Abstract
Italy was one of the most affected nations by coronavirus disease 2019 outside China. The infections, initially limited to Northern Italy, spread to all other Italian regions. This study aims to provide a snapshot of severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) epidemiology based on a single‐center laboratory experience in Rome. The study retrospectively included 6565 subjects tested for SARS‐CoV‐2 at the Laboratory of Virology of Sapienza University Hospital in Rome from 6 March to 4 May. A total of 9995 clinical specimens were analyzed, including nasopharyngeal swabs, bronchoalveolar lavage fluids, gargle lavages, stools, pleural fluids, and cerebrospinal fluids. Positivity to SARS‐CoV‐2 was detected in 8% (527/6565) of individuals, increased with age, and was higher in male patients (P < .001). The number of new confirmed cases reached a peak on 18 March and then decreased. The virus was detected in respiratory samples, in stool and in pleural fluids, while none of gargle lavage or cerebrospinal fluid samples gave a positive result. This analysis allowed to gather comprehensive information on SARS‐CoV‐2 epidemiology in our area, highlighting positivity variations over time and in different sex and age group and the need for a continuous surveillance of the infection, mostly because the pandemic evolution remains unknown.
Keywords: COVID‐19, epidemiology, laboratory diagnostics, SARS‐CoV‐2
Highlights
This is the first study reporting an epidemiological insight for the SARS‐CoV‐2 infection in Rome.
The containing measures have been effective to flatten epidemiological curve.
Gender and age are important contributor to lethality.
1. INTRODUCTION
A novel coronavirus, severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) causing human disease named coronavirus disease (COVID‐19), was first identified in Wuhan, China. 1 In early January 2020, this novel member of enveloped RNA coronavirus was detected in samples of bronchoalveolar lavage (BAL) fluid from a patient in Wuhan and subsequently confirmed by the Chinese Centre for Disease Control and Prevention as the cause of pneumonia cases of unknown origins emerged in December. 1 , 2
Despite the effort to stop the transmission of COVID‐19, the infection spread throughout mainland China, and in January 2020, cases were reported in Thailand, Japan, and South Korea. 3 , 4 On 11 March 2020 the infection reached the necessary epidemiological criteria to be declared a pandemic by the WHO, having spread to at least 114 countries worldwide. 5
The first Italian case of COVID‐19 has been assessed in Lombardy region on 20 February 2020. From that moment public health measures have been taken to contain the epidemic, initially located in some restricted areas, and extended by the government to all the Italian peninsula from 11 March 2020. 6 , 7
The territory was heterogeneously affected by the SARS‐CoV‐2 outbreak. Northern regions have experienced the highest burden, in contrast with the south and the islands where virus spread has been contained. 8 In fact, the Rome province showed, on the 4 May, a total of 4.948 positive cases compared to the 20.254 of Milan province on a national total of 211.938 positive cases. 9
In this scenario this paper aims to take a snapshot of the epidemiological characteristics of the population resulted positive for SARS‐CoV‐2 at Sapienza University Hospital “Policlinico Umberto I” in Rome starting from 6 March until 4 May.
2. MATERIALS AND METHODS
This study includes all individuals (n = 6565) who have been tested for SARS‐CoV‐2 at the Virology Laboratory of Sapienza University Hospital “Policlinico Umberto I” from 6 March through 4 May. A total of 9995 clinical specimens were analyzed. Of these, 9848 were upper and lower respiratory tract samples including nasopharyngeal swabs (n = 9461), BAL fluid (n = 367) and gargle lavage (n = 20), while 147 were nonrespiratory samples including stool (n = 134), pleural fluid (n = 8), and cerebrospinal fluid (CSF) (n = 5).
The RNA extraction from nasopharyngeal swabs and stool specimens was carried out with an automated sample preparation module using Versant SP 1.0 Reagents Kit (Siemens Healthcare Diagnostics Inc, Tarrytown, NY). The stool specimens were prepared as previously described. 10 RNA isolation and purification from BAL fluid, gargle lavage, pleural fluid, and CSF samples were achieved using the QIAamp viral RNA Mini Kit (Qiagen). The processing of all samples was accomplished following the technical guidelines on laboratory biosafety related to the COVID‐19 virus. 11
Detection of SARS‐CoV‐2 was performed by real‐time reverse transcription polymerase chain reaction (RT‐PCR) Assay (RealStar SARS‐CoV‐2 RT‐PCR; Altona Diagnostics) as indicated by WHO interim guidance to define a laboratory‐confirmed case. 12 Following logistical and clinical needs, other molecular methods were used (GeneFinder COVID‐19 Plus RealAmp Kit, Elitech; DiaSorin Molecular Simplexa COVID‐19 Direct EUA Assay, DiaSorin Molecular; Xpert Xpress SARS‐CoV‐2 assay, Cepheid). All tests and procedures were performed following the manufacturers' protocols.
The epidemiological and demographic data of infected patients, including age, gender, and residential district were acquired from diagnostic records.
Procedures performed in the study were in accordance with the ethical standards of the Institutional and National Research Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
2.1. Statistical analysis
χ 2 Test was used to analyze the differences in positivity between groups. The positivity trend over time was analyzed using the χ 2 test for trend. Mann‐Whitney U test was used to compare age between groups. Statistical tests were conducted two‐sided at a significance level of 0.05 using GraphPad Prism version 7.00 for Windows (GraphPad Software, La Jolla, CA).
3. RESULTS
Samples from 6565 individuals (males: 47.4%, 3110/6565) were processed for the detection of SARS‐CoV‐2 between 6 March 2020 and 4 May 2020. Data on age were available for 6327 patients (96.4%), which were divided in 10 age groups. The median age was 57 years (range: 1 day after birth‐99 years, interquartile range [IQR]: 41‐73). Most of the tested individuals (n = 4728, 72.0%) were hospitalized or admitted to the hospital emergency room of University Hospital Policlinico Umberto I; the remaining patients referred to other hospitals in Rome.
An overall increase was observed for the daily number of new confirmed cases and for the daily positivity rate during the first 2 weeks of observation. Both the parameters reached a peak on 18 March, with 34 newly diagnosed patients and a positivity rate of 29.6% (Figure 1). Thereafter, the number of positive cases declined, and after 29 March the positivity rate remained always below 10% (P < .0001).
Figure 1.
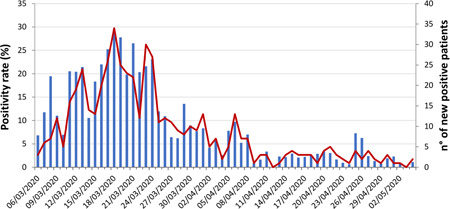
SARS‐CoV‐2 daily positivity during the period of observation. The arrow indicates the start of the nationwide COVID‐19 pandemic lockdown period in Italy. The bars represent positivity rate and the line represents number of positive patients. COVID‐19, coronavirus disease 2019; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2
During the 2 months long period of study, positivity to SARS‐CoV‐2 was found for 527 patients (8.0%) in at least one respiratory sample. Demographic characteristics of patients are summarized in Table 1. In Figure 2 a map of the city of Rome, subdivided in residential districts, highlights the number of positive cases from each district normalized for number of district population. Most part of positive patients referring to our center came from central and east areas of the city.
Table 1.
Patients' demographic data
| Sex | No. of SARS‐CoV‐2 positive patients (%) |
|---|---|
| Males | 305/3110 (9.8) |
| Females | 222/3455 (6.4) |
| Hospital | |
| Sapienza University Hospital Policlinico Umberto I | 394/4728 (8.3) |
| Others | 133/1837 (7.2) |
| Age, y | |
| 0‐10 | 0/142 (0) |
| 11‐20 | 3/121 (2.5) |
| 21‐30 | 26/705 (3.7) |
| 31‐40 | 45/850 (5.3) |
| 41‐50 | 61/1059 (5.8) |
| 51‐60 | 112/1228 (9.1) |
| 61‐70 | 105/847 (12.4) |
| 71‐80 | 85/644 (13.2) |
| 81‐90 | 76/591 (12.9) |
| 91‐99 | 12/139 (8.6) |
| Unknown | 2/238 (0.8) |
Abbreviation: SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2.
Figure 2.
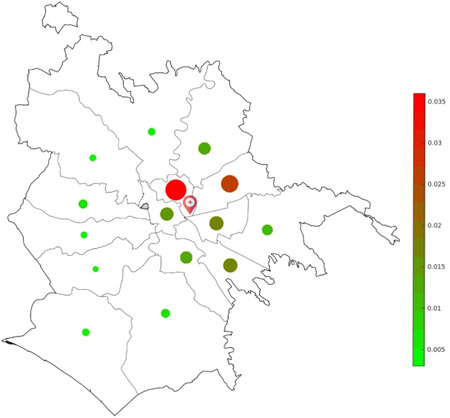
Prevalence of laboratory‐confirmed COVID‐19 cases in the different residential districts of Rome. The marker indicates the position of our hospital center. COVID‐19, coronavirus disease 2019
The median age of positive patients was 62 years (IQR: 50.5‐75). The number of positive individuals was significantly different stratifying patients for both sex and age (Figure 3). In particular, the positivity rate increased with age (P < .00001) and was higher in male patients compared to females (P < .00001). For all age groups the percentage of positive male individuals was always higher than in females, with a significant difference for the 41 to 50 (P < .05) and 51 to 60 years (P < .001) groups.
Figure 3.
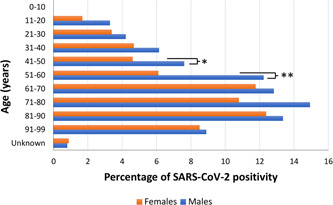
SARS‐CoV‐2 positive individuals stratified for sex and age (*P < .05, **P < .001). SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2
Among the positive patients hospitalized at Umberto I University Hospital, COVID‐19 related deaths, defined as those occurring in patients who tested positive for SARS‐CoV‐2 independently from pre‐existing diseases that may have caused or contributed to death, were estimated at 16.8% (66/393). According to sex, the number of deaths was higher in males (47/66, 71.2%) than in female patients (19/66, 28.8%). Median age of deceased patients was 74.5 (IQR: 66‐82.75), significantly higher compared to median age of positive patients (P < .00001). The lethality rate significantly increased with age (P < .00001), and the highest number of deaths was registered in the group aged 81 to 90 years (n = 21) (Table 2)
Table 2.
SARS‐CoV‐2 lethality according to age groups
| Age group, y | No. of deceased patients/positive patients (%) |
|---|---|
| 0‐20 | 0/1 (0) |
| 21‐30 | 1/6 (16.7) |
| 31‐40 | 0/20 (0) |
| 41‐50 | 2/31 (6.5) |
| 51‐60 | 6/66 (9.1) |
| 61‐70 | 18/69 (26.1) |
| 71‐80 | 15/57 (26.3) |
| 81‐90 | 21/51 (41.2) |
| 91‐99 | 3/6 (50.0) |
Abbreviation: SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2.
Considering the first patients' sample/s sent to our virology unit for the laboratory confirmation of SARS‐CoV‐2, most of the newly diagnosed cases were identified by nasopharyngeal swabs (512/527), while for a minor number of cases diagnosis was made by BAL (11/527). For four patients, both nasopharyngeal swabs and BAL were analyzed. One of these tested positive on both samples, and another resulted positive on the nasopharyngeal swab but not on BAL. Interestingly, for two patients the SARS‐CoV‐2 positivity was detected only in BAL but not in nasopharyngeal swab.
Overall, 9848 respiratory specimens were analyzed. Higher positivity was found in samples from lower respiratory tract (55/367, 15.0%). Around 8% (769/9461) of nasopharyngeal swabs tested positive, while all gargle lavage samples were negative to SARS‐CoV‐2 (0/20). Positivity was detected in 3/8 (37.5%) pleural fluids and in 26/134 (19.4%) stool samples. None of the five CSF samples gave a positive result.
4. DISCUSSION
COVID‐19 is a paradigmatic example of zoonosis capable of giving rise to a pandemic in a few months, exploiting the advantages of modern globalization and cross‐border diffusion.
Italy was the first European nation to be affected by COVID‐19; since COVID‐19 was first reported in Italy, the Government has progressively introduced restrictive measures to drastically limit social interactions and prevent virus diffusion, imposing a stringent lockdown from 11 March 2020.
In this study we retrospectively report epidemiological data regarding SARS‐CoV‐2 infected patients attending the laboratory of virology of one of the biggest hospitals in Italy from 6 March to 4 May 2020.
Since SARS‐CoV‐2 has been detected not only in respiratory samples, 13 , 14 we analyzed 9995 specimens, including nasopharyngeal swabs, BAL, gargle lavage, stool, pleural fluid, and CSF from 6565 suspected cases.
During the study period, in our center the prevalence of SARS‐Cov‐2 positivity was 8.0%. Most of positive patients came from nearby metropolitan areas. Among 527 SARS‐CoV‐2 positive patients, 305 were males (57.9%). The lethality rate was lower in female patients. These data confirm that males are disproportionately affected by COVID‐19 compared to females 15 , 16 , 17 , 18 ; indeed it has been suggested that biological sex is an important contributor to disease pathogenesis in multiple infectious diseases, including other coronavirus infections, 19 , 20 with a distinct genetic complement, hormonal environment, and behavioral and social context. The reasons for this gender difference remain unclear, but as reported by various authors androgens and estrogens appear to play an important role in COVID‐19 pathogenesis. 21 , 22 , 23 , 24 , 25
The median age of laboratory‐confirmed cases was 62 (IQR: 50.5‐75); the positivity increased with age, with the highest rates among adults older than 60 years (52.75%). These findings confirm that a high proportion of patients diagnosed with COVID‐19 are older and supposedly have underlying medical conditions.
Age is by now recognized as the strongest predictor of mortality and this association is firmly supported by our data. Multiple hypotheses have been suggested to explain this correlation. As known, older patients are more vulnerable due to underlying age‐related diseases. Moreover, aging is characterized by inflamm‐aging and immune senescence, which are defined respectively as a condition of chronic subclinical systemic inflammation and an impairment of the acquired immune system. All these clinical conditions appear to be involved in the worsening of COVID‐19 infection outcomes in elderly people, especially in males. 26
In our study only the 27.7% of subjects was younger than 40 years. Then it is not possible to rule out that a substantial number of asymptomatic cases remained underdiagnosed, especially among young people under 40. 27 , 28 , 29 Nowadays, the proportion of subclinical infections is unknown and should be derived from future serological studies.
Demographic characteristics of SARS‐CoV‐2 positive patients of this study are in agreement with the trends of other Italian areas, as reported by the Italian National Institute of Health. 30
Concerning the different types of clinical specimens, respiratory samples confirmed to have the greatest probability of detection, although the virus was found also in other specimens, including stool and pleural fluid, but not in CSF. 13 , 31 Interestingly, we found two cases of SARS‐CoV‐2 diagnosed from BAL samples despite a negative result from nasopharyngeal swabs, confirming that lower respiratory tract testing may increase the diagnostic yield for virus detection, especially in suspected patients with exposure history and clinical symptoms for 5 or more days, and with evidence of pneumonia. 32 , 33
Furthermore, we analyzed the trend of the daily incident cases in our hospital during the study period, enclosing the national lockdown; the trend slope was considerably reduced and containing measures seem to have been effective to flatten the epidemic curve of new notified infections.
Although our results derive from a small portion of the total population of a large city, the data confirm that Rome remains less affected than northern cities, 8 , 9 , 34 possibly because as previously estimated by Signorelli et al, 35 timing was a crucial factor in determining effect of mass‐measures adopted, especially in the central‐southern regions of Italy.
Despite this study refers to a single‐center experience, to the best of our knowledge, it is the first study reporting an epidemiological insight for the SARS‐CoV‐2 infection in our area, and the great number of samples included in the analysis allowed to gather comprehensive information on SARS‐CoV‐2 epidemiology. Considering that the results derived from one of the three largest public and academic Italian hospital, and the lack of studies on SARS‐CoV‐2 prevalence in Central Italy, this study provides a characterized epidemiologic outline and underlines the need for a continuous monitoring of the infection, mostly because the pandemic evolution remains unclear.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
The authors would like to thank the COVID‐19 ViroInfection Study Group of Sapienza University Hospital "Policlinico Umberto I" and particularly Gioacchino Galardo, Claudio M. Mastroianni, and Francesco Pugliese. A special thanks to Mauro Bucci, Maria Antonietta Lozzi, Sara Faraone, Antonella Battista, and Claudio Santarini for their contribution and support in SARS‐CoV‐2 laboratory diagnosis.
Turriziani O, Sciandra I, Mazzuti L, et al. SARS‐CoV‐2 diagnostics in the virology laboratory of a University Hospital in Rome during the lockdown period. J Med Virol. 2021;93:886–891. 10.1002/jmv.26332
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Tan W, Zhao X, Ma X, et al. A novel coronavirus genome identified in a cluster of pneumonia cases— Wuhan, China 2019−2020. China CDC Weekly. 2020;2:61‐62. [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gralinski LE, Menachery VD. Return of the coronavirus: 2019‐nCoV. Viruses. 2020;12(2):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim JY, Choe PG, Oh Y, et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci. 35, 2020:e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Callaway E. Time to use the p‐word? Coronavirus enter dangerous new phase. Nature. 2020;579:12. [DOI] [PubMed] [Google Scholar]
- 6.DPCM. http://www.governo.it/sites/new.governo.it/files/DPCM_20200311.pdf. Accessed June 30, 2020.
- 7.Gazzetta Ufficiale. https://www.gazzettaufficiale.it/eli/id/2020/03/11/20A01605/sg. Accessed July 8, 2020.
- 8.Istat. https://www.epicentro.iss.it/coronavirus/pdf/Rapporto_Istat_ISS.pdf. Accessed June 28, 2020.
- 9.Nuovo coronavirus. http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioNotizieNuovoCoronavirus.jsp?lingua=italiano&menu=notizie&p=dalministero&id=4677. Accessed July 10, 2020.
- 10. Sciandra I, Piccioni L, Coltella L, et al. Comparative analysis of 2 commercial molecular tests for the detection of gastroenteric viruses on stool samples. Diagn Microbiol Infect Dis. 2020;96(1):114893. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance. Accessed July 8, 2020.
- 12.World Health Organization. https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117. Accessed July 9, 2020.
- 13. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA. 2020;323(18):1843‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang Z, Zhang Y, Hang C, Ai J, Li S, Zhang W. Comparisons of viral shedding time of SARS‐CoV‐2 of different samples in ICU and non‐ICU patients. J Infect. 2020;81(1):147‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Distante C, Piscitelli P, Miani A. Covid‐19 outbreak progression in Italian regions: approaching the peak by the end of March in Northern Italy and first week of April in Southern Italy. Int J Environ Res Public Health. 2020;17(9):E3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nikpouraghdam M, Jalali Farahani A, Alishiri G, et al. Epidemiological characteristics of coronavirus disease 2019 (COVID‐19) patients in IRAN: a single center study. J Clin Virol. 2020;127:104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruggieri A, Anticoli S, D'Ambrosio A, Giordani L, Viora M. The influence of sex and gender on immunity, infection and vaccination. Ann Ist Super Sanita. 2016;52(2):198‐204. [DOI] [PubMed] [Google Scholar]
- 20. Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex‐based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198(10):4046‐4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wambier CG, Goren A. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection is likely to be androgen mediated. J Am Acad Dermatol. 2020;S0190‐9622(20):30608‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pozzilli P, Lenzi A. Commentary: testosterone, a key hormone in the context of COVID‐19 pandemic. Metabolism. 2020;108:154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Conti P, Younes A. Coronavirus COV‐19/SARS‐CoV‐2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents. 2020;34(2):71. [DOI] [PubMed] [Google Scholar]
- 24. Voinsky I, Baristaite G, Gurwitz D. Effects of age and sex on recovery from COVID‐19: analysis of 5,769 Israeli patients. J Infect. 2020.S0163‐4453(20):30303‐0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schroeder M, Tuku B, Jarczak D, et al. The majority of male patients with COVID‐19 present low testosterone levels on admission to Intensive Care in Hamburg, Germany: a retrospective cohort study. medRxiv, 2020:20073817. 10.1101/2020.05.07.20073817 [DOI] [Google Scholar]
- 26. Bonafè M, Prattichizzo F, Giuliani A, Storci G, Sabbatinelli J, Olivieri F. Inflamm‐aging: why older men are the most susceptible to SARS‐CoV‐2 complicated outcomes. Cytokine Growth Factor Rev. 2020;53:33‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID‐19 screened among close contacts in Nanjing, China. Sci China: Life Sci. 2020;63(5):706‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang R, Gui X, Xiong Y. Comparison of clinical characteristics of patients with asymptomatic vs symptomatic coronavirus disease 2019 in Wuhan, China. JAMA Netw Open. 2020;3(5):e2010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luo Y, Trevathan E, Qian Z, et al. Asymptomatic SARS‐CoV‐2 infection in household contacts of a healthcare provider, Wuhan, China. Emerg Infect Dis. 2020;26(8):1930‐1933. 10.3201/eid2608.201016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_3-giugno-2020.pdf. Accessed June 26, 2020.
- 31. Lescure FX, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID‐19 in Europe: a case series. Lancet Infect Dis. 2020;20(6):697‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Winichakoon P, Chaiwarith R, Liwsrisakun C, et al. Negative nasopharyngeal and oropharyngeal swabs do not rule out COVID‐19. J Clin Microbiol. 2020;58(5):e00297‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang J, Gong H, Chen X, et al. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019‐nCoV infections. medRxiv. 2020;20021493. 10.1101/2020.02.11.20021493 [DOI] [Google Scholar]
- 34. Michelozzi P, de'Donato F, Scortichini M, et al. Mortality impacts of the coronavirus disease (COVID‐19) outbreak by sex and age: rapid mortality surveillance system, Italy, 1 February to 18 April 2020. Euro Surveill. 2020;25(19):2000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Signorelli C, Scognamiglio T, Odone A. COVID‐19 in Italy: impact of containment measures and prevalence estimates of infection in the general population. Acta Biomed. 2020;91(3‐S):175‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


