clin Med 2020; 2: 19–24 [Google Scholar]
- 34. Sheth AN, Patel P, Peters PJ. Influenza and HIV: lessons from the 2009 H1N1 influenza pandemic. Curr HIV/AIDS Rep 2011; 8: 181–191. [DOI] [PubMed] [Google Scholar]
- 35. Cooper CL. Pandemic H1N12009 Influenza and HIV: a review of natural history, management and vaccine immunogenicity. Curr Opin Infect Dis 2012; 25: 26–35. [DOI] [PubMed] [Google Scholar]
- 36. Young JD, Patel M, Badowski M et al. Improved virologic suppression with HIV subspecialty care in a large prison system using telemedicine: an observational study with historical controls. Clin Infect Dis 2014; 59: 123–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ohl ME, Richardson K, Rodriguez‐Barradas MC et al. Impact of availability of telehealth programs on documented HIV viral suppression: a cluster‐randomized program evaluation in the veterans health administration. Open Forum Infect Dis 2019; 6: ofz206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rogers B, Coats C, Adams E, Murphy M, Stewart C, Arnold T, Chan P, Nunn A. Development of Telemedicine Infrastructure at an LGBTQ+ Clinic to Support HIV Prevention and Care in Response to COVID‐19, Providence, RI. AIDS and Behavior. 2020; 10.1007/s10461-020-02895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bigna JJR, Noubiap JJN, Kouanfack C, Plottel CS, Koulla‐Shiro S. Effect of mobile phone reminders on follow‐up medical care of children exposed to or infected with HIV in Cameroon (MORE CARE): a multicenter, single‐blind, factorial, randomized controlled trial. Lancet Infect Dis 2014; 14: 600–608. [DOI] [PubMed] [Google Scholar]
- 40. Lester RT, Ritvo P, Mills EJ et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomized trial. Lancet 2010; 376: 1838–1845. [DOI] [PubMed] [Google Scholar]
- 41. Mgbako O, Miller E, Santoro A, Remien R, Shalev N, Olender S, Gordon P, Sobieszczyk M. COVID‐19, Telemedicine, and Patient Empowerment in HIV Care and Research. AIDS and Behavior. 2020;24: 7:1990–1993. 10.1007/s10461-020-02926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shiau S, Krause K, Valera P, Swaminathan S, Halkitis P. The Burden of COVID‐19 in People Living with HIV: A Syndemic Perspective. AIDS and Behavior. 2020; 10.1007/s10461-020-02871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marziali M, Card K, McLinden T, Wang L, Trigg J, Hogg R. Physical Distancing in COVID‐19 May Exacerbate Experiences of Social Isolation among People Living with HIV. AIDS and Behavior. 2020; 10.1007/s10461-020-02872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. The Lancet HIV . The syndemic threat of food insecurity and HIV. Lancet HIV 2020; 7: e75. [DOI] [PubMed] [Google Scholar]
- 45. Sun S, Hou J, Chen Y, Lu Y, Brown L, Operario D. Challenges to HIV Care and Psychological Health During the COVID‐19 Pandemic Among People Living with HIV in China. AIDS and Behavior. 2020; 10.1007/s10461-020-02903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


