Abstract
Background
Thirty percent of Covid‐19 patients admitted to intensive care units present with thrombotic complications despite thromboprophylaxis. Bed rest, obesity, hypoxia, coagulopathy, and acute excessive inflammation are potential mechanisms reported by previous studies. Better understanding of the underlying mechanisms leading to thrombosis is crucial for developing more appropriate prophylaxis and treatment strategies.
Objective
We aimed to assess fibrinolytic activity and thrombin generation in 78 Covid‐19 patients.
Patients and Methods
Forty‐eight patients admitted to the intensive care unit and 30 patients admitted to the internal medicine department were included in the study. All patients received thromboprophylaxis. We measured fibrinolytic parameters (tissue plasminogen activator, PAI‐1, thrombin activatable fibrinolysis inhibitor, alpha2 anti‐plasmin, and tissue plasminogen activator‐modified ROTEM device), thrombin generation, and other coagulation tests (D‐dimer, fibrinogen, factor VIII, antithrombin).
Results and Conclusions
We observed two key findings: a high thrombin generation capacity that remained within normal values despite heparin therapy and a hypofibrinolysis mainly associated with increased PAI‐1 levels. A modified ROTEM is able to detect both hypercoagulability and hypofibrinolysis simultaneously in Covid‐19 patients with thrombosis.
Keywords: fibrinolysis, plasminogen activator inhibitor 1, tissue plasminogen activator, TAFI, thrombin generation, Covid‐19
ESSENTIALS
-
•
Covid‐19 patients have high risk of thrombosis and thromboprophylaxis is recommended in all hospitalized Covid‐19 patients.
-
•
Covid‐19 disease modifies the balance between coagulation and fibrinolysis and is associated with elevated levels of PAI‐1, TAFI and tPA resulting in hypofibrinolysis.
-
•
Covid‐19 patients have dramatically increased ex‐vivo thrombin generation.
-
•
TEM‐tPA might be a biomarker of interest to predict the risk of thrombosis in Covid‐19 patients.
Alt-text: Unlabelled Box
1. INTRODUCTION
Severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2 or Covid‐19) was first described in China in December 2019 and has rapidly spread to 187 countries around the world. The World Health Organization classified the Covid‐19 outbreak as a pandemic and the disease has contributed to significant mortality worldwide mainly explained by fatal acute respiratory distress syndrome. However, pulmonary damage is not the only reason for the high death risk; despite anticoagulant prophylaxis, several intensive care units (ICUs) have reported life‐threatening arterial and venous thrombosis, in particular frequent severe pulmonary embolisms.1., 2. These clinical observations led to the empirical treatment of hospitalized Covid‐19 patients with low molecular weight heparin (LMWH) in higher doses than usual thromboprophylaxis.3 However, some patients still exhibited thrombotic complications while they were on high‐dose prophylaxis.4 It was also demonstrated that high D‐dimers >3000 ng/mL constituted a biomarker for poor prognosis of the disease.5 The pathophysiology of Covid‐19‐related thrombosis is incompletely understood. Elevated fibrinogen, factor VIII (FVIII), von Willebrand factor levels, positive lupus anticoagulant, and antiphospholipid antibodies have been reported.6 The cytokine storm associated with Covid‐19 probably has a major impact on plasma levels of the previously mentioned coagulation proteins, creating a substantial shift in the balance between pro‐ and anticoagulant activities. As a consequence, pathological reports showed fibrin deposition within the lung.7 The discrepancy between the frequency of thrombotic complications and the intensity of antithrombotic prophylaxis administered to Covid‐19 patients is intriguing. We hypothesized that fibrinolytic abnormalities associated with increased thrombin generation might be key contributors to Covid‐19‐induced thrombosis. The aim of the present study was to assess thrombin generation capacity and fibrinolytic activity of patients hospitalized for Covid‐19 infection.
2. MATERIALS AND METHODS
Adult patients with a positive COVID‐19 polymerase chain reaction test hospitalized in Lyon Edouard Herriot University Hospital were included. The study was approved by the institutional ethics committee (CPP Est IV 20/41 [COVID]/ SI 20.04.23.41107) and informed consent was obtained from all participants. All patients received supportive management of the most common complications of severe Covid‐19: pneumonia, hypoxemic respiratory failure, sepsis, cardiomyopathy and arrhythmia, acute kidney injury, complications from prolonged hospitalization, including secondary bacterial infections, gastrointestinal bleeding, critical illness polyneuropathy, and appropriate thromboprophylaxis with LMWH (enoxaparin 40 mg once daily if 50‐100 kg and 40 mg twice daily [BID] if >100 kg or fibrinogen >8 g/L or D‐dimer >3000 ng/mL) or subcutaneous unfractionated heparin (5000 IU BID) according to their renal status. Clinical data were collected from the electronic patient medical file. Blood samples collected for D‐dimers and fibrinogen measurements were used for measuring fibrinolysis, thrombin generation, and other coagulation tests in the first 3 days following admission. All hemostasis tests were performed in the Haemostasis Laboratory of Lyon University Hospitals. D‐dimer, fibrinogen, FVIII, and antithrombin levels were measured on the ACL Top 750 analyser (Werfen) using HemosIL D‐Dimer HS 500, HemosIL QFA Thrombine, HemosIL Factor VIII‐deficient plasma, HemosIL Synthasil, and HemosIL Antithrombin reagents (Werfen), respectively. Thrombin generation was measured using the calibrated automated thrombography with PPP‐High reagent (Stago, Asnières‐sur‐Seine, France). Plasminogen activator inhibitor 1 (PAI‐1), thrombin activatable fibrinolysis inhibitor (TAFI; activated and inactivated TAFI), tissue plasminogen activator (tPA) were measured using ELISA kits (Asserachrome, Stago); alpha‐2 antiplasmin was measured using HemosIL Plasmin Inhibitor (Werfen) on ACLTOP 750. Clot formation and fibrinolysis were recorded using a ROTEM Delta device (Werfen, Le‐Pré‐Saint‐Gervais, France) and EXTEM reagent in the presence of 0.625 µg/mL tPA (Actilyse, Boehringer‐Ingelheim, Paris, France). Normal values were determined in the control population, which comprised 30 apparently healthy adult volunteers (14 men and 16 women) between 22 and 58 years (32.4 years ± 13.8; mean ± SD), nonsmokers, not using drugs known to affect the coagulation system, and without history of venous thromboembolism or bleeding disorder. Results were analyzed using GraphPad InStat and Prism 8, data were compared using Student's t and Mann‐Whitney tests. Spearman test was used to evaluate the correlation between anti‐Xa activity and thrombin generation. A P value <.05 was considered statistically significant.
3. RESULTS AND DISCUSSION
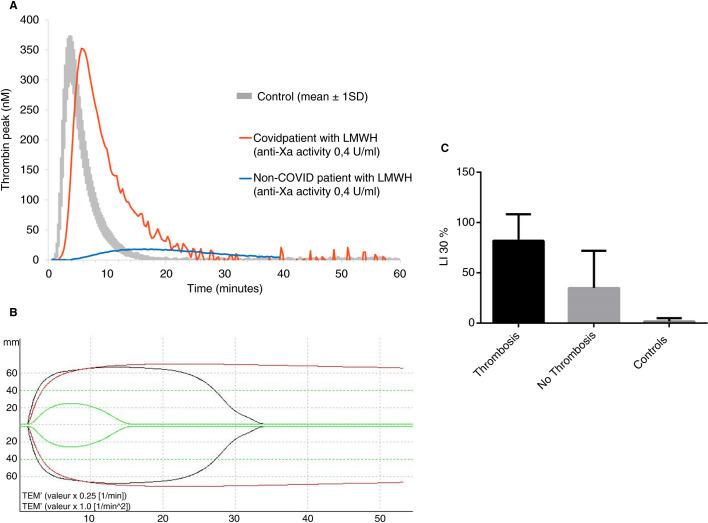
A total of 78 patients (51 males and 27 females) were included with a mean age of 60.2 ± 14.4 (mean ± SD). Forty‐eight patients were admitted to ICU; among them, 33 (66.7%) received invasive mechanical ventilation and 7 (14.6%) were treated with kidney replacement therapy. Thirty patients were admitted to the internal medicine department. Table 1 summarizes patient characteristics and laboratory data. All patients received prophylactic anticoagulation (71 with LMWH and 7 with unfractionated heparin) and 14 (29.1%) ICU patients developed thrombosis (8 pulmonary embolisms, 5 deep vein thromboses, and 1 acute aortic thrombosis) diagnosed with appropriate imaging tests. The incidence of thrombotic complications is in accordance with data reported by other groups.8 Among 64 patients who were on thromboprophylaxis, 50 received standard‐dose and 14 high‐dose prophylaxis. D‐dimers were increased (>500 ng/mL) in 80% of the patients, and ICU patients had significantly higher D‐dimer levels compared with non‐ICU patients. Despite anticoagulation, thrombin generation capacity was not decreased; peak thrombin and endogenous thrombin potential (ETP) were in the normal range (Figure 1A ). Sampling was not standardized relative to heparin administration. Nevertheless, in 23 patients for whom there was still sufficient volume of frozen plasma available, heparin plasma levels were substantial, with a mean anti‐Xa activity of 0.35 ± 0.20 U/mL. Among these 23 patients, 69% received high‐dose prophylaxis (enoxaparin 40 mg BID), and the mean ETP was 1670 ± 554 nmol/L/min (normal values = 1593 ± 206 nmol/L/min). We observed a statistically significant correlation between anti‐Xa levels and ETP (P = .02; Spearman correlation test). Thus, despite substantial heparin plasma levels, thrombin generation was normal, suggesting either a major hypercoagulability that could not be controlled with heparin therapy or a heparin resistance. Antithrombin activity was within the normal range in 91% (n = 71) of the population, making the hypothesis of heparin resistance unlikely. On the other hand, fibrinogen and FVIII levels were significantly elevated in all Covid‐19 patients, supporting the hypothesis of uncontrolled hypercoagulability, probably related to a major inflammatory syndrome.
Table 1.
Patient characteristics and laboratory results
| ICU | Non‐ICU | P | |
|---|---|---|---|
| Patients, n | 48 | 30 | ‐ |
| Age, y | 62.8 ± 13.1 | 60.2 ± 14.6 | .39 |
| BMI, kg/m2 | 29 ± 5.5 | 26.2 ± 4.8 | .07 |
| SOFA score | 5.4 ± 3.1 | ‐ | ‐ |
| SAPS II score | 37.9 ± 13 | ‐ | ‐ |
| Fibrinogen, g/L (normal values = 2‐4) | 6.1 ± 1.9 | 5.6 ± 1.7 | .38 |
| FVIII, % (normal values = 50‐150) | 199 ± 65 | 160 ± 28 | .22 |
| Antithrombin, % (normal values = 80‐120) | 87 ± 28 | 106 ± 14 | .016 |
| D‐dimers, ng/mL (normal values < 500) | 3456 ± 2641 | 874 ± 539 | .0019 |
| Peak thrombin, nM (normal values = 350 ± 39) | 312 ± 127 | 391 ± 76 | .004 |
| ETP, nM/min (normal values = 1593 ± 206) | 1682 ± 610 | 1815 ± 357 | .31 |
| t‐PA, ng/mL (normal values = 2‐12) | 23.9 ± 14.5 | 14.4 ± 7.5 | <.0001 |
| PAI‐1, ng/mL (normal values = 4‐43) | 96.3 ± 35 | 76.8 ± 40 | .017 |
| TAFIa/i, ng/mL (normal values = 1.76‐28.9) | 60.2 ± 42.3 | 39.8 ± 23.8 | .016 |
| Alpha 2 antiplasmin, UI/dL (normal values = 80‐120) | 124.6 ± 15.8 | 128 ± 9.2 | .41 |
| Patients, n | 19 | 4 | ‐ |
| TEM‐tPA MCF, mm (normal values = 32 ± 10.4) | 62.3 ± 10 | 61.5 ± 6.5 | .69 |
| TEM‐tPA alpha angle, (normal values = 69 ± 5) | 81 ± 1.4 | 79 ± 2.3 | .065 |
| Ly30, % (normal values = 1.8 ± 3.2) | 63 ± 39 | 18 ± 35 | .022 |
BMI, body mass index; SAPS II, Simplified Acute Physiology Score; SOFA, sequential organ failure assessment.
Figure 1.
Thrombin generation and TEM‐tPA results in Covid‐19 patients. A, Representative thrombin generation curves of a Covid‐19 patient receiving high‐dose LMWH prophylaxis (subcutaneous enoxaparin 40 mg BID, anti‐Xa activity 0.4 U/mL, red curve), and an obese patient without Covid‐19 infection at high risk of thrombosis, receiving the same treatment (subcutaneous enoxaparin 40 mg BID, anti‐Xa activity 0.4 U/mL, blue curve). The normal range of thrombin generation is represented in gray (mean ± 1 SD). Despite an overall correlation between anti‐Xa and ETP levels (P = .02; Spearman correlation test), the majority of Covid‐19 patients on prophylaxis with LMWH had normal or increased ETP (1670 ± 554 nmol/L/min [mean ± 2 SD]). Among 78 patients included in the study, 30 (38.5%) had an ETP above the reference range, 37 (47.4%) in the reference range, and 11 (14.1%) below the reference range. In vitro spiking of a plasma sample of a Covid‐19 patient with increasing concentrations of LMWH showed effective anticoagulation with expected anti‐Xa and ETP levels with high doses of LMWH (1 anti‐Xa U/mL). Taking together these results strongly suggests that patients with Covid‐19 infection were profoundly hypercoagulable at baseline. B, Representative TEM‐tPA curves from a normal control (green curve), a Covid‐19 patient with thrombosis (red curve), and a Covid‐19 patient without thrombosis (black curve). MCF corresponds to the maximal amplitude and reflects coagulation capacity of the patient. In Covid‐19 patients, MCF is increased. Ly30 is the lysis index at 30 minutes, which is very high in Covid‐19 patients with thrombosis and lower in other Covid patients. C, Ly30 results in Covid‐19 patients with and without thrombosis, compared with controls
In addition to very high thrombin generation, we observed impaired fibrinolysis in all Covid‐19 patients. ICU patients presenting with a severe form of the disease had significantly higher levels of tPA, PAI‐1, and TAFIa/i compared with non‐ICU patients. The hypercoagulable state existing in Covid‐19 infection can result in the generation of high concentrations of thrombin required to activate TAFI. It has been shown that increased TAFI antigen levels were associated with risk of arterial thrombosis9 and high TAFIa/i levels were associated with increased risk of cardiovascular death.10 Despite the short half‐life of TAFIa, data from animal models and clinical studies indicated that the amount of TAFIa might play a more crucial role in retarding fibrinolysis than the total amount of TAFI protein.10., 11. We observed high TAFIa/i levels in our patients with Covid‐19 compared with controls.
Inflammation promotes local release of tPA and PAI‐1 from endothelial cells.12 In addition, activated platelets may also release large amounts of PAI‐1 because platelets are the major circulating pool of PAI‐1 that can contribute to a high local concentration of PAI‐1 at the site of a growing fibrin clot. Increased PAI‐1 is responsible for hypofibrinolysis and fibrin persistence. Interestingly, increased PAI‐1 plasma levels were observed in patients during the SARS‐CoV epidemic in 2002.13 Persistent fibrin deposition in lung parenchyma and alveolar spaces of Covid‐19 patients strongly suggests that despite increased levels of tPA, high PAI‐1 levels can overcome local tPA release. In addition, plasma hypofibrinolysis from elevated levels of PAI‐1 and TAFI is a risk factor for venous thrombosis.12 One may think that high plasma levels of PAI‐1 found in ICU patients may be explained by the high incidence of severe Covid‐19 infection in obese patients as adipose tissue contributes to the production of PAI‐1.14 However, in our population, no significant difference was observed between the body mass index of ICU patients and others, suggesting that high PAI‐1 plasma levels are probably related to the severity of the disease and endothelial damage in the lungs. Our data clearly show that the balance between coagulation and fibrinolysis is lost in patients with Covid‐19 infection, who present with a significant hypercoagulability associated with hypofibrinolysis associated with high PAI‐1 and increased TAFI activation. However, PAI‐1 and TAFI measurements are not widely available in hospital laboratories. We therefore hypothesized that thromboelastography might be able to detect both hypercoagulability and hypofibrinolysis in patients with Covid‐19.
Thromboelastography is a global hemostasis assay, able to assess both the coagulation and fibrinolytic components simultaneously.15 This whole blood assay is easy to perform, fast, uses standardized reagents, and is available in an increasing number of hematology laboratories. The assay is routinely used to assess coagulation and it can detect hyperfibrinolysis in trauma patients. Two recent works reported that Covid‐19 patients present a severe hypercoagulability rather than consumptive coagulopathy using thromboelastography, but these studies did not reported any abnormality of fibrinolysis.16., 17. We modified a ROTEM assay (ROTEM delta device) by adding exogenous tPA 0.625 µg/mL (0.008 µmol/L) to the system and activated coagulation with the EXTEM reagent containing tissue factor and polybrene that neutralizes heparin. We determined a modified ROTEM (TEM‐tPA) intra‐assay precision in 10 measurements for 5 individual samples and the inter‐assay precision in 5 measurements for 5 individual samples by calculating the standard deviations and the corresponding coefficient of variation. In our hands, the inter‐assay coefficient of variation of the modified TEM‐tPA was 2.2%, 9.5%, and <1% for alpha angle, maximal clot firmness (MCF), and Ly30, respectively. The intra‐assay variability of the test was <5% for all three parameters. These low coefficient of variation percentages suggest a good reproducibility of the method, similar to those reported by Kuiper et al.18 The modified TEM‐tPA in whole blood was measured in a limited number of unselected patients (n = 23) who were still present in the hospital when TEM‐tPA assay was validated and ready to use. All Covid‐19 patients had increased coagulation capacity evidenced by elevated MCF. Interestingly, ICU patients with thrombotic complications had a more thrombogenic TEM‐tPA profile compared with Covid‐19 patients without thrombosis (Figure 1B). The Ly30 parameter that measures the extent of clot breakdown by assessing residual clot firmness 30 minutes after coagulation time was 1.8 ± 3.2% (mean ± SD) in 10 normal controls because the tPA‐induced fibrinolytic activity effectively eliminated clots. In 17 ICU Covid‐19 patients without thrombosis, a much less effective fibrinolysis with a Ly30 of 37 ± 35% was observed, which was in accordance with high PAI‐1 plasma levels and elevated TAFI activation. Finally, Ly30 of ICU patients with thrombosis was significantly higher (82 ± 26%; mean ± SD; P = .0029) than other ICU Covid‐19 patients with similar disease severity (Figure 1C). The present study has some limitations. First, because of the observational study design, time of sampling relative to LMWH administration was not standardized. However, despite this study design, chosen in an emergency pandemic situation, the study could achieve its goal and showed impaired fibrinolysis, responsible for high risk of thrombosis, in patients with Covid‐19. Second, the modified TEM‐tPA assay designed to detect hypofibrinolysis can be performed with the ROTEM delta and TEG 5000 (Haemonetics) devices but not with the ROTEM sigma or TEG‐6S devices that use a cartridge system. Finally, in this study, TEM‐tPA could be measured in 30% of patients only, but a prospective clinical trial to evaluate the ability of TEM‐tPA to predict thrombosis in Covid‐19 patients is currently ongoing (ClinicalTrials.gov NCT04366778).
In conclusion, Covid‐19 patients on heparin thromboprophylaxis present not only with hypercoagulability but also with impaired fibrinolysis that together may contribute to a risk of thrombosis in patients on adequate antithrombotic therapy. Future studies should assess efficacy and safety of escalated doses of heparin in patients with Covid‐19.
CONFLICT OF INTEREST
None of the authors declare conflicts of interest.
AUTHOR CONTRIBUTIONS
Christophe Nougier performed the research and analyzed the data; Remi Benoit performed the research; Marie Simon included patients and performed research; Helene Desmurs‐Clavel included patients and performed research; Guillaume Marcotte included patients and performed research; Laurent Argaud included patients and performed research; Jean S. David included patients and performed research; Aurelie Bonnet included patients and performed research; Claude Negrier critically revised the manuscript; and Yesim Dargaud designed the research study, analyzed the data, and wrote the paper.
Footnotes
Manuscript handled by: Ton Lisman
Final decision: Ton Lisman, 10 July 2020
REFERENCES
- 1.Connors J.M., Levy J.H. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID‐19 pneumonia detected by pulmonary CT angiography. Radiology. 2020:201544. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cattaneo M., Bertinato E.M., Birocchi S., et al. Pulmonary embolism or pulmonary thrombosis in COVID‐19? Is the recommendation to use high‐dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020 doi: 10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhai Z., Li C., Chen Y., et al. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020;120(06):937–948. doi: 10.1055/s-0040-1710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bruijne E.L., Gils A., Guimarães A.H., et al. The role of thrombin activatable fibrinolysis inhibitor in arterial thrombosis at a young age: the ATTAC study. J Thromb Haemost. 2009;7(6):919–927. doi: 10.1111/j.1538-7836.2009.03350.x. [DOI] [PubMed] [Google Scholar]
- 10.Tregouet D.A., Schnabel R., Alessi M.C., et al. Activated thrombin activatable fibrinolysis inhibitor levels are associated with the risk of cardiovascular death in patients with coronary artery disease: the AtheroGene Study. J Thromb Haemost. 2009;7:49–57. doi: 10.1111/j.1538-7836.2008.03221.x. [DOI] [PubMed] [Google Scholar]
- 11.Redlitz A., Nicolini F.A., Malycky J.L., Topol E.J., Plow E.F. Inducible carboxypeptidase activity. Circulation. 1996;93(7):1328–1330. doi: 10.1161/01.cir.93.7.1328. [DOI] [PubMed] [Google Scholar]
- 12.Meltzer M.E., Lisman T., de Groot P.G., et al. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI‐1. Blood. 2010;116(1):113–121. doi: 10.1182/blood-2010-02-267740. [DOI] [PubMed] [Google Scholar]
- 13.Prabhakaran P., Ware L.B., White K.E., Cross M.T., Matthay M.A., Olman M.A. Elevated levels of plasminogen activator inhibitor‐1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;285:L20–L28. doi: 10.1152/ajplung.00312.2002. [DOI] [PubMed] [Google Scholar]
- 14.Chen R., Yan J., Liu P., Wang Z., Wang C. Plasminogen activator inhibitor links obesity and thrombotic cerebrovascular diseases: the roles of PAI‐1 and obesity on stroke. Metab Brain Dis. 2017;32(3):667–673. doi: 10.1007/s11011-017-0007-3. [DOI] [PubMed] [Google Scholar]
- 15.Whiting D., DiNardo J.A. TEG and ROTEM: technology and clinical applications. Am J Hematol. 2014;89(2):228–232. doi: 10.1002/ajh.23599. [DOI] [PubMed] [Google Scholar]
- 16.Panigada M., Bottino N., Tagliabue P., et al. Hypercoagulability of COVID‐19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spiezia L., Boscolo A., Poletto F., et al. COVID‐19‐related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120(06):998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuiper G.J., Kleinegris M.C., van Oerle R., et al. Validation of a modified thromboelastometry approach to detect changes in fibrinolytic activity. Thromb J. 2016;14:1. doi: 10.1186/s12959-016-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]