Abstract
Aims
The wide variety of affected organ systems associated with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection highlights the need for tissue‐specific evaluation. We compared placentas from SARS‐CoV‐2‐positive and SARS‐CoV‐2‐negative women in our hospital in New York City, which became the epicenter of the coronavirus disease 2019 pandemic in March 2020. To date, some limited studies have been published on placentas from SARS‐CoV‐2‐positive women. The aim of our study, in addition to describing histomorphology, was to utilize in‐situ hybridization (ISH) for the S‐gene encoding the spike protein and immunohistochemistry (IHC) with the monoclonal SARS‐CoV‐2 spike antibody 1A9 for placental evaluation.
Methods and results
In this study, 51 singleton, third‐trimester placentas from SARS‐CoV‐2‐positive women and 25 singleton, third‐trimester placentas from SARS‐CoV‐2‐negative women were examined histomorphologically according to the Amsterdam Criteria and with ISH and/or IHC. The corresponding clinical findings and neonatal outcomes also were recorded. Although no specific histomorphologic changes related to SARS‐CoV‐2 were noted in the placentas, evidence of maternal–fetal vascular malperfusion was identified, with placentas from SARS‐CoV‐2‐positive women being significantly more likely to show villous agglutination (P = 0.003) and subchorionic thrombi (P = 0.026) than placentas from SARS‐CoV‐2‐negative women. No evidence of direct viral involvement was identified with ISH and IHC.
Conclusions
In this study, third‐trimester placentas from SARS‐CoV‐2‐positive women were more likely to show evidence of maternal–fetal vascular malperfusion; however, ISH and IHC provided no evidence of direct viral involvement or vertical transmission.
Keywords: COVID‐19, pregnancy, immunohistochemistry, in‐situ hybridization, SARS‐CoV‐2, third trimester, placental pathology
Introduction
An outbreak of pneumonia caused by the 2019 novel coronavirus severe acute respiratory syndrome (SARS) coronavirus 2 (SARS‐CoV‐2), first reported in Wuhan, China 1 in December 2019, rapidly spread throughout the world and was declared a pandemic by the World Health Organization on 11 March 2020. This led many hospitals, especially within SARS‐CoV‐2 epicenters, to initiate universal SARS‐CoV‐2 testing of women presenting to labor and delivery (L&D). At our academic hospital, Columbia University Irving Medical Center (CUIMC) in New York City, preliminary data showed that SARS‐CoV‐2 testing gave positive results in 15.4% of women presenting to L&D, 87.9% of whom were asymptomatic. 2
Pneumonias arising from any infectious etiology are important causes of morbidity and mortality among pregnant women. Adverse outcomes include premature rupture of membranes, preterm labor, intrauterine fetal demise, intrauterine growth restriction, and neonatal death. 3 Although most SARS‐CoV‐2 cases are mild to moderate, severe disease, termed coronavirus disease 2019 (COVID‐19), is devastating many communities. Many questions regarding its pathophysiology and impact on specific populations, including pregnant women, remain. Studies to date regarding SARS‐CoV‐2 and placental pathology have been limited by the number of SARS‐CoV‐2‐positive cases, 4 , 5 and only one, with a sample size of five cases, has utilized in‐situ hybridization (ISH) and immunohistochemistry (IHC). 6 In our study, we compared placental histopathology of 51 SARS‐CoV‐2‐positive and 25 SARS‐CoV‐2‐negative women in their third trimesters presenting to L&D, and tested placentas from SARS‐CoV‐2‐positive women with ISH and/or IHC.
Materials and methods
For this study, we examined placentas from 51 SARS‐CoV‐2‐positive women, as documented in their electronic medical records, from 23 March 2020 to 29 April 2020, the peak of SARS‐CoV‐2 infection in New York City. Twenty‐five selected consecutively received singleton third‐trimester placentas from SARS‐CoV‐2‐negative women from the same time period were reviewed for comparison. All women were tested in L&D with nasopharyngeal swabs by use of a reverse transcription polymerase chain reaction (RT‐PCR) test. 2 All neonates born to SARS‐CoV‐2‐positive women were tested for SARS‐CoV‐2 immediately after birth, with the same methodology.
All placentas were grossly examined, and hematoxylin and eosin‐stained sections of umbilical cords, membranes, and discs, including fetal and maternal plates, were reviewed. The placentas from SARS‐CoV‐2‐positive women were tested with ISH for the S‐gene encoding the spike protein (RNAscope‐ProbeV‐nCoV2019‐S; Advanced Cell Diagnostics, Hayward, CA, USA) according to the manufacturer’s protocol, and/or IHC with the monoclonal SARS‐CoV‐2 spike antibody 1A9 (1:1000 dilution; GeneTex, Irvine, CA, USA). For both ISH and IHC, on‐slide positive controls of lung tissue from SARS‐CoV‐2‐positive autopsy specimens were utilized. The 51 placentas from SARS‐CoV‐2‐positive women were tested with ISH and/or IHC: 32 with both ISH and IHC, five with ISH only, and 14 with IHC only. Slides were re‐reviewed in consensus according to the Amsterdam criteria. 7 Fisher’s exact test was performed to examine differences between morphological features in placentas from SARS‐CoV‐2‐positive and SARS‐CoV‐2‐negative women. The CUIMC Institutional Review Board approved this study.
18Results
CLINICAL DATA
For the 76 placentas in the study, maternal ages ranged from 19 years to 47 years (mean 29.2 ± 6.1 years) for SARS‐CoV‐2‐positive women and from 21 years to 42 years (mean 32.3 ± 5.7 years) for SARS‐COV‐2‐negative women. Of the 51 SARS‐CoV‐2‐positive women, 51% (26) were asymptomatic. Cough (61.5%), fever (53.8%), myalgia (26.9%), sore throat (11.5%) and fatigue (11.5%) were the most common symptoms. Although the majority of patients were either asymptomatic or had mild symptoms, four (15.4%) had severe disease necessitating supplemental oxygen, treatment with experimental therapies (hydroxychloroquine, azithromycin, tocilizumab, and remdesivir), and, in one patient, intubation. Comorbidities, including obesity, hypertension, pre‐eclampsia, diabetes, hypothyroidism, and asthma, were similar between SARS‐CoV‐2‐positive and SARS‐COV‐2‐negative women. Delivery methods and preterm delivery (gestational age of < 37 weeks) were also similar between these two groups (Table 1). No adverse perinatal outcomes were identified: all neonates from SARS‐CoV‐2‐positive women had high (>7) 5‐min Apgar scores. No deaths were reported.
Table 1.
Clinical information and histomorphology of third‐trimester placentas from severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐positive and SARS‐CoV‐2‐negative women
| Information | SARS‐CoV‐2‐positive (N = 51), N (%) | SARS‐CoV‐2‐negative (N = 25), N (%) | P‐value* |
|---|---|---|---|
| Clinical | |||
| Gestational age (years) | |||
| <37 | 10 (19.6) | 4 (16.0) | 1.00 |
| ≥37 | 41 (80.4) | 21 (84.0) | |
| Comorbidities | |||
| Yes | 21 (41.2) | 12 (48.0) | 0.63 |
| No | 30 (58.8) | 13 (52.0) | |
| Delivery method | |||
| Vaginal delivery | 26 (51.0) | 10 (40.0) | 0.47 |
| C‐section delivery | 25 (49.0) | 15 (60.0) | |
| Pathology | |||
| Ascending intrauterine infection | |||
| Maternal response | 17 (33.3) | 9 (36.0) | 1.00 |
| Fetal response | 9 (17.7) | 3 (12.0) | 0.74 |
| Maternal vascular malperfusion | |||
| DVA | 3 (5.9) | 1 (4.0) | 1.00 |
| ACCVM/DVH | 10 (19.6) | 1 (4.0) | 0.09 |
| VAG | 21 (41.2) | 2 (8.0) | 0.003 |
| INF | 7 (13.7) | 6 (24.0) | 0.33 |
| IVT | 8 (15.7) | 7 (28) | 0.23 |
| SCT | 9 (17.7) | 0 | 0.026 |
| Fetal vascular malformation | |||
| AVASCS | 5 (9.8) | 0 | 0.16 |
| FTV | 4 (7.8) | 0 | 0.30 |
| CHORS | 8 (15.7) | 2 (8.0) | 0.48 |
| Chronic villitis, unknown etiology | 2 (3.9) | 2 (8.0) | 0.59 |
ACCVM, accelerated villous maturity; AVASCS, avascular villi, segmental; CHORS, chorangiosis; DVA, decidual vasculopathy; DVH, distal villous hypoplasia; FTV, fetal thrombotic vasculopathy; INF, infarct; IVT, intervillous thrombus; SCT, subchorionic thrombus; VAG, villous agglutination.
P‐value based on Fisher’s exact test.
PLACENTAL PATHOLOGY
The majority of placental weights (PWs) and fetal/placental weight (FPR) ratios for SARS‐CoV‐2‐positive and SARS‐COV‐2‐negative women were within 10th–90th percentile reference ranges. Placentas from SARS‐CoV‐2‐positive women showed non‐specific evidence of maternal–fetal vascular malperfusion, including subchorionic thrombi (Figure 1A), intervillous thrombi (Figure 1B), infarction (Figure 1C), chorangiosis, segmental avascular villi (Figure 1D), fetal thrombotic vasculopathy (Figure 1E), and villous agglutination (Figure 1F). Of these, villous agglutination and subchorionic thrombi were significantly more likely to occur in placentas from SARS‐CoV‐2‐positive women than in those from SARS‐CoV‐2‐negative women (P = 0.026 and P = 0.003, respectively). We found no statistically significant differences between placentas from symptomatic and asymptomatic SARS‐CoV‐2‐positive women in terms of intervillous thrombi, infarction, chorangiosis, accelerated villous maturation, etc. (Table 2). No lesions associated with direct viral involvement (viral cytopathic changes) were noted, and both ISH (Figure 1G) and IHC (Figure 1H) gave negative results in all tested placentas from SARS‐CoV‐2‐positive women. All neonates born to SARS‐CoV‐2‐positive women tested negative for SARS‐CoV‐2.
Figure 1.
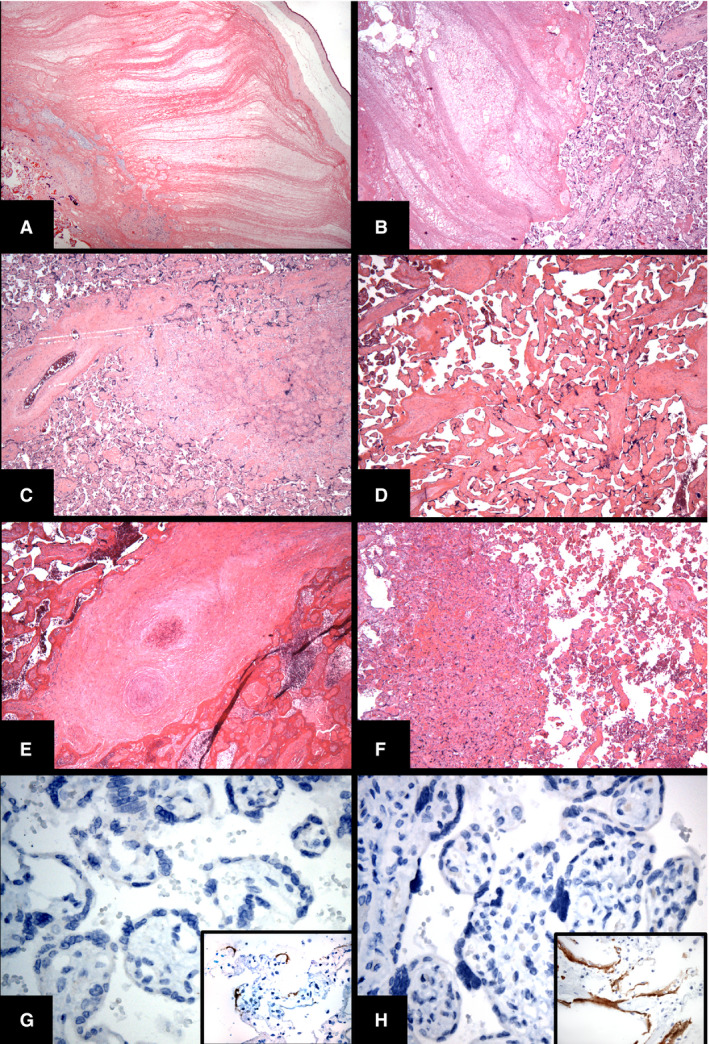
Histologic findings in placentas from severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐positive women. A, Subchorionic thrombus. B, Intervillous thrombi. C, Infarction. D, Segmental avascular villi. E, Thrombotic vasculopathy. F, Villous agglutination. G, Negative spike protein in‐situ hybridization result in placental tissue from a SARS‐CoV‐2‐positive woman; inset shows a positive control from autopsy lung tissue. H, Negative result for monoclonal SARS‐CoV‐2 spike antibody 1A9 immunohistochemistry in placental tissue from a SARS‐CoV‐2‐positive woman; inset shows a positive control from autopsy lung tissue. [Colour figure can be viewed at wileyonlinelibrary.com]
Table 2.
Clinical information and histomorphology of third‐trimester placentas from symptomatic and asymptomatic severe acute respiratory syndrome coronavirus 2‐positive women
| Information | Symptomatic (N = 25), N (%) | Asymptomatic (N = 26), N (%) | P‐value* |
|---|---|---|---|
| Clinical | |||
| Gestational age (weeks) | |||
| <37 | 6 (24) | 4 (15) | 0.50 |
| ≥37 | 19 (76) | 22 (85) | |
| Comorbidities | |||
| Yes | 12 (48) | 9 (35) | 0.40 |
| No | 13 (52) | 17 (65) | |
| Delivery method | |||
| Vaginal delivery | 12 (48) | 14 (54) | 0.78 |
| C‐section delivery | 13 (52) | 12 (46) | |
| Pathology | |||
| Ascending intrauterine infection | |||
| Maternal response | 7 (28) | 10 (38) | 0.56 |
| Fetal response | 2 (8) | 7 (27) | 0.14 |
| Maternal vascular malperfusion | |||
| DVA | 1 (4) | 2 (7) | 1.00 |
| ACCVM/DVH | 4 (16) | 6 (23) | 0.73 |
| VAG | 12 (48) | 9 (35) | 0.40 |
| INF | 3 (12) | 4 (15) | 1.00 |
| IVT | 2 (8) | 6 (23) | 0.25 |
| SCT | 7 (28) | 2 (8) | 0.07 |
| Fetal vascular malformation | |||
| AVASCS | 0 | 5 (19) | 0.051 |
| FTV | 0 | 4 (15) | 0.11 |
| CHORS | 3 (12) | 5 (19) | 0.70 |
| Chronic villitis, unknown etiology | 1 (4) | 1 (4) | 1.00 |
ACCVM, accelerated villous maturity; AVASCS, avascular villi, segmental; CHORS, chorangiosis; DVA, decidual vasculopathy; DVH, distal villous hypoplasia; FTV, fetal thrombotic vasculopathy; INF, infarct; IVT, intervillous thrombus; SCT, subchorionic thrombus; VAG, villous agglutination.
Discussion
SARS‐CoV‐2 infects target cells by binding to angiotensin‐converting enzyme II (ACE2) via its surface spike protein, in a similar fashion to the SARS coronavirus responsible for the epidemic of SARS in 2002–2003. 8 ACE2 has been reported to be present in decidual cells, syncytiotrophoblasts, cytotrophoblasts, and endothelium and vascular smooth muscle of primary and secondary villi. 9 , 10 Although the presence of placental ACE2 suggests the possibility of SARS‐CoV‐2 infection of these cells, we did not detect direct viral presence of SARS‐CoV‐2 in placentas by the use of morphology, IHC, and ISH targeting the spike protein, similarly to other studies. 6
Thus far, studies have reported similar clinical symptoms and outcomes between SARS‐CoV‐2‐positive pregnant and SARS‐CoV‐2‐positive non‐pregnant women. 11 However, to date, there have been no reported cases of definitive vertical transmission. 12 , 13 , 14 Studies from the previous SARS outbreak indicated that SARS was associated with higher incidences of spontaneous miscarriage, preterm delivery, and intrauterine growth restriction, but without vertical transmission. 15 Placental pathologic features described in association with SARS, e.g. increases in intervillous or subchorionic fibrin and avascular fibrotic villi, 16 are similar to our histomorphologic findings. As compared with SARS‐CoV‐2‐negative placentas, in the SARS‐CoV‐2‐positive placentas there was an increased incidence of villous agglutination, subchorionic thrombi, accelerated villous maturity, chorangiosis, fetal thrombotic vasculopathy, and avascular villi, suggestive of fetal stress and warranting further investigation (Table 1). In view of the fact that retroplacental and intraplacental hemorrhages are considered to constitute evidence of maternal vascular malperfusion (MVM) according to the Amsterdam Criteria and other studies, 7 , 17 , 18 , 19 we considered subchorionic thrombi to constitute evidence of MVM in our study.
PW is closely related to fetal growth and is affected by various pregnancy‐related conditions. 20 The FPR is commonly used to assess the possibility of underlying pathological conditions and poor perinatal outcomes. 21 In our study, the majority of PWs and FPRs from SARS‐CoV‐2‐positive and SARS‐CoV‐2‐negative women were within the 10th–90th percentile reference range. 22
In our limited study of 51 placentas from SARS‐CoV‐2‐positive women in the third trimester, ISH and IHC showed no definite evidence of SARS‐CoV‐2 in the placentas, and we noted non‐specific histomorphologic changes suggestive of maternal–fetal vascular malperfusion. All neonates tested negative for SARS‐CoV‐2, and all women recovered clinically. Further studies, including more sensitive techniques for viral infection (e.g. RT‐PCR), are warranted.
Conflicts of interest
All authors state that they have no conflicts of interest.
Author contributions
M. C. Smithgall, D. Hamele‐Bena, and X. Chen: conception, design, primary data acquisition, data analysis, and writing of the manuscript. X. Liu‐Jarin, A. Cimic, and L. Debelenko: conception, design, and data acquisition. M. Mourad: clinical data acquisition.
Acknowledgements
The authors would like to thank Denice Tsao‐Wei for the statistical analyses.
Smithgall MC, Liu‐Jarin X, Hamele‐Bena D, Cimic A, Mourad M, Debelenko L & Chen X. (2020) Histopathology 77, 994–999. 10.1111/his.14215 Third‐trimester placentas of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐positive women: histomorphology, including viral immunohistochemistry and in‐situ hybridization
References
- 1. Zhu N, Zhang D, Wang W et al. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2020; 382; 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sutton D, Fuchs K, D’Alton M, Goffman D. Universal screening for SARS‐CoV‐2 in women admitted for delivery. N. Engl. J. Med. 2020; 382; 2163–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schwartz DA, Graham A. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019‐nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses 2020; 12; 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baergen RN, Heller DS. Placental pathology in Covid‐19 positive mothers: preliminary findings. Pediatr. Dev. Pathol. 2020; 23; 177–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID‐19. Am. J. Clin. Pathol. 2020; 154; 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mulvey JJ, Magro CM, Ma LX, Nuovo GJ, Baergen RN. Analysis of complement deposition and viral RNA in placentas of COVID‐19 patients. Ann. Diagn. Pathol. 2020; 46; 151530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khong TY, Mooney EE, Ariel I et al. Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group consensus statement. Arch. Pathol. Lab. Med. 2016; 140; 698–713. [DOI] [PubMed] [Google Scholar]
- 8. Lu R, Zhao X, Li J et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origin and receptor binding. Lancet 2020; 395; 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004; 203; 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Valdes G, Neves LAA, Anton L et al. Distribution of angiotensin‐(1–7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta 2006; 27; 200–207. [DOI] [PubMed] [Google Scholar]
- 11. Chen H, Gui J, Wang C et al. Clinical characteristics and intrauterine vertical transmission potential of COVD‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020; 395; 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Y, Peng H, Wang L et al. Infants born to mothers with a new coronavirus (COVID‐19). Front. Pediatr. 2020; 8; 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen H, Gui J, Wang C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020; 395; 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu H, Wang L, Fang C et al. Clinical analysis of 10 neonates born to mothers with 2019‐nCoV pneumonia. Transl. Pediatr. 2020; 9; 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong SF, Chow KM, Leung TN et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004; 191; 292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ng WF, Wong SF, Lam A et al. The placentas of patients with severe acute respiratory syndrome: a pathophysiological evaluation. Pathology 2006; 38; 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bustamante Helfrich B, Chilukuri N, He H et al. Maternal vascular malperfusion of the placental bed associated with hypertensive disorders in the Boston Birth Cohort. Placenta 2017; 52; 106–113 [published correction appears in Placenta 2019; 86; 52–53]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alanjari A, Wright E, Keating S et al. Prenatal diagnosis, clinical outcomes and associated pathology in pregnancies complicated by massive subchorionic thrombohematoma (Breus’ mole). Prenat. Diagn. 2013; 33; 973–978. [DOI] [PubMed] [Google Scholar]
- 19. Armstrong‐Wells J, Post MD, Donnelly M, Manco‐Johnson MJ, Fisher BM, Winn VD. Patterns of placental pathology in preterm premature rupture of membranes. J. Dev. Orig. Health Dis. 2013; 4; 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ness RB, Bass D, Hill L, Klebanoff MA, Zhang J. Diagnostic test characteristics of placental weight in the prediction of small‐for‐gestational‐age neonates. J. Reprod. Med. 2007; 52; 793–800. [PubMed] [Google Scholar]
- 21. McNamara H, Hutcheon JA, Platt RW, Benjamin A, Kramer MS. Risk factors for high and low placental weight. Paediatr. Perinat. Epidemiol. 2014; 28; 97–105. [DOI] [PubMed] [Google Scholar]
- 22. Redline RW, Boyd TK, Roberts DJ eds. Placental and gestational pathology. Cambridge: Cambridge University Press, 2019; 336–337. [Google Scholar]


