Abbreviations
- ACE2
angiotensin‐converting enzyme‐2
- anti‐CCP
antibodies to cyclic citrullinated peptide
- COVID‐19
novel coronavirus disease 2019
- NSAID
nonsteroidal anti‐inflammatory drug
- ReA
reactive arthritis
- RF
Rheumatoid factor
- SARS‐CoV‐2
novel coronavirus
1. INTRODUCTION
New type pneumonia, later termed the novel coronavirus disease 2019 (COVID‐19) arose from Wuhan Province in China in December 2019, has quickly become a global public health emergency. 1 The etiological agent was identified as a new type coronavirus, named as novel coronavirus (SARS‐CoV‐2). 2 Outbreak has rapidly spread all over the world 3 and the first confirmed COVID‐19 patient in Turkey was reported on 11th March 2020. 2 A syndrome of dysregulated and systemic immune overactivation described as a cytokine storm or hyperinflammatory syndrome may develop during of the course COVID‐19. 4 As a result, coagulation and inflammation can significantly affect disease progression. 4 , 5 In this case, we report the first reactive arthritis associated with COVID‐19 infection.
2. CASE PRESENTATION
A 73‐year‐old man with diabetes mellitus, hypertension, and coronary heart disease presented to the emergency department with a history of fever, weakness, and dry cough lasting 1 week. He had not traveled or direct contacted COVID‐19 patients recently. Also, his wife had similar symptoms for 3 days.
On admission to the hospital, the temperature was 36.7°C, the pulse rate 78 beats per minute, the blood pressure 100/70 mm Hg, respiratory rate of 22 breaths per minute, and the oxygen saturation 96% at ambient air. Physical examination findings were normal.
Laboratory examination showed white blood cell count of 465 × 10^9/L, neutrophil count of 316 × 10^9/L, lymphocyte count of 101 × 10^9/L, a platelet count of 206 × 10^9/L, aspartate aminotransferase 51 U/L, alanine aminotransferase 44 U/L, creatinine 0.79 mg/dL.
The patient's serum C‐reactive protein, procalcitonin, D‐dimer concentrations were 0.0256 g/L, 0.03 µg/L, and 0.55 mg/L, respectively. The other laboratory examinations revealed that creatine phosphokinase level was 1161 U/L and lactate dehydrogenase level was 246 U/L. Nasopharyngeal and oropharyngeal swabs were positive for COVID‐19, negative for influenza and other respiratory viral infections. Chest computed tomography showed that bilateral, peripherally located ground‐glass opacities at upper and lower lobes.
Ceftriaxone, hidroxychloroquine, and azitromycin were administered. His cough, fever, and fatigue were improved gradually. He completed the 5‐day course of hydroxychloroquine and azithromycin, 7 days of ceftriaxone. In the follow‐up, repeated polymerase chain reaction tests for SARS‐CoV‐2 from nasopharyngeal and oropharyngeal swabs were negative twice. The patient received enoxaparin sodium prophylaxis during the hospitalization period.
Eight days after completion of COVID‐19 treatment swelling, redness, pain, and tenderness in the left first metatarsophalangeal, proximal and distal interphalangeal joints were developed (Figure 1A). Two days later similar findings appeared in the right second proximal and distal interphalangeal joints. At the same time markedly elevated CRP, ferritin, and D‐dimer levels were detected. Radiographic examinations of effected joints revealed normal findings. Venous and arterial Doppler ultrasound imaging of bilateral lower extremities showed no pathological signs. The screening laboratory tests for arthritis were unremarkable; rheumatoid factor, antibodies to cyclic citrullinated peptide were negative and uric acid level was within the normal range. Because of a typical pattern of clinical presentation, the patient was diagnosed with reactive arthritis (ReA) caused by COVID‐19. The patient was treated with a nonsteroidal anti‐inflammatory drug (NSAID). Figure 1B the symptoms of arthritis were completely resolved with NSAID therapy. All the laboratory tests were in normal limits. The patient was discharged from hospital on day 22.
Figure 1.
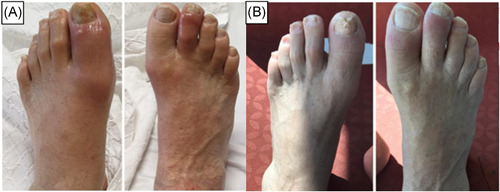
A, Images of arthritis in the left first metatarsophalangeal, proximal, distal interphalangeal joints, and right second proximal and distal interphalangeal joints. B, Images of affected joints after NSAID treatment. NSAID, nonsteroid anti‐inflammatory drug
3. DISCUSSION
To our knowledge, this is the first ReA case caused by COVID‐19. ReA belongs to a group of diseases known as spondyloarthritis is a sterile joint inflammation triggered by a distant infection in susceptible hosts. 5 , 6 The time required for the development of ReA after infection takes a few days to 4 weeks. 5 ReA is characterized with typically asymmetric monoarthritis or oligoarthritis of the lower extremities as seen in our patient. 5 Although classical ReA is associated with urogenital and gastrointestinal infections, nonclassical ReA can be triggered by most other infections. 6 Because of a typical pattern of clinical presentation of arthritis plus the evidence of COVID‐19 infection in the preceding 2 weeks; the patient was diagnosed with ReA caused by COVID‐19. Also, gastrointestinal manifestation was reported in approximately 12% of COVID‐19 patients. 7 Gastrointestinal system may serve as a secondary site for COVID‐19 infection in relation to the expression of angiotensin‐converting enzyme‐2 in the gastrointestinal tract. 8 ReA may be related to this involvement. COVID‐19 is not a disease that is fully understood yet. In the course of time, clinicians all over the world experienced several different forms of COVID‐19. This first reported ReA complication of COVID‐19 will raise the awareness of the physicians.
KEYWORDS
COVID‐19, oligoarticular, reactive arthritis, rheumatic involvement
REFERENCES
- 1. Le HT, Nguyen LV, Tran DM, et al. The first infant case of COVID‐19 acquired from a secondary transmission in Vietnam. Lancet Child Adolesc Health. 2020;4(5):405‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tufan ZK, Kayaaslan B. Crushing the curve, the role of national and international institutions and policy makers in COVID‐19 pandemic. Turk J Med Sci. 2020;50(SI‐1):495‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang W, Zhao Y, Zhang F, et al. The use of anti‐inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID‐19): The experience of clinical immunologists from China. Clin Immunol. 2020;214:108393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ingraham NE, Lotfi‐Emran S, Thielen BK, et al. Immunomodulation in COVID‐19. Lancet Respir Med. 2020;8(6):544–546. 10.1016/S2213-2600(20)30226-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Toivanen A, Toivanen P. Reactive arthritis. Best Pract Res Clin Rheumatol. 2004;18(5):689‐703. [DOI] [PubMed] [Google Scholar]
- 6. Kim T‐H, Uhm W‐S, Inman RD. Pathogenesis of ankylosing spondylitis and reactive arthritis. Curr Opin Rheumatol. 2005;17(4):400‐405. [DOI] [PubMed] [Google Scholar]
- 7. Parasa S, Desai M, Thoguluva C, et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019. JAMA Network Open. 2020;3(6):e2011335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gheblawi M, Wang K, Viveiros A, et al. Angiotensin‐converting enzyme 2: SARS‐CoV‐2 receptor and regulator of the renin‐angiotensin system. Circ Res. 2020;126:1457‐1475. [DOI] [PMC free article] [PubMed] [Google Scholar]


