Abstract
The current COVID‐19 pandemic has a tremendous impact on daily life world‐wide. Despite the ability to dampen the spread of SARS‐CoV‐2, the causative agent of the diseases, through restrictive interventions, it is believed that only effective vaccines will provide sufficient control over the disease and revert societal live back to normal. At present, a double‐digit number of efforts are devoted to the development of a vaccine against COVID‐19. Here, we provide an overview of these (pre)clinical efforts and provide background information on the technologies behind these vaccines. In addition, we discuss potential hurdles that need to be addressed prior to mass scale clinical translation of successful vaccine candidates.
Keywords: COVID-19, pandemic, SARS-CoV-2, vaccine
A mass vaccination campaign against the SARS‐CoV‐2 virus is likely to be the most effective measure to fight the current COVID‐19 pandemic. Here the current COVID‐19 vaccine development efforts in (pre‐)clinical stage are reviewed.
The ongoing 2019–2020 pandemic of coronavirus disease 2019 (COVID‐19) is due to an infectious respiratory disease caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2). Although non‐pharmaceutical interventions, such as social distancing and confinement, appear to slow down the spread of SARS‐CoV‐2, there are no signs that these measures will completely stop the pandemic.1 Hence, there is common ground for the hypothesis that only an effective vaccination campaign will have the power to stop the current pandemic. The goal of such campaign is to establish broad protective immunity in a sufficiently large number of individuals in order to reach herd immunity which stops further transmission of the pathogen. Based on animal model research from previous SARS (SARS‐CoV‐1) and MERS (Middle East Respiratory Syndrome) outbreaks, it is believed that a suitable vaccine should induce humoral immunity through neutralizing antibodies. In addition, induction of cellular immunity by such vaccines might increase its efficacy.2, 3, 4 Although some SARS‐CoV‐1 and MERS clinical trials have resulted in the induction of promising immune responses, these have been small, and no efficacy trial has been conducted to date for any human coronavirus vaccine.5, 6 Given the urgent need for a vaccine against COVID‐19, so far over 200 vaccine candidates are under development and in different stages of (pre)clinical testing. In this report, we aim at giving an overview of the general approaches and challenges regarding the current COVID‐19 vaccine development endeavors and discuss the progress of specific candidates and their (pre)clinical evaluation based on the antigen source, formulation strategy, epitope and route of administration (Table 1).
Table 1.
Selected candidate vaccines against COVID‐19.
|
Type |
Current stage of development |
Formulation strategies |
Epitope |
Route of administration |
Developer |
|---|---|---|---|---|---|
|
mRNA/ saRNA |
Clinical trial Phase I/II (NCT04368728) |
Formulated in LNP |
SP/RBD |
IM |
BioNTech/Fosun Pharma/Pfizer30 |
|
|
|
|
|
|
|
|
mRNA |
Clinical trial phase II (NCT04283461) |
Formulated in LNP |
prefusion stabilized SP |
IM |
Moderna/NIAID28 |
|
Clinical trial phase I (NCT04449276) |
Formulated in LNP |
SP |
IM |
Curevac75 |
|
|
Clinical trial phase I (ChiCTR200003411) |
|
RBD |
|
Academy of Military Sciences/ Walvax Biotech |
|
|
Pre‐clinical |
Naked mRNA |
SP |
|
China CDC/Tongji University76 |
|
|
Pre‐clinical |
Encoding VLP/SP |
SP/RBD |
|
Fudan University/ Shanghai JiaoTong University77 |
|
|
Pre‐clinical |
Liposome RNA Encoding VLP |
SP |
|
Translate Bio/ Sanofi Pasteur78 |
|
|
|
|
|
|
|
|
|
saRNA |
Pre‐clinical |
Formulated in LNP |
SP |
|
Arcturus/Duke‐NUS34 |
|
Clinical trial phase I (ISRCTN17072692) |
Formulated in LNP |
SP |
IM |
Imperial College London79 |
|
|
|
|
|
|
|
|
|
DNA |
Clinical trial phase I (NCT04336410) |
Plasmid DNA |
SP |
ID and EP |
Inovio Pharmaceuticals22 |
|
Clinical trial phase I (NCT04334980) |
Genetically modified probiotic bacteria with a plasmid DNA |
SP |
Oral |
symvivo38 |
|
|
Clinical trial phase I (NCT04445389) |
|
SP |
IM |
Genexine,Inc. |
|
|
Clinical trial phase I/II (CTRI/2020/07/02635) |
Plasmid DNA |
|
ID |
Zydus Cadila |
|
|
Clinical trial phase I (JapicCTI‐205328) |
Plasmid DNA |
|
IM |
Osaka University/ AnGes |
|
|
Pre‐clinical |
A fragment of DNA |
|
IM and EP |
Takis/Applied DNA Sciences/Evvivax79 |
|
|
Pre‐clinical |
Plasmid DNA |
SP |
|
Zydus Cadila80 |
|
|
Pre‐clinical |
Delivered by bacteriophage |
|
IN |
University of Waterloo81 |
|
|
|
|
|
|
|
|
|
Non‐ Replicating Viral Vector |
Clinical trial phase II (NCT04313127) |
Ad5 Vector |
SP |
IM |
CanSino/Beijing Institute of Biotechnology43 |
|
Clinical trial phase II/ III (NCT04400838) |
Chimpanzee adenovirus vaccine vector (ChAdOx1) |
SP |
IM |
University of Oxford |
|
|
Clinical trial phase I (NCT04437875) |
Ad26 Vector |
SP |
IM |
Gamaleya Research Institute |
|
|
Pre‐clinical |
Ad vectors, alone or with MVA boost |
|
IM |
Janssen Pharmaceutical Companies47 |
|
|
Pre‐clinical |
Modified Vaccinia Virus Ankara vector Encoding VLP |
|
|
GeoVax/BravoVax82 |
|
|
Pre‐clinical |
Ad vector |
SP |
IN |
Altimmune83 |
|
|
Pre‐clinical |
Ad vector |
|
|
Greffex84 |
|
|
Pre‐clinical |
Ad5 vector |
SP |
Oral |
||
|
Pre‐clinical |
DelNS1 live attenuated influenza virus (LAIV) vector |
SP |
IN |
University of Hong Kong87 |
|
|
Pre‐clinical |
MVA vector |
SP |
|
DZIF—German Center for Infection Research88 |
|
|
Pre‐clinical |
MVA vector; Encoding structural proteins |
SP |
|
Centro Nacional Biotecnología89 |
|
|
|
|
|
|
|
|
|
Replicating Viral Vector |
Pre‐clinical |
Recombinant measles virus (rMV) |
Codon‐optimised SP |
|
Zydus Cadila80 |
|
Pre‐clinical |
Measles virus vector |
SP |
|
Institute Pasteur/Themis/Univ. of Pittsburg90 |
|
|
Pre‐clinical |
Horsepox virus vector |
SP |
|
Tonix Pharma/Southern Research91 |
|
|
Pre‐clinical |
Recombinant vesicular stomatitis virus (VSV) vector |
SP |
|
IAVI/Batavia92 |
|
|
|
|
|
|
|
|
|
Protein Subunit |
Clinical trial phase I (NCT04405908) |
Adjuvant: AS03, Alum |
Trimeric SP |
IM |
Clover Biopharmaceuticals Inc./GSK93 |
|
Clinical trial phase I (NCT04453852) |
Adjuvant: Advax‐SM |
SP |
|
Vaxine Pty Ltd |
|
|
Clinical trial phase I (NCT04445194) |
|
|
IM |
Anhui Zhifei Longcom Biologic Pharmacy Co., Ltd. |
|
|
Pre‐clinical |
|
RBD |
|
Vaxil Bio94 |
|
|
Pre‐clinical |
|
SP |
|
AJ Vaccines95 |
|
|
Pre‐clinical |
Activated by T‐Cell called li‐Key |
RBD |
|
Generex/EpiVax96 |
|
|
Pre‐clinical |
|
SP |
|
EpiVax/Univ. of Georgia97 |
|
|
Pre‐clinical |
Baculovirus expression system;Adjuvant: AS03 |
SP |
IM |
Sanofi Pasteur/GSK55 |
|
|
Pre‐clinical |
GP‐96 backbone |
SP |
ID |
||
|
Pre‐clinical |
Molecular clamp stabilized trimeric SP;Adjuvant: AS03 |
SP |
|
University of Queensland/GSK57 |
|
|
Pre‐clinical |
Chimeric soluble protein |
SP and N protein |
Oral |
MIGAL Galilee Research Institute100 |
|
|
Pre‐clinical |
MPLA adjuvant |
SP |
ID |
University of Pittsburgh School of Medicine58 |
|
|
Pre‐clinical |
Formulated as microspheres |
Peptide antigen |
|
Flow Pharma Inc101 |
|
|
Pre‐clinical |
|
RBD |
|
Baylor College of Medicine102 |
|
|
Pre‐clinical |
Formulated in LNP |
Peptide antigen |
|
||
|
Pre‐clinical |
Adjuvant:AS03 |
truncated SP |
|
Innovax/Xiamen Univ./GSK105 |
|
|
Pre‐clinical |
|
SP |
|
WRAIR/USAMRIID106 |
|
|
|
|
|
|
|
|
|
VLP |
Clinical trial phase I (NCT04368988) |
Baculovirus/insect cells expression system; Adjuvant: Matrix‐M™ |
SP |
IM |
Novavax60 |
|
Pre‐clinical |
Drosophila S2 insect cells expression system |
SP |
IM |
||
|
Pre‐clinical |
Plant derived VLP |
SP |
IM |
Medicago Inc109 |
|
|
Pre‐clinical |
FastPharming System™; Coated with oligomannose |
|
|
iBio/CC‐Pharming110 |
|
|
|
|
|
|
|
|
|
Live Attenuated Virus |
Pre‐clinical |
Gene rationally designed vaccines |
Viral proteins |
|
Codagenix/Serum Institute of India62 |
|
|
|
|
|
|
|
|
Inactivated virus |
Clinical trial phase I/II (NCT04352608) |
Chemically inactivated whole virus; Adjuvant:CpG 1018 ™ |
Viral proteins |
IM |
Sinovac/Dynavax Technologies Corporation63 |
|
Clinical trial phase I/II (NCT04412538) |
|
Viral proteins |
|
Chinese Academy of Medical Sciences |
|
|
Clinical trial phase I (ChiCTR2000032459) |
|
Viral proteins |
|
Beijing Institute of Biological Products |
|
|
Clinical trial phase I (ChiCTR2000031809) |
|
Viral proteins |
|
Wuhan Institute of Biological Products |
IM: Intramuscular; ID: Intradermal; IN: Intranasal; EP: Electroporation; LNP: lipid nanoparticle; VLP: virus‐like particle; SP: spike glycoprotein; N protein: nucleocapsid protein; RBD: receptor bind domain.
In general, the vaccine candidates group into five different platforms, comprising classical approaches such as inactivated or live attenuated viruses, protein subunits, and more recent technologies such as engineered viral vectors and nucleic acid based antigens including DNA and mRNA (Figure 1). Besides the identification of a suitable antigen against which neutralizing antibodies should be mounted, formulation strategies will also determine the success of the multitude of vaccine candidates. These strategies encompass the use of adjuvants, encapsulation methods to deliver the vaccine at the right tissue and intracellular compartments. Moreover, vaccination approaches need to be compatible with the existence of scalable production systems. Adjuvants generally act by activating the innate immune system and by skewing adaptive immune responses and are usually essential vaccine components. These include mainly aluminum‐based or saponin‐based adjuvants and Toll‐like receptor (TLR) agonists.7, 8 In this context, GlaxoSmithKline (GSK) plays an important role with its proprietary adjuvant technology. GSK has reported to provide its collaborators Sanofi Pasteur, Clover Biopharmaceuticals Inc., University of Queensland and Innovax with their AS03 adjuvant.9 AS03 contains α‐tocopherol and squalene in an oil‐in‐water (o/w) emulsion, and promotes spatio‐temporal co‐localisation of antigen, resulting in longer‐lasting immunity and a reduction in the amount of antigen required per dose.10, 11 Biomaterials, including polymers and lipids, are crucial for the construction of delivery systems that adequately address extracellular stability and intracellular delivery challenges associated to nucleic acid based antigens, and also for some protein subunit vaccines. In addition, delivery systems can also be used to prolong the exposure of the vaccine to the immune system.
Figure 1.
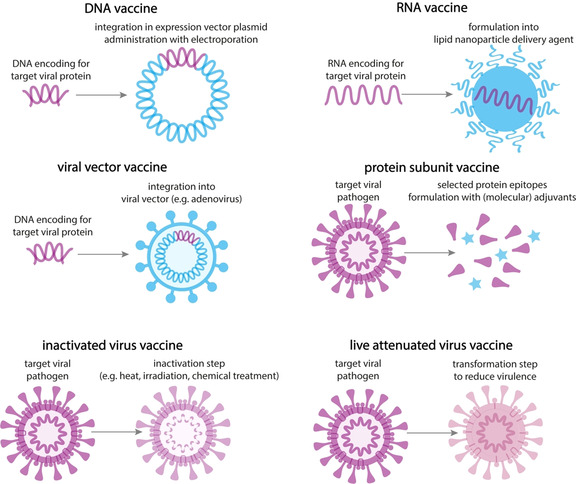
Schematic overview of the different types of vaccines.
Coronaviruses are enveloped, single and positive stranded RNA viruses. Their genomic RNA (≈26–32 kilobases) encodes a nonstructural replicase polyprotein and structural proteins, including spike, envelope, membrane and nucleocapsid proteins (Figure 2). The spike glycoprotein (SP) is naturally presented as a heavily glycosylated trimer and is required for both target cell binding and viral membrane fusion, making it or its minimal receptor‐binding domain (RBD) an ideal vaccine target epitope, while nucleocapsid protein (N protein) also holds the potential to elicit immune protection according to experience with SARS vaccine development.12, 13
Figure 2.
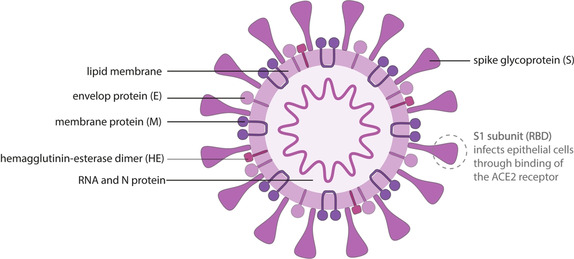
Schematic illustration of the SARS‐CoV‐2 virus structure.
Finally, the route of administration could also affect the quality of the induced immune response. SARS‐CoV‐2 depends for entry on angiotensin‐converting enzyme 2 (ACE2) which is expressed mainly on the surface of epithelial cells in the respiratory tract and also in the intestine.1, 14 Therefore, oral or nasal delivery of the vaccine, instead of parenteral injection, is being considered to elicit robust mucosal immune responses. However, the physicochemical barriers created by mucosal surfaces are a formidable hurdle to overcome, and can be hugely different between individuals, hence advocating less for these routes of administration.15 Intramuscular injection is the most common route of vaccine administration since it allows for induction of immune responses while being less prone to local adverse reactions compared to subcutaneous or intradermal injection. However, novel vaccine delivery methods such as microneedle technology have also shown great potential because of their safety, comfort for patients and effectiveness, and could offer distinct advantages in less developed regions in case they are designed in way that the cold chain can be avoided.16
Nucleic acid‐based vaccines
Nucleic acid (DNA and RNA) based vaccines carry a nucleotide sequence encoding the protein of interest. This approach uses the host cellular machinery to generate foreign antigens that can be presented in the context both major histocompatibility complex (MHC) class I and class II molecules of antigen‐presenting cells (APCs), thereby eliciting both humoral and cellular immune responses. Interestingly, although there is currently no nucleic acid‐based vaccine available on the market, nucleic acid vaccines do show great promise in the COVID‐19 vaccine race. Firstly, they have the potential for rapid, inexpensive and scalable manufacture, which is essential in setting of a pandemic outbreak. Secondly, nucleic acids instruct host cells to synthesize SP without the struggling with protein folding, purification, solubility and inappropriate glycosylation issues that are commonly associated with recombinant protein synthesis, thereby matching better with virus’ own glycoprotein coat.17, 18 Importantly, DNA or RNA requires a delivery system to reach its target (i.e. cell nucleus for DNA and cytoplasm for RNA). In the context of nucleic acid based COVID‐19 vaccine development, liposomes and lipid nanoparticles (LNP), together with electrophysiological methods such as electroporation are the most explored system to deliver the DNA or RNA at the required intracellular location.19, 20, 21, 22 In this context an important role exists for so‐called ionizable cationic lipids, with a pK a typically below neutral pH, that condense mRNA into nanoparticles and deliver their payload into the cellular cytoplasm upon endocytosis.
RNA vaccines
mRNA technology has emerged as a novel platform for vaccine development. mRNA‐based vaccines hold several attractive advantages. For example, mRNA triggers rapid and immediate antigen expression in the cytoplasm without the need for crossing the nuclear membrane. In addition, there is no potential risk of infection or insertional mutagenesis. mRNA expression is rapid and transient as mRNA can be safely degraded by normal cellular processes.23 But it also involves concerns related to its instability, high innate immunogenicity and inefficient in vivo delivery. At present, these drawbacks are becoming resolved to some extent by engineering of the RNA sequence, combing novel adjuvants and developing highly efficient and non‐toxic carrier systems that mediate intracellular delivery of mRNA. Two major types of RNA are currently studied in a vaccine context: non‐replicating mRNA and virally derived, self‐amplifying RNA (saRNA). Conventional mRNA‐based vaccine encodes the antigen of interest, whereas saRNA also encodes a viral replication machinery that enables intracellular RNA amplification and abundant protein expression, hence minimizing the required dose of RNA.24, 25 A saRNA‐LNP vaccine expressing SP of SARS‐CoV‐2 has been recently tested in animals and demonstrated that it can lead to comparable levels of SP‐specific antibody titers and viral neutralization as found in recovered COVID‐19 patients.26
In March 2020, the first SARS‐CoV‐2 vaccine candidate, mRNA‐1273, was announced by the NIAID and Moderna to be evaluated in a clinical trial (NCT04283461) fueled by their prior efforts on coronavirus vaccine development. After the genetic information of SARS‐CoV‐2 recently became available, mRNA encoding SP of the virus was synthesized and formulated into LNP using their existing mRNA delivery system. The latter is composed of a proprietary ionizable lipid (SM‐102) and three commercially available lipids.27, 28 Accomplishing a phase I trial with promising outcome, Moderna has started clinical trial II (NCT04405076) with amended dose levels. Meanwhile the phase III study protocol is finished and the study is expected to be launched in July 2020.29
BioNTech and Pfizer are jointly developing BNT162 as a SARS‐CoV‐2 mRNA vaccine into clinical trial on April 14th (NCT04368728). They will evaluate four vaccine candidates representing different mRNA formats including a nucleoside modified mRNA (modRNA), a uridine containing mRNA (uRNA) and a saRNA, to express the larger SP or the smaller optimized RBD. All the mRNAs will be formulated as LNP.30 Recently, the companies have released the safety and immunogenicity data of one candidate, that is, BNT162b1, expressing trimerized RBD. In this case, the mRNA was modified with 1‐methyl‐pseudouridine to dampen innate immune sensing and increase mRNA translation in vivo. Their safety data showed that, upon intramuscular injection, only mild to moderate local reactions and systemic events were observed, with the severity being in a dose‐dependent manner. After receiving two shots (10 or 30 μg) of BNT162b1 vaccine, SARS‐CoV‐2 specific neutralizing titers reached, respectively 1.8‐ and 2.8‐fold of that of a panel of COVID‐19 convalescent human sera. These results support further evaluation of this mRNA vaccine candidate.31
CureVac's mRNA vaccine candidate utilizes nucleotides without chemical modifications in the mRNA, encodes the full‐length SP and is formulated as LNP. This vaccine has been evaluated in various animal models and the obtained data indicated that the induced neutralizing antibody titers were comparable to sera from patients who recovered from COVID‐19. Additionally, it also elicited specific T cell responses that may contribute to combating viral infection. Starting in June 2020, a clinical trial I (NCT04449276) is conducted in Germany and Belgium.32
Arcturus and their collaborator at Duke‐NUS are developing a saRNA based on Arcturus's STARR (Self‐Transcribing And Replicating RNA) technology and take advantage of a delivery platform developed at Duke‐NUS allowing for rapid effectiveness and safety screening of vaccines. The saRNA is encapsulated with the LUNAR® (lipid‐enabled and unlocked nucleic acid modified RNA), an ionizable and biodegradable LNP platform.33 They expect their vaccine to elicit an immune response using much lower doses than traditional mRNA vaccines due to a sustained protein expression though the use of saRNA, as it was observed for their other products in development.34
DNA vaccines
DNA vaccines are relatively easy to manufacture and are fairly stable. However, DNA needs to cross the nuclear membrane to become translated, which is a major barrier for DNA‐based therapeutics. Moreover, DNA vaccines could possibly result in insertional mutagenesis and cell transformation because of chromosomal integration by non‐homologous recombination. Hence, formulation and delivery strategies are of particular relevance for DNA vaccines.35
Inovio initiated their clinical phase I trial of DNA vaccine candidate INO‐4800 (NCT04336410), fueled by their experience gathered from INO‐4700, a MERS‐CoV vaccine which was shown to be well‐tolerated and capable to induce strong antibody and T cell responses.6 INO‐4800 is composed of optimized DNA plasmids that are designed to express the SP of SARS‐CoV‐2. Moreover, to improve the DNA vaccine potency, Inovio's DNA medicines deliver plasmids intradermally by electroporation (CELLECTRA®), which uses a brief electrical pulse to reversibly open small pores in the cell membrane to allow the plasmids to enter.22 In this context it is noteworthy that in vivo electroporation of DNA has reported to transfect both immune cells and stromal cells, including fibroblasts and endothelial cells.36 Currently, following immunization of mice and guinea pigs with INO‐4800, both SP‐specific antibody responses and T cell responses were observed. Furthermore, antigen‐specific IgG has been detected in bronchoalveolar lavage fluid and serum and showed functional neutralizing activity against SARS‐CoV‐2. The preliminary dataset identifies INO‐4800 as a potential COVID‐19 vaccine candidate, supporting further translational study.37
Symvivo's bacTRL platform designed orally administered, genetically modified probiotic bacteria that colonize the gut, directly bind to intestinal epithelial cells and constitutively replicate, secrete and deliver plasmid DNA encoding antigenic transgenes and neutralizing nanobodies. They have concluded preclinical testing and initiated clinical trial (NCT04334980) in April 2020 evaluating a bifidobacterial monovalent SARS‐CoV‐2 DNA vaccine called bacTRL‐Spike to rapidly induce both cellular and humoral immunity against SP.38
Viral vector vaccines
Viral vector‐based vaccines rely on the delivery of one or more antigens encoded in the context of an unrelated, modified virus. This strategy either employs live (replicating but often attenuated) or non‐replicating vectors, among which adenovirus (Ad) vectors, measles virus, vesicular stomatitis virus (VSV) and modified vaccinia Ankara (MVA) are the most commonly used.18 Adenovirusses allow insertion of up to 7–8 kb, supporting the expression of most target antigens as a multivalent vaccine. Ad type‐5 (Ad5) has been extensively used and shows superior ability to induce exceptionally potent CD4+ and CD8+ T cell as well as antibody responses. Moreover, pre‐existing immunity against the viral vector itself, which is often considered as a factor to hamper the vaccine potency, only has limited influence on the immunogenicity of Ad5‐based vaccines.39, 40, 41 Innovatively, researchers from the KU Leuven made use of the live‐attenuated yellow fever 17D (YF17D) vaccine as a vector to express SARS‐CoV‐2 SP, which elicited potent immune responses in mice.42 Non‐replicating vectors are more used as they cannot cause an ongoing infection in the vaccinated individual, but may need more doses than replicating viral vectors. Viral vector vaccines also have specific advantages. Indeed, delivery of the target antigen as genetic information allows for faithful antigen generation. Furthermore, viral vectors mimic a natural infection and induce potent immune responses, often without the need for additional adjuvants. However, viral vectors are genetically modified organisms (GMOs) and are therefore treated with caution.
The Chinese biotech company CanSino Bio is developing a COVID‐19 vaccine based on a non‐replicating Ad5 vector. Their platform has been previously successfully applied for the development of a vaccine against Ebola. A single intramuscular vaccination of the Ad5‐nCoV was found sufficient to induce humoral and T‐cell responses, while no serious adverse events were noted within 28 days post‐vaccination according to the accomplished the phase I trial outcome (NCT04313127).43 A phase II clinical trial subsequently started on April 10th 2020 (NCT04341389). This study will examine adverse reactions as well as serum levels of neutralizing antibodies against the SP for up to six months.44
A chimpanzee adenovirus vaccine vector (ChAdOx1) was developed at the Jenner Institute in Oxford. As a non‐replicating and non‐human adenoviral vector, it largely avoids pre‐existing immunity. Hence, the chimpanzee adenoviral vector is safe in use and has proven to elicit a robust humoral and cellular immune response in mice and in rhesus macaques upon administration of a single injection.45 This team of researchers has previously developed a vaccine for MERS which showed promise in clinical trial I, in which ChAdOx1 MERS was safe and well tolerated, whereas a single dose was able to elicit both humoral and cellular responses against MERS‐CoV.46 The phase I trial (NCT04324606) of ChAdOx1 COVID‐19, involving over 1000 participants, is currently completed, and a phase II/III trial (NCT04400838) is launched to determine the efficacy, safety and immunogenicity of the ChAdOx1 candidate in a population with a broader age range.
Janssen Pharmaceutical Companies of Johnson & Johnson is expediting its COVID‐19 vaccine program based on Janssen's AdVac® and PER.C6® technologies which provide a cost‐effective manufacturing system for high‐yield, faster and large‐scale production of Ad vector vaccines. This approach has also been used for the development of an Ebola vaccine, which is currently deployed in Africa. The Ebola vaccine is a heterologous two‐dose vaccine with Ad26.ZEBOV and MVA‐BN‐Filo, the first to prime the immune system and the second to boost the immune system to mount long‐lasting immunity.47, 48 Its selected non‐replicating Ad26 vector‐based vaccine candidate will enter clinical evaluation in the U.S. and in Belgium in the second half of July 2020.
Protein subunit vaccines
Subunit vaccines are based on only a limited number of viral proteins, often the major protein in the capsid or the envelope needed to confer protective immunity. Examples of these include the SP or RBD of SARS‐CoV‐2. Therefore, subunit vaccines are considered safer than full pathogen‐based vaccines. However, single proteins, when expressed and purified in the absence of other viral components, are much less immunogenic. Besides lack of immune‐stimulatory viral compounds, also a partial misfolded conformation of the protein, relative to the native protein, could be responsible for this reduced immunogenicity. Therefore, subunit vaccines often require higher dosing, booster administrations and co‐administration of adjuvants to enhance antigen‐specific immunity.49, 50 Virus‐like particles (VLPs) are a highly effective type of subunit vaccines that mimic the overall structure of a virus. VLPs are formed by structural viral proteins which have the inherent property to self‐assemble into protein nanoparticles, and mimic the morphology of the pathogen. Conferring improved delivery to lymphoid tissue and multivalent antigen display, usually mean that VLP elicits stronger humoral and cellular response.51, 52 To date, subunit vaccines have been produced for a number of targets using mammalian, plant, insect or bacterial cells.53
Sanofi Pasteur is leveraging their previous work on the development a SARS‐CoV‐1 vaccine to unlock a fast path for developing a COVID‐19 vaccine. Sanofi Pasteur uses its recombinant DNA in vitro platform that produces an exact genetic match to SP. The DNA sequence will be combined into a baculovirus expression platform, the basis of Sanofi's licensed recombinant influenza vaccine, which allows for rapid production of large quantities of antigen.54 Sanofi Pasteur plans to initiate phase I clinical trials in the second half of 2020.55
A team from the University of Queensland announced a subunit vaccine candidate for which they used a technique called a “molecular clamp”. It's a novel thermally‐stable trimerisation domain, that can constrain a recombinant protein (i.e. SP of SARS‐CoV‐2) to its prefusion, trimeric conformation, expecting to expose the conserved stem domain to the immune system, while simultaneously stimulating an improved cross‐reactive immune response to conserved epitopes.56 Clinical trials are expected to be initiated in July 2020.57
The University of Pittsburgh School of Medicine has announced a potential vaccine against SARS‐CoV‐2 using the SP as antigen based, on their previous experience on SARS and MERS vaccine development. When tested in mice, the vaccine produced antibodies specific to SARS‐CoV‐2 at quantities thought to be sufficient for viral neutralization.58 This lab uses a microneedle array, that is, a fingertip‐sized patch of 400 tiny needles that delivers the antigen into the skin. The patch is applied as band‐aid, and subsequently the needles, which are entirely composed of sugar, and antigen dissolve into the skin. This system was reported to be scalable, by manufacturing of the antigen by a “cell factory”, that is, layer‐upon‐layer of cultured cells engineered to express SP. Researchers are now in the process of applying for an investigational new drug approval from the FDA in anticipation of starting a phase I clinical trial.59
Novavax, a late‐stage biotech company, has developed a SARS‐CoV‐2 subunit vaccine (NVX‐CoV2373) produced from the full‐length SP, stabilized in the prefusion conformation. Matrix‐M was used as adjuvant to enhance the immune response by promoting antigen absorption.7 In mice and nonhuman primate (baboons) models, NVX‐CoV2373 with Matrix‐M adjuvant induced a Th1 dominant B‐ and T‐cell response, hACE2 receptor blocking antibodies as well as SARS‐CoV‐2 neutralizing antibodies. This vaccine successfully protected immunized mice against SARS‐CoV‐2 challenge without evidence of vaccine‐associated enhanced respiratory disease.60 These results support the ongoing phase I/II clinical evaluation of the safety and immunogenicity of NVX‐CoV2327 (NCT04368988).
Inactivated and live attenuated virus
The majority of the currently available human vaccines are based on inactivated or live attenuated viruses. Live attenuated vaccines, engineered from much less virulent version of the pathogen, are often able to induce strong, long‐lasting cellular and humoral immunity as observed with the natural live virus. However, risk exists of reversion to virulent wild‐type strains which can lead to disease, especially in immunocompromised individuals. On the other hand, inactivated vaccines (or killed vaccines) cannot replicate and cause disease and thus, are non‐infectious and safer. One animal trial on SARS‐CoV‐2 inactivated vaccine has been conducted, resulted in specific neutralizing antibodies while no significant T‐cell immunity was found.61 As these a less potent than live vaccines in inducing protective immunity, multiple doses and administration of boosters of the inactivated vaccine may be required to ensure durable protection.49 Additionally, the efficacy of inactivated virus vaccine can be dampened when crucial viral neutralizing epitopes are destroyed during the inactivation process.
Codagenix and the Serum Institute of India are co‐developing a live‐attenuated vaccine against SARS‐CoV‐2. Codagenix uses viral deoptimization to synthesize a “rationally designed,” live‐attenuated vaccine, starting from the digital sequence of the viral genome. This viral vaccine still requires animal testing prior to initiating clinical trials.62
Dynavax Technologies is collaborating with Sinovac Biotech to develop an inactivated COVID‐19 vaccine in combination with the FDA approved adjuvant CpG 1018 (NCT04352608). The latter is a short, unmethylated CpG oligodeoxynucleotide with immunostimulatory function through TLR9 signalling.7 Mid of June 2020, Sinovac revealed promising preliminary data, reporting that in a phase I/II study, more than 90 % of the 600 healthy volunteers showed antigen‐specific immune response. In July 2020, Phase III trials are expected to start in Brazil.63
Conclusion and perspective
The global efforts in vaccine development in response to the COVID‐19 pandemic are unprecedented in terms of scale and speed, with a licensed vaccine hopefully to become available in one year and a half. However, several technical difficulties in this race are noted. First of all, studies with SARS and MERS suggested COVID‐19 vaccine research should focus on establishing animal models that recapitulate replication, pathogenesis and transmission in humans. Animal models that include ferrets and NHPs are good choices but with high cost and limited source. The golden Syrian hamster model may be good alternative as they are smaller, easier to handle and pathology with coronavirus infection has been described.64 However, human ACE2 transgenic mice developed encephalitis when used as a model to study SARS‐CoV, which was not observed in humans.65 Secondly, a paradoxical phenomenon called antibody‐dependent enhancement (ADE) has been described for flaviviruses.66, 67 During ADE, viruses exploits virus‐specific antibodies in the human host to enter into host cells. This phenomenon complicates vaccine development for flaviviruses, and should be considered as a potential risk when developing a vaccine against a new emerging pathogen like SARS‐CoV‐2. It is important to find out the level of protection and absence of ADE during (re‐)infection provided by immunity against SARS‐CoV‐2, induced by natural infection or vaccination. This to optimize protection and to avoid that vaccines or convalescent people develop more severe disease than non‐vaccinated individuals.68 Previous animal experiments on vaccines against SARS and MERS also report the occurrence of ADE.69, 70 Hence, prior to clinical trials, the vaccine's potential risk to aggravate disease should be fully evaluated in animal models.
Another possible complication is whether SARS‐CoV‐2 can change antigenically through genetic mutations and thereby avoid immunity.71 At present it is not yet known whether SARS‐CoV‐2 mutations will affect viral antigenicity and if this would allow the virus to escape neutralizing antibodies as witnessed for influenza viruses (antigenic drift). In that case, a vaccine that targets more conserved epitopes such as RBD on the virus would be necessary for long‐term protection by vaccination. Robbiani et al. recently reported that most convalescent plasmas obtained from individuals who recover from COVID‐19 do not contain elevated levels of neutralizing antibodies, but RBD‐specific neutralizing antibodies were found in all tested individuals.72 Despite the urgent pursuit for a vaccine to master the current COVID‐19 pandemic, we still do not know how long specific immunity will last in the human body after being vaccinated. Evidences showed that antibody titers in individuals that survived SARS‐CoV or MERS‐CoV infections often waned after 2–3 years.73, 74 Longitudinal studies of host immune responses to SARS‐CoV‐2 are required to understand the best immune correlates of protection, which in turn will guide further vaccine development.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Tingting Ye graduated as a Master of Clinical Medicine at Zhejiang University, China. In 2019 she started her PhD research in the research group of Prof. Dr. Bruno De Geest at Ghent University. In her PhD research she focuses on self‐assembled nanomaterials for anti‐tumor immuno‐engineering.

Biographical Information
Zifu Zhong obtained a PhD in Veterinary Sciences at Ghent University in 2020. In this work he focused on the design and the in vivo delivery (using electroporation and nanoparticles) of novel vaccines based on self‐replicating RNA. He is currently a postdoctoral researcher at the in the group of Prof. Dr. Bruno De Geest at Department of Pharmaceutics (Ghent University). He is currently exploring functional nanomaterials for the delivery of RNA for cancer immunotherapy.

Biographical Information
Adolfo García‐Sastre is Professor in the Departments of Microbiology and Medicine, and Director of the Global Health and Emerging Pathogens Institute of Icahn School of Medicine at Mount Sinai in New York, NY. He is also Principal Investigator for the Center for Research on Influenza Pathogenesis (CRIP), one of five NIAID Centers of Excellence for Influenza Research and Surveillance (CEIRS). For the past 30 years, his research interest has been focused on the molecular biology, virus‐host interactions, innate immunity and pathogenesis of influenza viruses and several other RNA viruses, as well as on the development of new vaccines and antivirals.

Biographical Information
Michael Schotsaert is an Assistant Professor in the Department of Microbiology at the Icahn School of Medicine at Mount Sinai in New York, NY, USA. His laboratory focuses on host‐pathogen interactions (influenza virus, Zika virus, Staphylococcus aureus, SARS‐CoV‐2), the complex interplay between innate and adaptive immune responses in the infected host as wells as on vaccine and adjuvant development and validation in preclinical animal models.

Biographical Information
Bruno De Geest graduated as chemical engineer in 2003 and obtained his PhD in pharmaceutical sciences in 2006, both at Ghent University, Belgium. After 2 years of postdoctoral research at Utrecht University (The Netherlands), Bruno returned to the Ghent University and was appointed as assistant professor in 2012 and promoted to associate professor in 2017, and in February 2019 he became full Professor at the Department of Pharmaceutics at Ghent University. He is currently a recipient of an ERC Consolidator Grant. The endeavors of his research group are situated at the interface between materials chemistry and immunology, aiming at the design of novel immunotherapies and vaccines.

Acknowledgements
T.T. acknowledges the CSC for funding (grant N 201906320081). M.S. acknowledges the NIH/NIAID (grant N 1R21AI151229‐01). Research on COVID‐19 is supported at the A.G.S. lab by NIH supplements to grants U19AI135972, U19AI142733 and R35 HL135834, and to contract HHSN272201800048C, by a DoD supplement to grant W81XWH‐20‐1‐0270, by DARPA project HR0011‐19‐2‐0020, by CRIP (Center for Research on Influenza Pathogenesis), a NIAID funded Center of Excellence for Influenza Research and Surveillance (CEIRS, contract HHSN272201400008C), by an NIAID funded Collaborative Influenza Vaccine Innovation Center (SEM‐CIVIC, contract 75N93019C00051) and by the generous support of the JPB Foundation, the Open Philanthropy Project (research grant 2020‐215611(5384)) and anonymous donors. B.G.D.G. acknowledges funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant N 817938).
T. Ye, Z. Zhong, A. García-Sastre, M. Schotsaert, B. G. De Geest, Angew. Chem. Int. Ed. 2020, 59, 18885.
References
- 1. Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., Yuan M.-L., Zhang Y.-L., Dai F.-H., Liu Y., Wang Q.-M., Zheng J.-J., Xu L., Holmes E. C., Zhang Y.-Z., Nature 2020, 579, 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fett C., DeDiego M. L., Regla-Nava J. A., Enjuanes L., Perlman S., J. Virol. 2013, 87, 6551–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim E., Okada K., Kenniston T., Raj V. S., AlHajri M. M., Farag E. A. B. A., AlHajri F., Osterhaus A. D. M. E., Haagmans B. L., Gambotto A., Vaccine 2014, 32, 5975–5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang L.-T., Peng H., Zhu Z.-L., Li G., Huang Z.-T., Zhao Z.-X., Koup R. A., Bailer R. T., Wu C.-Y., Clin. Immunol. 2006, 120, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martin J. E., Louder M. K., Holman L. A., Gordon I. J., Enama M. E., Larkin B. D., Andrews C. A., Vogel L., Koup R. A., Roederer M., Bailer R. T., Gomez P. L., Nason M., Mascola J. R., Nabel G. J., Graham B. S., Vaccine 2008, 26, 6338–6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Modjarrad K., Roberts C. C., Mills K. T., Castellano A. R., Paolino K., Muthumani K., Reuschel E. L., Robb M. L., Racine T., Oh M., Lamarre C., Zaidi F. I., Boyer J., Kudchodkar S. B., Jeong M., Darden J. M., Park Y. K., Scott P. T., Remigio C., Parikh A. P., Wise M. C., Patel A., Duperret E. K., Kim K. Y., Choi H., White S., Bagarazzi M., May J. M., Kane D., Lee H., Kobinger G., Michael N. L., Weiner D. B., Thomas S. J., Maslow J. N., Lancet Infect. Dis. 2019, 19, 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ho N. I., Huis in′t Veld L. G. M., Raaijmakers T. K., Adema G. J., Front. Immunol. 2018, 9, 10.3389/fimmu.2018.02874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee S., Nguyen M. T., Immune Netw. 2015, 15, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.“Update on GSK actions to support the global response to COVID-19,” can be found under https://us.gsk.com/en-us/media/covid-19-response-and-updates/.
- 10. Morel S., Didierlaurent A., Bourguignon P., Delhaye S., Baras B., Jacob V., Planty C., Elouahabi A., Harvengt P., Carlsen H., Vaccine 2011, 29, 2461–2473. [DOI] [PubMed] [Google Scholar]
- 11. Standaert B., Dort T., Linden J., Madan A., Bart S., Chu L., Hayney M. S., Kosinski M., Kroll R., Malak J., Meier G., Segall N., Schuind A., Health Qual. Life Outcomes 2019, 17, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao J., Zhao J., Mangalam A. K., Channappanavar R., Fett C., Meyerholz D. K., Agnihothram S., Baric R. S., David C. S., Perlman S., Immunity 2016, 44, 1379–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Du L., He Y., Zhou Y., Liu S., Zheng B.-J., Jiang S., Nat. Rev. Microbiol. 2009, 7, 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X., Nature 2020, 581, 215–220. [DOI] [PubMed] [Google Scholar]
- 15. Zhang L., Wang W., Wang S., Expert Rev. Vaccines 2015, 14, 1509–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lambert P. H., Laurent P. E., Vaccine 2008, 26, 3197–3208. [DOI] [PubMed] [Google Scholar]
- 17. Leitner W. W., Ying H., Restifo N. P., Vaccine 1999, 18, 765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rauch S., Jasny E., Schmidt K. E., Petsch B., Front. Immunol. 2018, 9, 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Vrieze J., Louage B., Deswarte K., Zhong Z., De Coen R., Van Herck S., Nuhn L., Kaas Frich C., Zelikin A. N., Lienenklaus S., Sanders N. N., Lambrecht B. N., David S. A., De Geest B. G., Angew. Chem. Int. Ed. 2019, 58, 15390–15395; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 15535–15541. [Google Scholar]
- 20. Wadhwa A., Aljabbari A., Lokras A., Foged C., Thakur A., Pharmaceutics 2020, 12, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bookstaver M. L., Tsai S. J., Bromberg J. S., Jewell C. M., Trends Immunol. 2018, 39, 135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.“Inovio Accelerates Timeline for COVID-19 DNA Vaccine INO-4800,” can be found under http://ir.inovio.com/news-and-media/news/press-release-details/2020/Inovio-Accelerates-Timeline-for-COVID-19-DNA-Vaccine-INO-4800/default.aspx.
- 23. Zhong Z., Mc Cafferty S., Combes F., Huysmans H., De Temmerman J., Gitsels A., Vanrompay D., Portela Catani J., Sanders N. N., Nano Today 2018, 23, 16–39. [Google Scholar]
- 24. Pardi N., Hogan M. J., Porter F. W., Weissman D., Nat. Rev. Drug Discovery 2018, 17, 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blakney A. K., McKay P. F., Ibarzo Yus B., Hunter J. E., Dex E. A., Shattock R. J., ACS Nano 2019, 13, 5920–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McKay P. F., Hu K., Blakney A. K., Samnuan K., Bouton C. R., Rogers P., Polra K., Lin P. J. C., Barbosa C., Tam Y., bioRxiv 2020, 10.1101/2020.04.22.055608. [DOI] [Google Scholar]
- 27. Richner J. M., Himansu S., Dowd K. A., Butler S. L., Salazar V., Fox J. M., Julander J. G., Tang W. W., Shresta S., Pierson T. C., Ciaramella G., Diamond M. S., Cell 2017, 168, 1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.“NIH clinical trial of investigational vaccine for COVID-19 begins,” can be found under https://www.nih.gov/news-events/news-releases/nih-clinical-trial-investigational-vaccine-covid-19-begins.
- 29.“Moderna Completes Enrollment of Phase 2 Study of its mRNA Vaccine Against COVID-19 (mRNA-1273),” can be found under https://investors.modernatx.com/news-releases/news-release-details/moderna-completes-enrollment-phase-2-study-its-mrna-vaccine.
- 30.“BioNTech and Pfizer announce regulatory approval from German authority Paul-Ehrlich-Institut to commence first clinical trial of COVID-19 vaccine candidates,” can be found under https://investors.biontech.de/news-releases/news-release-details/biontech-and-pfizer-announce-regulatory-approval-german.
- 31. Mulligan M. J. et al., MedRxiv 2020, DOI: 10.1101/2020.06.30.20142570. [DOI] [Google Scholar]
- 32.“CureVac Receives Regulatory Approval from German and Belgian Authorities to Initiate Phase 1 Clinical Trial of its SARS-CoV-2 Vaccine Candidate,” can be found under https://www.curevac.com/news/curevac-receives-regulatory-approval-from-german-and-belgian-authorities-to-initiate-phase-1-clinical-trial-of-its-sars-cov-2-vaccine-candidate.
- 33. Ramaswamy S., Tonnu N., Tachikawa K., Limphong P., Vega J. B., Karmali P. P., Chivukula P., Verma I. M., Proc. Natl. Acad. Sci. USA 2017, 114, E1941–E1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.“Arcturus Therapeutics and Duke-NUS Medical School Partner to develop a coronavirus (COVID-19) vaccine using STARR TechnologyTM,” can be found under https://www.duke-nus.edu.sg/allnews/media-releases/arcturus-dukenus-covid-19-vaccine-using-starr-technology.
- 35. Kutzler M. A., Weiner D. B., Nat. Rev. Genet. 2008, 9, 776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Drabick J. J., Glasspool-Malone J., Somiari S., King A., Malone R. W., Mol. Ther. 2001, 3, 249–255. [DOI] [PubMed] [Google Scholar]
- 37. Smith T. R. F., Patel A., Ramos S., Elwood D., Zhu X., Yan J., Gary E. N., Walker S. N., Schultheis K., Purwar M., Xu Z., Walters J., Bhojnagarwala P., Yang M., Chokkalingam N., Pezzoli P., Parzych E., Reuschel E. L., Doan A., Tursi N., Vasquez M., Choi J., Tello-Ruiz E., Maricic I., Bah M. A., Wu Y., Amante D., Park D. H., Dia Y., Ali A. R., Zaidi F. I., Generotti A., Kim K. Y., Herring T. A., Reeder S., Andrade V. M., Buttigieg K., Zhao G., Wu J.-M., Li D., Bao L., Liu J., Deng W., Qin C., Brown A. S., Khoshnejad M., Wang N., Chu J., Wrapp D., McLellan J. S., Muthumani K., Wang B., Carroll M. W., Kim J. J., Boyer J., Kulp D. W., Humeau L. M. P. F., Weiner D. B., Broderick K. E., Nat. Commun. 2020, 11, 2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.“symvivo:CVID-19 PROGRAM VISION,” can be found under https://www.symvivo.com/covid-19.
- 39. Lauer K. B., Borrow R., Blanchard T. J., Clin. Vaccine Immunol. 2017, 24, 10.1128/CVI.00298-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Humphreys I. R., Sebastian S., Immunology 2018, 153, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mast T. C., Kierstead L., Gupta S. B., Nikas A. A., Kallas E. G., Novitsky V., Mbewe B., Pitisuttithum P., Schechter M., Vardas E., Wolfe N. D., Aste-Amezaga M., Casimiro D. R., Coplan P., Straus W. L., Shiver J. W., Vaccine 2010, 28, 950–957. [DOI] [PubMed] [Google Scholar]
- 42. Sanchez Felipe L., Vercruysse T., Sharma S., Ma J., Lemmens V., van Looveren D., Arkalagud Javarappa M. P., Boudewijns R., Malengier-Devlies B., Kaptein S. F., Liesenborghs L., De Keyzer C., et al., bioRxiv 2020, 10.1101/2020.07.08.193045. [DOI] [Google Scholar]
- 43. Zhu F.-C., Li Y.-H., Guan X.-H., Hou L.-H., Wang W.-J., Li J.-X., Wu S.-P., Wang B.-S., Wang Z., Wang L., Jia S.-Y., Jiang H.-D., Wang L., Jiang T., Hu Y., Gou J.-B., Xu S.-B., Xu J.-J., Wang X.-W., Wang W., Chen W., Lancet 2020, 395, 1845–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.“China's CanSino Bio advances COVID-19 vaccine into phase 2 on preliminary safety data,” can be found under https://www.fiercepharma.com/vaccines/china-s-cansino-bio-advances-covid-19-vaccine-into-phase-2-preliminary-safety-data.
- 45. van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J. N., Port J. R., Avanzato V., Bushmaker T., Flaxman A., Ulaszewska M., Feldmann F., Allen E. R., Sharpe H., Schulz J., Holbrook M., Okumura A., Meade-White K., Pérez-Pérez L., Bissett C., Gilbride C., Williamson B. N., Rosenke R., Long D., Ishwarbhai A., Kailath R., Rose L., Morris S., Powers C., Lovaglio J., Hanley P. W., Scott D., Saturday G., de Wit E., Gilbert S. C., Munster V. J., bioRxiv 2020, https://doi.org/2020.05.13.093195. [Google Scholar]
- 46. Folegatti P. M., Bittaye M., Flaxman A., Lopez F. R., Bellamy D., Kupke A., Mair C., Makinson R., Sheridan J., Rohde C., Halwe S., Jeong Y., Park Y.-S., Kim J.-O., Song M., Boyd A., Tran N., Silman D., Poulton I., Datoo M., Marshall J., Themistocleous Y., Lawrie A., Roberts R., Berrie E., Becker S., Lambe T., Hill A., Ewer K., Gilbert S., Lancet Infect. Dis. 2020, 20, 816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anywaine Z., Whitworth H., Kaleebu P., Praygod G., Shukarev G., Manno D., Kapiga S., Grosskurth H., Kalluvya S., Bockstal V., Anumendem D., Luhn K., Robinson C., Douoguih M., Watson-Jones D., J. Infect. Dis. 2019, 220, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Draper S. J., Heeney J. L., Nat. Rev. Microbiol. 2010, 8, 62–73. [DOI] [PubMed] [Google Scholar]
- 49. Liljeqvist S., Ståhl S., J. Biotechnol. 1999, 73, 1–33. [DOI] [PubMed] [Google Scholar]
- 50. Roy P., Noad R., Hum. Vaccines 2008, 4, 5–12. [DOI] [PubMed] [Google Scholar]
- 51. Chackerian B., Expert Rev. Vaccines 2007, 6, 381–390. [DOI] [PubMed] [Google Scholar]
- 52. Deml L., Speth C., Dierich M. P., Wolf H., Wagner R., Mol. Immunol. 2005, 42, 259–277. [DOI] [PubMed] [Google Scholar]
- 53. Kushnir N., Streatfield S. J., Yusibov V., Vaccine 2012, 31, 58–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Treanor J. J., Schiff G. M., Hayden F. G., Brady R. C., Hay C. M., Meyer A. L., Holden-Wiltse J., Liang H., Gilbert A., Cox M., JAMA J. Am. Med. Assoc. 2007, 297, 1577. [DOI] [PubMed] [Google Scholar]
- 55.“Sanofi joins forces with U. S. Department of Health and Human Services to advance a novel coronavirus vaccine,” can be found under https://www.sanofi.com/en/media-room/press-releases/2020/2020-02-18-16-00-00.
- 56.“Molecular Clamp: a Novel Protein Vaccine for Influenza, RSV, Ebola and Other Human and Veterinary Viruses,” can be found under https://www.pharmalicensing.com/detail.php?uid=66499.
- 57.“$17 m shot in the arm for UQ′s COVID-19 vaccine research,” can be found under https://stories.uq.edu.au/news/2020/17m-shot-in-the-arm-for-uq-covid-19-vaccine-research/index.html.
- 58. Kim E., Erdos G., Huang S., Kenniston T. W., Balmert S. C., Carey C. D., Raj V. S., Epperly M. W., Klimstra W. B., Haagmans B. L., Korkmaz E., Falo L. D., Gambotto A., EBioMedicine 2020, 55, 102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.“COVID-19 vaccine candidate shows promise in first peer-reviewed research,” can be found under https://medicalxpress.com/news/2020-04-covid-vaccine-candidate-peer-reviewed.html.
- 60. Tian J. H., et al., bioRxiv 2020, 10.1101/2020.06.29.178509. [DOI] [Google Scholar]
- 61. Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., Li Y., Zhu L., Wang N., Lv Z., Gao H., Ge X., Kan B., Hu Y., Liu J., Cai F., Jiang D., Yin Y., Qin C., Li J., Gong X., Lou X., Shi W., Wu D., Zhang H., Zhu L., Deng W., Li Y., Lu J., Li C., Wang X., Yin W., Zhang Y., Qin C., Science 2020, eabc1932. [Google Scholar]
- 62.“Codagenix and Serum Institute of India Initiate Co-Development of a Scalable, Live-Attenuated Vaccine Against the 2019 Novel Coronavirus, COVID-19,” can be found under https://www.prnewswire.com/news-releases/codagenix-and-serum-institute-of-india-initiate-co-development-of-a-scalable-live-attenuated-vaccine-against-the-2019-novel-coronavirus-covid-19–301004654.html?tc=eml cleartime.
- 63.“Sinovac COVID-19 Vaccine Collaboration with Butantan Receives Approval from Brazilian Regulator for Phase III, Trial,” can be found under http://http://www.sinovac.com/?optionid=754&auto_id=907.
- 64. Bao L., Deng W., Huang B., Gao H., Liu J., Ren L., Wei Q., Yu P., Xu Y., Qi F., Qu Y., Li F., Lv Q., Wang W., Xue J., Gong S., Liu M., Wang G., Wang S., Song Z., Zhao L., Liu P., Zhao L., Ye F., Wang H., Zhou W., Zhu N., Zhen W., Yu H., Zhang X., Guo L., Chen L., Wang C., Wang Y., Wang X., Xiao Y., Sun Q., Liu H., Zhu F., Ma C., Yan L., Yang M., Han J., Xu W., Tan W., Peng X., Jin Q., Wu G., Qin C., Nature 2020, 10.1038/s41586-020-2312-y. [DOI] [Google Scholar]
- 65. Tseng C.-T. K., Huang C., Newman P., Wang N., Narayanan K., Watts D. M., Makino S., Packard M. M., Zaki S. R., Chan T., Peters C. J., J. Virol. 2007, 81, 1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bardina S. V., Bunduc P., Tripathi S., Duehr J., Frere J. J., Brown J. A., Nachbagauer R., Foster G. A., Krysztof D., Tortorella D., Stramer S. L., García-Sastre A., Krammer F., Lim J. K., Science 2017, 356, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Halstead S. B., Russell P. K., Vaccine 2016, 34, 1643–1647. [DOI] [PubMed] [Google Scholar]
- 68. Peeples L., Proc. Natl. Acad. Sci. USA 2020, 117, 8218–8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wan Y., Shang J., Sun S., Tai W., Chen J., Geng Q., He L., Chen Y., Wu J., Shi Z., Zhou Y., Du L., Li F., J. Virol. 2019, 94, 10.1128/JVI.02015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tseng C.-T., Sbrana E., Iwata-Yoshikawa N., Newman P. C., Garron T., Atmar R. L., Peters C. J., Couch R. B., PLoS One 2012, 7, e35421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Phan T., Infect. Genet. Evol. 2020, 81, 104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Robbiani D. F., et al., Nature 2020, 10.1038/s41586-020-2456-9. [DOI] [Google Scholar]
- 73. Liu W., Fontanet A., Zhang P., Zhan L., Xin Z., Baril L., Tang F., Lv H., Cao W., J. Infect. Dis. 2006, 193, 792–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wu L.-P., Wang N.-C., Chang Y.-H., Tian X.-Y., Na D.-Y., Zhang L.-Y., Zheng L., Lan T., Wang L.-F., Liang G.-D., Emerging Infect. Dis. 2007, 13, 1562–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.“CureVac Receives Regulatory Approval from German and Belgian Authorities to Initiate Phase 1 Clinical Trial of its SARS-CoV-2 Vaccine Candidate,”.
- 76.“Tongji University speeds up research for epidemic prevention and control,” can be found under https://en.tongji.edu.cn/info/1009/3995.htm.
- 77.“Towards an effective mRNA vaccine against 2019-nCoV,” can be found under https://www.fudan.edu.cn/en/2020/0307/c344a104281/page.htm.
- 78.“Sanofi Pasteur and Translate Bio to Collaborate to Develop a Novel mRNA Vaccine Candidate Against COVID-19,” can be found under http://investors.translate.bio/news-releases/news-release-details/sanofi-pasteur-and-translate-bio-collaborate-develop-novel-mrna.
- 79.“The Imperial lab developing a COVID-19 vaccine,” can be found under https://www.imperial.ac.uk/news/196313/in-pictures-imperial-developing-covid-19-vaccine/.
- 80.“Zydus Cadila launches a fast tracked programme to develop vaccine for the novel coronavirus, 2019-nCoV (COVID-19,” can be found under https://zyduscadila.com/public/pdf/pressrelease/Zydus Cadila launches a fast tracked programme to develop vaccine for the novel coronavirus 2019-nCoVCOVID-19).pdf.
- 81.“University of Waterloo developing DNA-based COVID-19 vaccine,” can be found under https://uwaterloo.ca/stories/news/university-waterloo-developing-dna-based-covid-19-vaccine.
- 82.“GeoVax Progresses in Coronavirus (COVID-19) Vaccine Development Program,” can be found under https://www.geovax.com/investors/news/geovax-progresses-in-coronavirus-covid-19-vaccine-development-program.
- 83.“Altimmune Completes First Development Milestone Toward a Single-Dose Intranasal COVID-19 Vaccine,” can be found under https://ir.altimmune.com/node/12501/pdf.
- 84.“Greffex, Inc. Completes COVID-19 Vaccine and Prepares for Testing,” can be found under https://www.greffex.com/news/greffex-inc-completes-covid-19-vaccine-and-prepares-for-testing/.
- 85.“Vaxart announces it entered into an agreement with Emergent Biosolutions for the development and manufacturing of oral coronavirus (COVID-19) vaccine candidate,” can be found under https://investors.vaxart.com/news-releases/news-release-details/vaxart-announces-it-entered-agreement-emergent-biosolutions.
- 86. Liebowitz D., Gottlieb K., Kolhatkar N. S., Garg S. J., Asher J. M., Nazareno J., Kim K., McIlwain D. R., Tucker S. N., Lancet Infect. Dis. 2020, 20, 435–444. [DOI] [PubMed] [Google Scholar]
- 87.“HKU State Key Laboratory for Emerging Infectious Diseases joins global effort to develop COVID-19 vaccine,” can be found under https://www.hku.hk/press/news detail 20788.html.
- 88.“Promising MERS coronavirus vaccine trial on humans—useful insights for vaccine development against SARS-CoV-2,” can be found under https://www.dzif.de/en/promising-mers-coronavirus-vaccine-trial-humans-useful-insights-vaccine-development-against-sars.
- 89.“El CSIC recibe 350.000 Euros de grupo catalana occidengte para cintribuir a lograr una vacuna contra el SARS-COV2,” can be found under https://www.cnb.csic.es/index.php/es/cultura-cientifica/noticias/item/1710-el-csic-recibe-350-000-euros-del-grupo-catalana-occidente-para-contribuir-a-lograr-una-vacuna-contra-el-sars-cov2.
- 90.“CEPI collaborates with the Institut Pasteur in a consortium to develop COVID-19 vaccine,” can be found under https://www.pasteur.fr/en/press-area/press-documents/cepi-collaborates-institut-pasteur-consortium-develop-covid-19-vaccine.
- 91.“Tonix Pharmaceuticals Reports Fourth Quarter and Full Year 2019 Financial Results and Operational Highlights,” can be found under https://ir.tonixpharma.com/press-releases/detail/1195/tonix-pharmaceuticals-reports-fourth-quarter-and-full-year.
- 92.“IAVI and Batavia Biosciences Announce Collaboration on VSV-vector Based Epidemic Preparedness Vaccines,” can be found under https://www.iavi.org/newsroom/press-releases/2020/iavi-and-batavia-announce-collaboration-vsv-vector-epidemic-preparedness-vaccines.
- 93.“Clover Successfully Produced 2019-nCoV Subunit Vaccine Candidate and Detected Cross-Reacting Antibodies from Sera of Multiple Infected Patients,” can be found under http://www.cloverbiopharma.com/index.php?m=content&c=index&a=show&catid=11&id=41&langId=1.
- 94.“Vaxil announces the identification of a potential corona virus vaccine and provides an update in the previously announced debt financing,” can be found under https://vaxil-bio.com/vaxil-announces-the-identification-of-a-potential-corona-virus-vaccine-and-provides-an-update-in-the-previously-announced-debt-financing/.
- 95.“Breakthrough in the global battle against polio: AJ Vaccines granted WHO prequalification for new polio vaccine,” can be found under https://ajvaccines.com/news/.
- 96.“Coronavirus Vaccine Development Barrels Ahead,” can be found under https://www.biocompare.com/Editorial-Articles/561652-Coronavirus-Vaccine-Development-Barrels-Ahead/.
- 97.“EpiVax Accelerates COVID-19 Vaccine Development with UGA′s Center for Vaccines and Immunology,” can be found under https://www.prnewswire.com/news-releases/epivax-accelerates-covid-19-vaccine-development-with-ugas-center-for-vaccines-and-immunology-301016648.html?tc=eml cleartime.
- 98.“Heat Biologics Announces Research Collaboration with University of Miami to Develop Vaccine Designed to Protect Against COVID-19 Coronavirus,” can be found under https://www.heatbio.com/news-media/news-releases/detail/649/heat-biologics-announces-research-collaboration-with.
- 99. Strbo N., Vaccari M., Pahwa S., Kolber M. A., Doster M. N., Fisher E., Gonzalez L., Stablein D., Franchini G., Podack E. R., J. Immunol. 2013, 190, 2495–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.“MIGAL′s Coronavirus Vaccine Project,” DOI https://www.migal.org.il/en/node/7010.
- 101.“Flow Pharma Announces Strategic Partnership with Oakwood Labs for GMP Manufacturing of FlowVax COVID-19 Vaccine,” can be found under https://www.accesswire.com/585181/Flow-Pharma-Announces-Strategic-Partnership-with-Oakwood-Labs-for-GMP-Manufacturing-of-FlowVax-COVID-19-Vaccine.
- 102.“Baylor, Texas Children's Hospital developing COVID-19 vaccine,” can be found under Baylor, Texas Children's Hospital developing COVID-19 vaccine.
- 103.“IMV Inc. Launches Plans to Advance Clinical Development of a Vaccine Candidate Against COVID-19,” can be found under https://ir.imv-inc.com/press-releases/detail/635/imv-inc-launches-plans-to-advance-clinical-development-of.
- 104.“One Platform. Multiple Opportunities.,” can be found under https://imv-inc.com/platform/.
- 105.“GSK Strikes Deals with Vir Biotechnology and China's Innovax Biotech Against COVID-19,” can be found under https://www.biospace.com/article/gsk-strikes-vaccine-deal-with-china-s-innovax-against-covid-19/.
- 106.“WRAIR Pivots to Combat COVID-19,” can be found under https://www.wrair.army.mil/node/318.
- 107.“ExpreS2ion announces EU grant award for the COVID-19 vaccine development programme,” can be found under https://news.cision.com/expres2ion-biotechnologies/r/expres2ion-announces-eu-grant-award-for-the-covid-19-vaccine-development-programme,c3054055.
- 108. Mordmüller B., Sulyok M., Egger-Adam D., Resende M., de Jongh W. A., Jensen M. H., Smedegaard H. H., Ditlev S. B., Soegaard M., Poulsen L., Dyring C., Calle C. L., Knoblich A., Ibáñez J., Esen M., Deloron P., Ndam N., Issifou S., Houard S., Howard R. F., Reed S. G., Leroy O., Luty A. J. F., Theander T. G., Kremsner P. G., Salanti A., Nielsen M. A., Clin. Infect. Dis. 2019, 69, 1509–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.“Medicago: COVID-19 Vaccine Development Program,” can be found under https://www.medicago.com/en/covid-19-programs/.
- 110.“iBio Announces Development of Proprietary COVID-19 Vaccine Candidates,” can be found under https://www.ibioinc.com/news/ibio-announces-development-of-proprietary-covid-19-vaccine-candidates.


