Abstract
Objectives
To assess changes in characteristics and management among ST‐elevation myocardial infarction (STEMI) patients with coronavirus disease (COVID‐19) who underwent primary percutaneous coronary intervention.
Methods
Our prospective, monocentric study enrolled all STEMI patients who underwent PPCI during the COVID‐19 outbreak (n = 83). This cohort was first compared with a previous cohort of STEMI patients (2008–2017, n = 1,552 patients) and was then dichotomized into a non‐COVID‐19 group (n = 72) and COVID‐19 group (n = 11).
Results
In comparison with the pre‐outbreak period, patients during the outbreak period were older (59.6 ± 12.9 vs. 62.6 ± 12.2, p = .03) with a delayed seek to care (mean delay first symptoms‐balloon 3.8 ± 3 vs. .7.4 ± 7.7, p < .001) resulting in a two‐fold higher in‐hospital mortality (non COVID‐19 4.3% vs. COVID‐19 8.4%, p = .07). Among the 83 STEMI patients admitted during the outbreak period, 11 patients were infected by COVID‐19. Higher biological markers of inflammation (C‐reactive protein: 28 ± 39 vs. 98 ± 97 mg/L, p = .04), of fibrinolysis (D‐dimer: 804 ± 1,500 vs. 3,128 ± 2,458 μg/L, p = .02), and antiphospholipid antibodies in four cases were observed in the COVID‐19 group. In this group, angiographic data also differed: a thrombotic myocardial infarction nonatherosclerotic coronary occlusion (MINOCA) was observed in 11 cases (1.4% vs. 54.5%, p < .001) and associated with higher post‐procedure distal embolization (30.6% vs. 72.7%, p = .007). The in hospital mortality was significantly higher in the COVID‐19 group (5.6% vs. 27.3%, p = .016).
Conclusion
The COVID‐19 outbreak implies deep changes in the etiopathogenesis and therapeutic management of STEMI patients with COVID‐19. The impact on early and long‐term outcomes of systemic inflammation and hypercoagulability in this specific population is warranted.
Keywords: Acute myocardial infarction/STEMI, Coronary Artery Disease, Percutaneous Coronary Intervention
1. INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic, also known as coronavirus disease 2019 (COVID‐19), has significantly impacted the healthcare system worldwide. To preserve patients' health and resources, including hospital beds to care for COVID‐19 patients, health authorities recommend deferral of elective cardiac procedures, including coronary angiography and percutaneous coronary intervention for stable coronary artery disease. 1
Timely reperfusion of primary percutaneous coronary intervention (PPCI) is the standard of care for patients who develop an ST‐elevation myocardial infarction (STEMI). 2 Thus, the Society for Cardiac Angiography and Interventions and the American College of Cardiology continue to recommend primary percutaneous coronary intervention as the standard treatment of STEMI patients during the current pandemic. 3
Despite these recommendations for treatment of STEMI, different reports suggest a reduction in the number of STEMI cases treated in cardiac catheterization laboratories by up to 40%. 4
Moreover, little is known about changes in characteristics and management of STEMI patients who underwent PPCI during the outbreak, and especially in patients infected by COVID‐19.
We described the experience concerning STEMI patients during the complete period of the COVID‐19 confinement at the University Hospital of Nancy (France) located in the North‐Eastern region of France, one of the French epicenters of the pandemia.
2. METHODS
We prospectively collected all STEMI patients admitted at the University Hospital of Nancy from February 26, 2020, corresponding to the date of the first patient with COVID‐19 was diagnosed in the “Grand Est”, the administrative name of the northeastern region of France to May 10th 2020 corresponding to the official end of confinement in France. During this time period all patients admitted to the catheterization laboratory were routinely tested for COVID‐19 by nasopharyngeal swabs or respiratory samples as well as thoracic CT in case of respiratory symptoms. The COVID‐19 diagnosis was confirmed by a positive real‐time PCR (RT‐PCR) or by the result of an evocative CT scan associated with typical clinical features, that is, acute respiratory distress and fever. Viral RNA was extracted using the NucliSens easyMAG (bioMerieux France) according to the manufacturer's instructions, and amplified by RT‐PCR protocols developed by La Charité (E gene) and the Institut Pasteur (RdRp gene) on CFX96 Touch™ (Bio‐Rad). Quantified positive controls were kindly provided by the French National Reference Center for Respiratory Viruses, Institut Pasteur, Paris.
STEMI patients during the outbreak were stratified in two groups: a non COVID‐19 group and a COVID‐19 group.
STEMI was defined according to the universal definition by 1 continuous chest pain for at least 20 min, 2 ST‐segment elevation ≥1 mm (0.1 mV) in ≥2 contiguous leads on the 12‐lead ECG, and 3 new onset left bundle branch block. All study patients underwent invasive coronary angiography and transthoracic echocardiography, whereas other diagnostic imaging modalities (optical coherence tomography, and intravascular ultrasound) were used according to the treating physician's decision.
Two experienced operators reviewed all coronary angiograms. At least two orthogonal angiographic projections were used to assess the culprit vessel and lesion. The angiographic definition of thrombotic MINOCA was the presence of a thrombus defined as a non‐calcified filling defect outlined on at least three sides by contrast media and the absence of coronary lesions (underlying the thrombus as well as throughout the coronary tree) defined as a luminal narrowing >25% by visual estimate.
The case definition of thrombotic MINOCA used in this study consisted of major and minor criteria according to Shibata al as described elsewhere. 5 We excluded myocardial infarction based on the following findings: (a) etio‐pathological evidence of atherosclerotic thrombus, (b) coronary artery ectasia, (c) plaque disruption or coronary erosion detected by intravascular ultrasound or optic coherence tomography of the culprit lesion, (d) coronary artery stenosis outside the culprit lesion (≥25%) on the coronary angiography, and (e) other causes of STEMI without non atherosclerotic disease (vasospasm).
2.1. Procedural characteristics
In the pre‐hospital phase all patients received aspirin 250–500 mg intravenously and heparin (70 UI/kg IV bolus) as well as an oral loading dose of a P2Y12 inhibitor. During the procedure, heparin dose was adapted according to the use of an glycoprotein IIb/IIIa inhibitor according to the physician's decision. Dual antiplatelet therapy (aspirin 70–160 mg/day and P2Y12 inhibitors) was maintained for the first year.
2.1.1. Clinical events
Clinical outcome included death from all causes during the in hospital period. If death occurred, the patient's physician or the hospital records were consulted to determine the cause. The retrospective study of patients' files was approved by the Commission Nationale Informatique et Liberte (CNIL), in keeping with French law for single‐center usual care observational studies. Each patient and 2 the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
2.1.2. Statistical analysis
Continuous variables are presented as mean ± SD or median and in‐terquartile range and compared using a Student t test or Wilcoxon test. Categorical data are presented as numbers and percentages and compared using a χ2 or Fisher exact test, according to conditions of application. Because of the small sample size and outcome events, multivariable adjustment was considered inappropriate. The significance level was set at .05. All statistical analyses were performed using SPSS software.
3. RESULTS
During the COVID‐19 outbreak, 83 STEMI patients who underwent PPCI were admitted in our hospital (Group 1) and were compared with 1,552 STEMI patients who were treated with PPCI in between the years 2008 and 2017. Patients' characteristics, angiographic and procedural characteristics, according to the study period, are summarized in Table 1. In comparison with the pre‐outbreak period, we observed a delayed hospital presentation during the outbreak period (mean delay first symptoms‐balloon in hours: 3.8 ± 3 vs. 7.4 ± 7.7, p < .001) with near one quarter of the patients admitted more than 8 h after symptom onset. Epicardial perfusion assessed by corrected TIMI frame count [cTFC] (optimal cTFC post procedure <23:42.3% vs. 53.8%, p = .044) and microvascular perfusion by ECG (optimal ST resolution [STR]): >70%: 53.9% vs. 40.7%, p = .02) were significantly lower in the outbreak group. LVEF <45% was observed in more than half in the outbreak patients with near a twofold higher in hospital mortality (4.3% vs. 8.4%, p = .07).
TABLE 1.
Baseline and procedural characteristics according to the study period
| n | Non outbreak period (n = 1,552) | n | Outbreak period (n = 83) | p value | |
|---|---|---|---|---|---|
| % | % | ||||
| Age, years, mean ± SD | 1,552 | 59.6 ± 12.9 | 83 | 62.6 ± 13.2 | .03 |
| Sex, male | 1,552 | 76.1% | 83 | 56.3% | .14 |
| Body mass index (kg/m2) mean ± SD | 1,552 | 26.6 ± 4.6 | 83 | 26.8 ± 5.26 | .67 |
| Cardiovascular risk factors | |||||
| Diabetes | 231 | 14.9% | 16 | 19.3% | .28 |
| Hypertension | 620 | 40.1% | 36 | 43.4% | .55 |
| Hyperlipidemia | 544 | 37.1% | 31 | 37.8% | .96 |
| Smoker (current or past) | 897 | 58% | 44 | 53% | .37 |
| Atrial fibrillation | 123 | 7.9% | 8 | 9.6% | .57 |
| Delay first symptoms –balloon (hours) | 1,552 | 3.8 ± 3 | 83 | 7.4 ± 7.7 | <.001 |
| Delay call to balloon | 1,552 | 1.7 ± 1.5 | 83 | 2.4 ± 3.2 | .10 |
| Delay door to balloon | 1,552 | 1.2 ± 2.3 | 83 | 1.3 ± 2.3 | .5 |
| Pre hospital cardiogenic shock | 120 | 7.7% | 8 | 9.6% | .53 |
| Prehospital cardiac arrest | 180 | 11.6% | 14 | 16.9% | .14 |
| Angiographic results | |||||
| Main culprit lesion treated with PCI: | <.001 | ||||
| Left main | 9 | 0.6% | 1 | 1.2% | |
| Left anterior descending | 695 | 44.8% | 37 | 44.6% | |
| Left circumflex | 479 | 30.9% | 6 | 7.2% | |
| Right coronary artery | 355 | 22.9% | 39 | 47% | |
| Triple coronary disease | 193 | 12.4% | 9 | 10.8% | .66 |
| Pre PCI flow grade | .12 | ||||
| TIMI 0/1 | 846 | 68.7% | 58 | 69.9% | |
| TIMI 2 | 164 | 11.9% | 9 | 10.8% | |
| TIMI 3 | 267 | 19.4% | 16 | 19.3% | |
| Primary angioplasty (balloon and/or stent) | 1,459 | 94% | 80 | 96.4% | .36 |
| Stent implantation | 1,374 | 91.7% | 69 | 83.1% | .008 |
| Direct stenting | 534 | 48.6% | 59 | 72% | <.001 |
| Distal embolization | 209 | 15.9% | 30 | 36.1% | <.01 |
| Post PCI flow grade | .40 | ||||
| TIMI 0/1 | 54 | 3.9% | 2 | 2.4% | |
| TIMI 2 | 113 | 8.1% | 10 | 12% | |
| TIMI 3 | 1,220 | 88% | 71 | 85.5% | |
| TIMI frame count <23 | 567 | 42.3% | 43 | 53.8% | .044 |
| Post PPCI results | |||||
| CPK (UI/L) | 1,552 | 2,697 ± 2,702 | 83 | 2,418 ± 2,663 | .8 |
| ECG: ST resolution >70% | 705 | 53.9% | 33 | 40.7% | .022 |
| LVEF (%) | .2 | ||||
| <35% | 146 | 9.4% | 12 | 14.5% | |
| 35–45% | 586 | 37.8% | 30 | 36.1% | |
| >45% | 806 | 51.9% | 41 | 49.4% | |
Among the STEMI patients admitted during the outbreak period, 72 patients did not have COVID‐19 (non‐COVID‐19 group) and 11 patients (13%) had COVID‐19 (COVID‐19 group). Patients' baseline, angiographic and procedural characteristics in non COVID‐19 and COVID‐19 patients during the outbreak period are summarized in Table 2. Angiographic data differed in COVID‐19 group with myocardial infarction secondary to a non‐atherosclerotic coronary occlusion (MINOCA) in six cases (1.4% vs. 54.5%, p < .001) needing stent implantation in less than two third of the cases (86.1% vs. 63.9%, p = .007) and associated with higher post‐procedure distal embolization (30.6% vs. 72.7%, p = .007). Further, in two cases, multiple thrombotic coronary occlusions were observed and one of them with a concomitant pulmonary embolism (Figures 1 and 2).
TABLE 2.
Baseline and procedural characteristics in non COVID‐19 and COVID‐19 patients during the outbreak
| n | Non COVID‐19 group (n = 72) | n | Covid‐19 group (n = 11) | p value | |
|---|---|---|---|---|---|
| % | % | ||||
| Age, years, mean ± SD | 72 | 62.5 ± 12.6 | 11 | 63.6 ± 17.4 | .79 |
| Sex, male | 53 | 73.6% | 11 | 63.9% | .49 |
| Body mass index (kg/m2) mean ± SD | 72 | 27.02 ± 4.8 | 11 | 25.1 ± 8.1 | .5 |
| Cardiovascular risk factors | |||||
| Diabetes | 14 | 19.4% | 8 | 18.2% | .92 |
| Hypertension | 31 | 43.1% | 5 | 45.5% | .88 |
| Hyperlipidemia | 28 | 38.9% | 4 | 27.3% | .46 |
| Smoker (current or past) | 40 | 55.6% | 4 | 36.4% | .23 |
| Atrial fibrillation | 6 | 8.3% | 2 | 18.2% | .3 |
| Delay first symptoms –balloon (hours) | 72 | 7.48 ± 7.7 | 11 | 7.39 ± 7.6 | .91 |
| Delay call to balloon | 72 | 2.49 ± 1.56 | 11 | 1.56 ± 1.01 | .51 |
| Delay door to balloon | 72 | 1.42 ± 2.4 | 11 | 0.89 ± 0.78 | .41 |
| Pre hospital cardiogenic shock | 5 | 4.2% | 3 | 36.4% | .03 |
| Prehospital cardiac arrest | 10 | 13.9% | 4 | 36.4% | .064 |
| Angiographic results | |||||
| Thrombotic MINOCA | 5 | 6.9% | 6 | 54.5% | <.001 |
| Main culprit lesion treated with PCI: | .74 | ||||
| Left main | 1 | 1.4% | 0 | 0 | |
| Left anterior descending | 32 | 44.4% | 5 | 45.5% | |
| Left circumflex | 8 | 8.3% | 0 | 0 | |
| Right coronary artery | 33 | 45.8% | 6 | 54.5% | |
| Triple coronary disease | 9 | 12.5% | 0 | .21 | |
| Pre PCI flow grade | .31 | ||||
| TIMI 0/1 | 49 | 68.1% | 9 | 81.8% | |
| TIMI 2 | 7 | 9.7% | 2 | 18.2% | |
| TIMI 3 | 16 | 22.2% | 0 | 0 | |
| Glycoprotein IIb/IIIa blocker | 9 | 12.9% | 1 | 9.1% | .72 |
| Primary angioplasty (balloon and/or stent) | 70 | 97.2% | 10 | 90.9% | .29 |
| Stent implantation | 62 | 86.1% | 7 | 63.9% | .007 |
| Direct stenting | 52 | 73.2% | 7 | 63.6% | .5 |
| Distal embolization | 22 | 30.6% | 8 | 72.7% | .04 |
| Post PCI TIMI flow grade: | <.001 | ||||
| TIMI 0/1 | 0 | 0 | 2 | 18.2% | |
| TIMI 2 | 7 | 9.7% | 3 | 27.3% | |
| TIMI 3 | 65 | 90.3% | 6 | 54.5% | |
| Post PPCI results | |||||
| CPK (UI/L) | 72 | 2,071 ± 2,079 | 11 | 4,681 ± 4,582 | .09 |
| Troponin Ic (ng/ml) | 72 | 80,202 ± 95,900 | 11 | 230,000 ± 31,000 | .13 |
| ECG: ST resolution >70% | 32 | 45.7% | 1 | 9.1% | .022 |
| TIMI frame count <23 | 42 | 59.2% | 1 | 9.1% | .006 |
| LVEF | .02 | ||||
| <35% | 7 | 19.7% | 5 | 45.5% | |
| 35–45% | 27 | 37.5% | 3 | 27.3% | |
| 45% | 38 | 52.8% | 3 | 27.3% |
FIGURE 1.
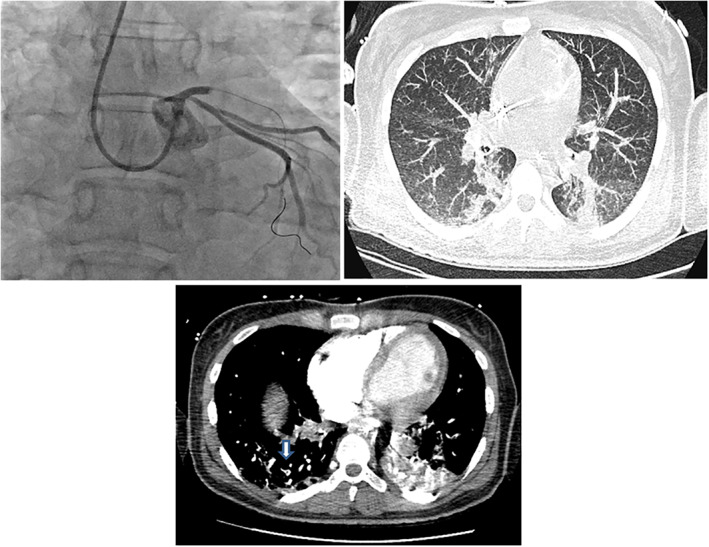
The clinical course of a 38‐year‐old patient with acute respiratory distress syndrome due to SARS‐CoV‐2 infection was complicated by concomitant anterior ST‐elevation myocardial infarction and pulmonary embolism
FIGURE 2.
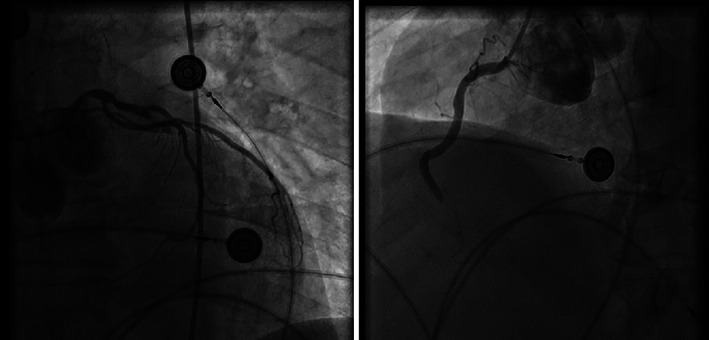
Representative multivessel thrombotic MINOCA involving LAD and RCA in a 49‐year‐old patient with acute respiratory distress
The in hospital mortality was significantly higher in the COVID‐19 group. (5.6% vs. 27.3%, p = .016). Higher biological markers of inflammation (C‐reactive protein: 28 ± 39 vs. 98 ± 97 mg/L, p = .04), of fibrinolysis (D‐dimer: 804 ± 1,500 vs. 3,128 ± 2,458 μg/L, p = .02), and more frequently antiphospholipid antibodies in four cases were also observed in the COVID‐19 group (Table 3). Among six COVID‐19 patients who experienced STEMI secondary to thrombotic MICOCA, inflammation and recorded coagulation parameters were increased in all cases and antiphospholipid antibodies were observed in three patients.
TABLE 3.
Biological and coagulation parameters in non COVID‐19 and COVID‐19 patients during the outbreak
| Non COVID‐19 patients (n = 72) | COVID‐19 patients (n = 11) | p | |
|---|---|---|---|
| Hemoglobin (g/dl) normal range 12–16 | 13.9 ± 1.9 | 11.9 ± 2.9 | .05 |
| Platelet count (Giga/L) normal range 150–450 | 266 ± 80 | 287 ± 67 | .41 |
| Lymphocyte (Giga/L) normal range 1–4 | 1.59 ± 1.61 | 0.45 ± 0.93 | .03 |
| Prothrombin time (s) normal range | 14.3 ± 2.6 | 17.6 ± 4.5 | .04 |
| Fibrinogen (g/L) normal range: 1.7–4 g/L | 3.6 ± 1.4 | 4.3 ± 2.2 | .20 |
| D‐dimer (μg/L) normal range < 500 μg/L | 864 ± 1,500 | 3,128 ± 2,498 | .02 |
| Protein C (%) normal range ≥ 70 | 102 ± 20 | 84 ± 28 | .10 |
| Protein S (%) normal range ≥ 75 | 104 ± 20 | 79 ± 24 | .02 |
| Antithrombin activity (%) normal range:50%–150% | 89 ± 11 | 83 ± 17 | .7 |
| Antiphospholipid antibody syndrome: | 7 | 4 | |
| Anticardiolipin antibodies | 7 | 3 | |
| antibeta2 glycoprotein I antibodies | 7 | 1 | |
| Creatininemia (μmol/L) normal range 49–90 | 76 ± 24 | 135 ± 125 | .15 |
| Total bilirubin (μmol/L) normal range < 10 | 11 ± 6 | 10 ± 5 | .41 |
| ALAT (U/L) normal range 7–40 | 70 ± 80 | 239 ± 328 | .11 |
| ASAT (U/L) normal range 13–40 | 184 ± 217 | 499 ± 409 | .03 |
| NT‐proBNP (pg/ml) normal range < 400 | 2,670 ± 3,557 | 12,082 ± 28,628 | .30 |
| CRP (mg/L) normal range < 5 | 28 ± 39 | 98 ± 97 | .04 |
4. DISCUSSION
The major findings of this prospective study evaluating a consecutive series of STEMI patients during the COVID‐19 confinement period were: (a) an increase in delay between symptoms onset and PPCI in the overall population with a significant proportion beyond the recommended timelines; (b) although the atherothrombotic mechanism of coronary thrombosis by plaque rupture remains important, a higher prevalence of coronary non‐atherosclerotic obstructive disease was observed which concerned more than half of patients in the COVID‐19 group, and (c) the incidence of abnormalities in conventional coagulation function parameters was higher in COVID‐19 group who experienced nonatherosclerosis induced MI, suggesting that coagulopathy is more frequent. (d) Lastly prognosis in these patients seems worse with more severe early outcomes.
A delayed hospital presentation was the greatest contributor to postponed treatment of acute myocardial infarction (MI) and a critical determinant of short‐ and long‐term mortality. 6
The most significant component in delay from the onset of coronary symptoms to reaching definitive coronary care has been identified in the delay calling for medical assistance because other pre‐hospital medical management delays, that is, call to balloon and door to balloon are unchanged. It is understandable that people are reluctant to go to a hospital during the COVID‐19 outbreak, which explains the potential delays in care‐seeking. Another concern that we were unable to evaluate is whether some patients with STEMI did not seek care at all. Strategies to reduce patient delay times in a MI setting during this confinement period depend on educating the public on the recognition and diversity of coronary symptoms and the benefits of presenting promptly to the hospital by way of the emergency ambulance service. It will certainly also depend on information campaigns certifying the presence of a COVID‐19 free standard care to patients.
Although respiratory distress symptoms were the main clinical manifestation in COVID‐19 patients admitted to the intensive care unit, this viral infection may result in acute thrombotic cardiovascular events. Excess in mortality from cardio‐vascular disease during bacterial or virus epidemics, such as the influenza epidemic, was first recognized early in the 20th century, and the specific association between a variety of infective pathogens (viral and bacterial) and the development of MI was characterized later. 7 , 8
Atherosclerotic plaques contain inflammatory cells, and infection generates circulating inflammatory cytokines, such as interleukin‐1, 6, and 8 and tumor necrosis factor‐α, that can activate inflammatory cells in atherosclerotic plaques. 9 Studies in animals have shown that inflammatory activity in atheromatous plaques increases after infective stimuli which contributes to plaque‐destabilization. 10 Additional to plaque rupture, thrombotic MINOCA may be a clinical manifestation of a hypercoagulable state in relation with an inherited and/or an acquired disorder.
Atherothrombosis by a ruptured plaque remained an important cause of MI during the confinement period, but the proportion of non‐atherosclerotic acute coronary occlusion dramatically increased in COVID‐19 group (54.5% of the cases). Indeed, there is extensive literature concerning the hypercoagulability in COVID‐19 infection as well as potential direct inflammatory involvement of the endothelial cells. 11
For the first time, our study described a large profile of coagulation function parameters in COVID‐19 patients who experienced an MI. Compared with non‐COVID patients, hypercoagulability is more frequently observed in COVID‐19 patients and in a subgroup of COVID‐19 patients who experienced non‐atherosclerosis induced MI. This hypercoagulability is attested by usual coagulation parameters (longer pro‐thrombotic time and higher fibrinolysis) and reinforced by antiphospholipid antibodies. In various systemic autoimmune diseases, a pro‐thrombotic state was also shown to be associated with the presence of antiphospholipid antibodies increasing the likelihood of coronary thrombotic events, including thrombotic MINOCA. 12 These antibodies can also arise transiently in patients with critical illness and various infections. 13 Moreover, a marked elevation in measured C‐reactive protein (CRP) levels seen in patients with significant pulmonary or systemic inflammation may interfere with these results. 14 Further studies for evaluation of the impact of antiphospholipid antibodies on adverse events in COVID‐19 patients are still warranted. Non‐atherosclerotic disease as pathogenesis of MI may also imply specific peri‐procedural conditions and therapeutic management. 15
A carefully conducted patient history may also avoid an overaggressive reperfusion strategy, as angioplasty with stenting may be deleterious leading to more distal embolization 15 in addition with antithrombotic drugs. 15
However, the treatment efficiency of antithrombotic drugs in patients with hemodynamic impairment frequently remains challenging.
4.1. Limitations
Our retrospective, monocentric study and the limited number of STEMI patients during the confinement period might have resulted from certain methodological bias. However, it remains, to our knowledge, one of the first series in the literature that has studied the etiopathogenesis of STEMI during this outbreak.
Despite a restrictive thrombotic MINOCA definition, intravascular imaging modalities including optical coherence tomography and intravascular ultrasound may be very helpful tools to identify plaque rupture and ulceration, in proximal coronary lesions especially. However, intravascular imaging may be challenging or inappropriate in the specific clinical settings of STEMI secondary to an extensive occlusive coronary thrombosis requiring multiple balloon inflations to obtain a coronary flow or secondary to a distal coronary occlusion.
Further paradoxical coronary thromboembolism through a patent foramen ovale as the cause of thrombotic MINOCA may have been underestimated by a nonsystematic transesophageal or contrast‐enhanced echocardiography.
5. CONCLUSION
The COVID‐19 outbreak implies deep changes in the clinical profile and therapeutic management of STEMI patients who underwent PPCI. Systemic inflammation and hypercoagulability characterized STEMI patients infected with COVID‐19 and their impact on early and long‐term outcomes should be investigated.
Popovic B, Varlot J, Metzdorf PA, Jeulin H, Goehringer F, Camenzind E. Changes in characteristics and management among patients with ST‐elevation myocardial infarction due to COVID‐19 infection. Catheter Cardiovasc Interv. 2021;97:E319–E326. 10.1002/ccd.29114
Each co‐author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation
REFERENCES
- 1. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidancehcf. html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019‐ncov%2Fhealthcare‐facilities%2Fguidance‐hcf.html.
- 2. Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119‐177. [DOI] [PubMed] [Google Scholar]
- 3. Mahmud E, Dauerman HL, Welt FG, et al. Management of acute myocardial infarction during the COVID‐19 pandemic. J Am Coll Cardiol. 2020. 10.1016/j.jacc.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST‐segment elevation cardiac catheterization laboratory activations in the United States during COVID‐19 pandemic. J Am Coll Cardiol. 2020;75:2871‐2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shibata T, Kawakami S, Noguchi T, et al. Prevalence, clinical features, and prognosis of acute myocardial infarction attributable to coronary artery embolism. Circulation. 2015;132(4):241‐250. [DOI] [PubMed] [Google Scholar]
- 6. Cannon CP, Gibson CM, Lambrew CT, et al. Relationship of symptom‐onset‐to‐balloon time and door‐to‐balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction. JAMA. 2000;283(22):2941‐2947. [DOI] [PubMed] [Google Scholar]
- 7. Musher DM, Abers MS, Corrales‐Medina VF. Acute infection and myocardial infarction. Reply N Engl J Med. 2019;380(15):e21. [DOI] [PubMed] [Google Scholar]
- 8. Warren‐Gash C, Geretti AM, Hamilton G, Rakhit RD, Smeeth L, Hayward AC. Influenza‐like illness in acute myocardial infarction patients during the winter wave of the influenza a H1N1 pandemic in London: a case‐control study. BMJ Open. 2013;3(5):e002604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mauriello A, Sangiorgi G, Fratoni S, et al. Diffuse and active inflammation occurs in both vulnerable and stable plaques of the entire coronary tree: a histopathologic study of patients dying of acute myocardial infarction. J Am Coll Cardiol. 2005;45(10):1585‐1593. [DOI] [PubMed] [Google Scholar]
- 10. Kaynar AM, Yende S, Zhu L, et al. Effects of intra‐abdominal sepsis on atherosclerosis in mice. Crit Care. 2014;18(5):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vergallo R, Aguirre AD, Abtahian F, et al. Recurrent myocardial infarctions and premature coronary atherosclerosis in a 23‐year‐old man with antiphospholipid syndrome. Thromb Haemost. 2016;115(2):237‐239. [DOI] [PubMed] [Google Scholar]
- 13. Vila P, Hernandez MC, Lopez‐Fernandez MF, Batlle J. Prevalence, follow‐up and clinical significance of the anticardiolipin antibodies in normal subjects. Thromb Haemost. 1994;72(2):209‐213. [PubMed] [Google Scholar]
- 14. Schouwers SM, Delanghe JR, Devreese KM. Lupus anticoagulant (LAC) testing in patients with inflammatory status: does C‐reactive protein interfere with LAC test results? Thromb Res. 2010;125(1):102‐104. [DOI] [PubMed] [Google Scholar]
- 15. Popovic B, Agrinier N, Bouchahda N, et al. Coronary embolism among ST‐segment‐elevation myocardial infarction patients: mechanisms and management. Circ Cardiovasc Interv. 2018;11(1):e005587. [DOI] [PubMed] [Google Scholar]


