Based on the known and emerging biology of autoimmune diseases and COVID‐19, it was hypothesised that whilst B‐cell depletion should not necessarily expose people to severe SARS‐CoV‐2‐related issues, it may inhibit or blunt the protective immunity following infection and vaccination. This is supported clinically, as the majority of SARS‐CoV‐2 infected, CD20‐depleted people with autoimmunity, have recovered. However, in CD‐20 treated people until naïve B‐cells repopulate, based on B‐cell repopulation‐kinetics and vaccination responses, from published rituximab, and unpublished ocrelizumab (NCT00676715, NCT02545868) trial data shown here suggests that it may be possible to undertake dose‐interruption to maintain inflammatory disease control, whilst allowing effective vaccination against SARS‐CoV‐29, if and when an effective vaccine is available.
Keywords: autoimmunity, B cell, CD20, COVID‐19, immunotherapy, multiple sclerosis, ocrelizumab, rheumatoid arthritis, rituximab
Summary
Although most autoimmune diseases are considered to be CD4 T cell‐ or antibody‐mediated, many respond to CD20‐depleting antibodies that have limited influence on CD4 and plasma cells. This includes rituximab, oblinutuzumab and ofatumumab that are used in cancer, rheumatoid arthritis and off‐label in a large number of other autoimmunities and ocrelizumab in multiple sclerosis. Recently, the COVID‐19 pandemic created concerns about immunosuppression in autoimmunity, leading to cessation or a delay in immunotherapy treatments. However, based on the known and emerging biology of autoimmunity and COVID‐19, it was hypothesised that while B cell depletion should not necessarily expose people to severe SARS‐CoV‐2‐related issues, it may inhibit protective immunity following infection and vaccination. As such, drug‐induced B cell subset inhibition, that controls at least some autoimmunities, would not influence innate and CD8 T cell responses, which are central to SARS‐CoV‐2 elimination, nor the hypercoagulation and innate inflammation causing severe morbidity. This is supported clinically, as the majority of SARS‐CoV‐2‐infected, CD20‐depleted people with autoimmunity have recovered. However, protective neutralizing antibody and vaccination responses are predicted to be blunted until naive B cells repopulate, based on B cell repopulation kinetics and vaccination responses, from published rituximab and unpublished ocrelizumab (NCT00676715, NCT02545868) trial data, shown here. This suggests that it may be possible to undertake dose interruption to maintain inflammatory disease control, while allowing effective vaccination against SARS‐CoV‐29, if and when an effective vaccine is available.
Introduction
Although many people consider CD4 T helper type 17 (Th17) cells to be central effectors in autoimmunity, response to therapy has indicated that B cell‐depleting drugs exhibit high efficacy in autoimmune and neuroimmunological diseases [1, 2, 3]. As such, not only are CD20‐depleting agents approved for B cell‐related cancers, but they are increasingly being used on‐ and off‐label in autoimmune diseases [1, 4]. Ocrelizumab has recently been licensed for the treatment of multiple sclerosis (MS), and antibodies including ofatumumab and ublituximab are in development for MS [5, 6, 7]. In addition, rituximab, which is approved for rheumatoid arthritis (RA) and pemphigus vulgaris, is frequently used off‐label in MS, neuromyelitis optica spectrum disorders (NMOSD) and a variety of other autoimmunities [1, 3, 5, 8]. Such off‐label use provides valuable insight into the biology of CD20‐depleting therapy [3]. For this reason, cells within the memory B cell subsets appear to be important targets for disease control, and their depletion and slow repopulation may, in part, account for the long‐term disease control seen from short‐term treatment cycles with alemtuzumab, cladribine, ocrelizumab and rituximab [2, 3, 9, 10]. Using rituximab to deplete repopulating memory B cells when they reach predefined levels can maintain clinical remission while reducing the frequency of infusions in RA, NMO, MS and other conditions [3, 11, 12, 13]. Translating this knowledge may help to improve the benefit : risk balance of ocrelizumab [9]. This is currently highly relevant, as repeated 6‐monthly CD20 depletion is associated with immunoglobulin (Ig)M and then IgA and IgG hypogammaglobulinaemia in some individuals, and also a small but increased risk of severe infections [14, 15, 16].
Immunological issues of COVID‐19
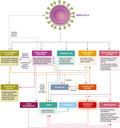
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) causing coronavirus disease 2019 (COVID‐19) has killed hundreds of thousands of humans in a global pandemic [17]. Severe COVID‐19 is often associated with lymphopenia [18], initially causing great concern over the use of immunosuppressive agents. In some cases, this led to the cessation or delay of treatment of autoimmunity [19, 20]. However, it is increasingly evident that lymphopenia is a consequence rather than a cause of infection [20, 21]. While the immune system eliminates SARS‐CoV‐2 in most individuals (Fig. 1), viral escape, immune exhaustion and elevated cytokine release can lead to hyperactivation of the innate immune response, vascular damage and hypercoagulation (Fig. 1), which can lead to significant morbidity, acute respiratory distress, multi‐organ failure and, in some cases, death [17, 18, 22]. While immunotherapy may have some value in treating severe COVID‐19 [23], the development of a SARS‐CoV‐2 vaccine is considered to be important for protecting the uninfected [24]. A vaccination programme should help to create herd immunity against the COVID‐19 virus [25]. Therefore, not only is it relevant to determine how disease‐modifying treatments (DMT) influence susceptibility to infection and length of the carrier state, it is also important to consider how DMT may influence immunity to reinfection and potential vaccine responses [20].
Fig. 1.
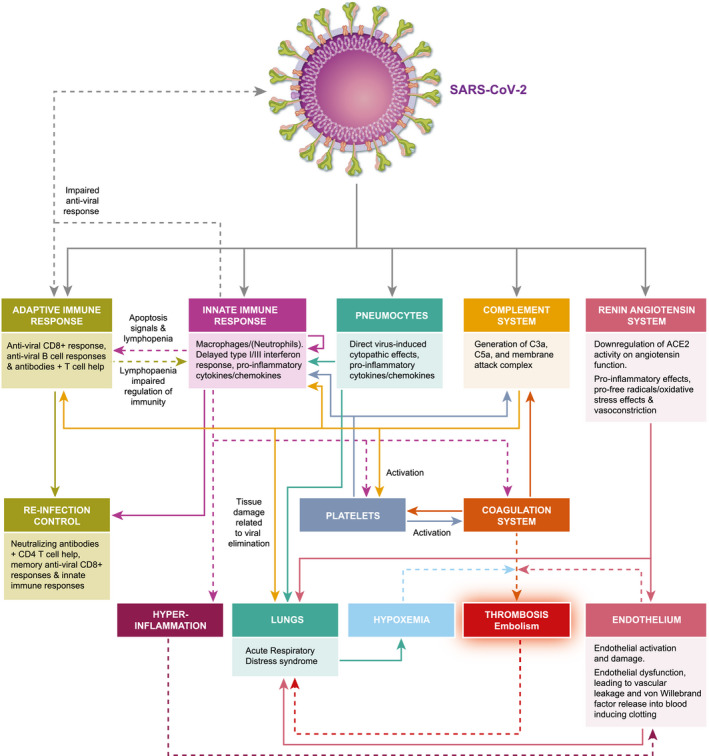
Pathobiology of COVID‐19. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infects cells in the lung and the gut via the angiotensin‐converting enzyme 2 (ACE2). This blocks ACE2‐induced formation of anti‐oxidant angiotensin, facilitating oxygen free‐radical formation and vascular damage. The innate immune response provides the initial line of defence against the virus, while a CD8 anti‐viral T cell response and neutralizing and complement‐fixing antibody response serve to remove the virus in the majority of people. However, the virus triggers suppression of interferon responses and other viral escape mechanisms that in a minority of people stimulate the innate immune response leading to lymphocyte apoptosis that blocks their regulatory signals and, in some cases, releases a cytokine storm that drives hyper‐innate inflammation. This, in part, causes acute respiratory distress. Importantly, this augments vascular damage that accentuates the respiratory distress and leads to von Willebrand factor release into the blood. This contributes to the formation of microthrombi, contributing to respiratory distress and vascular embolism that may be fatal. Adapted from Henry et al. 2020 [22].
Surprisingly, there are limited published data concerning the influence of ocrelizumab on immune subsets, vaccine responses, durability of response and the safety of extending infusion intervals. This prompted us to report data available within the public domain that addressed some of these safety concerns [9]. These data indicate that delaying ocrelizumab [9] and rituximab [10, 26] re‐infusion should be associated with minor risk of disease reactivation, based on B cell subset depletion and repopulation kinetics [9].
CD20‐B cell‐depleting agents do not markedly expose people to life‐threatening COVID‐19
Based on previous understanding of the immune response to SARS‐CoV and SARS‐CoV‐2, animal studies of the elimination of coronaviruses, informative COVID‐19 case reports and preliminary reports of COVID‐19 pathology [20, 27, 28, 29], the biology of MS, MS treatments and COVID‐19 have suggested that halting treatment may cause more harm than good through ineffective disease control [20, 30]. This indicates that a more pragmatic approach, supported by others in the field of MS and other conditions, may be of value [9, 31, 32]. It appears that the innate immune response, and perhaps later anti‐viral CD8 T cell responses, could eliminate the SARS‐CoV2 before significant antibody responses have developed [20, 28, 33] (Fig. 1), suggesting that most MS treatments that largely exhibit limited persistent effects on the innate immune and CD8 T cell responses would have limited influence on COVID‐19. SARS‐CoV‐2 is eliminated by the majority of people with MS and other autoimmunities on immunotherapies, without significant consequences [34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56] (Table 1). Anti‐viral antibodies, notably those targeting the receptor binding domain of the viral spike protein, clearly neutralize the virus [57, 58] and can contribute to the elimination of the primary SARS‐CoV‐2 infection in humans [58, 59]. However, B cells do not appear to be an absolute requirement for recovery. This is shown by the recovery of people genetically lacking B cells, such as with X‐linked hypogammaglobulinaemia [60, 61], and is reinforced by the finding that the vast majority of people treated with CD20 B cell‐depleting agents in MS recover from COVID‐19 [37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56] (Table 1). Furthermore, B cell depletion is unlikely to influence, or be significantly influenced by, the vascular pathology and hypercoagulopathy that are major pathological features in COVID‐19 that contribute to the acute respiratory distress syndrome, cardiovascular, cerebrovascular and other non‐pulmonary morbidities [9, 20, 22, 62, 63] (Fig. 1). Importantly, it provides another rationale as to why immunosuppressive treatments in MS and other autoimmunities [44, 64, 65] have not noticeably influenced COVID‐19 susceptibility and prognosis. People with MS appear to respond to SARS‐CoV‐2 in a similar way to the general population, where severe disease is notably influenced by age and comorbidities, such as diabetes and obesity [18, 43, 44, 66]. While this information is further consolidated by biology and the clinical evidence (Table 1), it may focus attention away from issues of being infected with SARS‐CoV‐2 [20] to methods of avoiding SARS‐CoV‐2 infection in uninfected individuals, as discussed below.
Table 1.
Infection with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in people treated with CD20‐depleting antibodies in multiple sclerosis
| CD20 antibody | Total no. infected | No. hospitalised | No. in intensive care | No. of deaths | Reference |
|---|---|---|---|---|---|
| Ocrelizumab | 1 | 1 | 0 | 0 | [37] |
| Ocrelizumab | 1 | 1 | 0 | 0 | [38] |
| Ocrelizumab | 2 | 0 | 0 | 0 | [39] |
| Ocrelizumab | 34 | 2 | 0 | 0 | [40] |
| Ocrelizumab | 100 | 26 | 5 | n.r. | [41] |
| Ocrelizumab | 1 | 1 | 0 | 0 | [42] |
| Ocrelizumab | 1 | 0 | 0 | 0 | [43] |
| Ocrelizumab | 11 | 5 | 2 | n.r. | [44] |
| Ocrelizumab | 26 | n.r. | 2 | 0 | [45] |
| Ocrelizumab | 59 | n.r. | n.r. | 2 | [46] |
| Ocrelizumab | 10 | n.r. | n.r. | n.r. | [47] |
| Ocrelizumab | 2 | 0 | 0 | 0 | [48] |
| Ocrelizumab | 38 | 10 | 3 | 0 | [49] |
| Ocrelizumab | 7 | 3 | 0 | 0 | [50] |
| Ocrelizumab | 1 | 1 | 0 | 0 | [51] |
| Ocrelizumab | 2 | 0 | 0 | 0 | [52] |
| Ocrelizumab | 1 | 0 | 0 | 0 | [53] |
| Subtotal | 297 | 50 | 12 | 2 | |
| Rituximab | 7 | 1 | 0 | 0 | [39] |
| Rituximab | 21 | ≤ 2 | 0 | 0 | [40] |
| Rituximab | 2 | n.r. | 1 | 1 | [45] |
| Rituximab | 9 | n.r. | n.r. | 0 | [46] |
| Rituximab | 6 | n.r. | n.r. | n.r. | [47] |
| Rituximab | 17 | 9 | 3 | 1 | [49] |
| Rituximab | 1 | 1 | 1 | 1 | [54] |
| Rituximab | 41 | 9 | 6 | n.r. | [55] |
| Subtotal | 104 | 22 | 11 | 3 | |
| Anti‐CD20 | 34 | 9 | n.r. | 2 | [56] |
| Total | 435 | 81 | 23 | 7 |
Number of people that have been infected with the COVID‐19 virus that have been documented in case reports and registries from published and social media reports. It is not possible to exclude that people reported in case reports, registries and pharmacovigilance studies are repeat reporting. In addition, infection was defined by symptoms and was not always confirmed via viral nucleic acid testing or serology. n.r. = not reported.
Ocrelizumab is the only DMT that is licenced across the spectrum of primary progressive and relapsing MS [5]. In comparison to other high‐efficacy DMT used in MS it has limited monitoring requirements, fewer restrictions on usage compared to cladribine and alemtuzumab and off‐label alternatives are widely used, and pharmacovigilance reports have been released [8, 40, 67]. Therefore, it is perhaps not surprising that there is currently more information about the influence of CD20 depletion and COVID‐19 disease outcome than for other high‐efficacy DMT in MS [44, 49] (Table 1). This is consistent with, albeit limited, information in people with rheumatic diseases [66, 68] indicating that people generally recover, while the few reported deaths may be linked to co‐morbidities [44]. The suggestion that rituximab treatment may increase risk of infection should be considered in the context of possible sampling biases, although this could be supported by data reported in social media from Sweden [40, 54] (Table 1). It is evident that both rituximab and ocrelizumab cause IgM hypogammaglobulinaemia in some people within a few treatment cycles, and this and IgA and IgG hypogammaglobulinaemia increases with repeated infusions, potentially contributing to infection [9, 14, 15, 16]. A delayed IgM response to SARS‐CoV‐2, which usually appears a few days after symptom onset, may contribute to disease severity [69, 70]. With time, CD20 depletion is also associated with reduced IgA responses [15], and similarly early IgA responses may also be important for efficient clearance of SARS‐CoV‐2 [15, 71, 72]. However, as yet there is no compelling evidence that CD20 depletion increases the severity of COVID‐19 compared to the general population [40, 41], although in people with genetically dysfunctional B cells this has been suggested [62]. In addition, hypogammaglobulinaemia may inhibit SARS‐CoV‐2 cross‐reactive, protective antibodies generated from immunity from previous coronavirus infection, which has been shown at the T and B cell level [73, 74], and seen previously with SARS and common cold‐causing coronaviruses [75]. In contrast, it has been questioned whether benefit may be imparted [37, 43], as B cell depletion could lead to limited antibody‐mediated enhancement of macrophage activity and complement‐mediated damage and antibody levels have been associated with severe COVID‐19 [71, 76, 77]. Although some people seroconvert and generate an anti‐SARS‐CoV‐2 response, this is expected to be, and sometimes is, blunted or absent due to the inhibition of antibody responses by anti‐CD20 B cell depletion [39, 50, 51, 52, 53]. The antibody titre required for protection against SARS‐CoV‐2 and the quality and the neutralizing potential of the antibody response after CD20 depletion are currently unknown [39, 50, 51, 52, 53]. However, even in non‐immunosuppressed, notably asymptomatic, cases that produce low‐titre antibody responses, many people do not produce a marked or long‐lasting neutralizing antibody response [78, 79, 80]. Perhaps benefit may be achieved by vaccination to boost immunity.
Infection of SARS‐CoV‐2 infection induces immunity
Although there is much hope for the impact of vaccination on generating immunity to SARS‐CoV‐2, there is no guarantee of protection or prolonged protection [24, 80]. Repeated infection is observed with other endemic human coronaviruses that cause common colds, suggesting that recurrent reinfections may also occur with SARS‐CoV‐2 [81]. This strongly supports the contribution of macrophages in viral control and the limited and transient induction of adaptive immunity [81]. Possible reinfection with SARS‐Cov‐2 has also been suggested by the finding of positive, polymerase chain reaction (PCR)‐detected SARS‐CoV‐2 nucleic acid swabs after a number of negative swabs [82]. However, it is clear that this may be due to non‐infective viral particles or artefacts created by sampling location and the testing systems used. PCR‐positive swabs can be found in faeces long after loss of nasopharyngeal‐positive PCR findings, indicating that the virus may persist for some time and that the PCR test detects fragments of the viral nucleic acid and not necessarily infective virus [83, 84]. Importantly, contact tracing of hundreds of people with positive tests after previous negative tests and hospital discharge failed to detect any evidence of the production of infective virus and subsequent viral spread to contacts [84]. Importantly, animal model studies show that immunity to SARS‐COV‐2 develops after primary infection that can rapidly eliminate the virus on re‐exposure [28, 29, 85]. This can be stimulated via vaccination in animals and in humans to generate neutralizing antibodies [86, 87]. In most, but not all cases, neutralizing antibodies persist for a number of months [79, 80], and following SARS coronavirus infection SARS‐CoV‐specific antibodies were detectable for a year or two before they disappeared [88], due probably to lack of antigenic stimulation following elimination of the virus. However, as new responses will be generated from CD20+ naive B cells, these responses would be anticipated to be blunted by B cell depleting agents.
CD20 antibodies inhibit vaccine responses
It has been shown that rituximab depletes naive B cells in the blood, lymphoid tissue and, to some extent, the bone marrow, and can also disrupt germinal centre formation in secondary lymphoid tissues [3, 89, 90]. Although the influence of ocrelizumab on B cell subsets and vaccine responses have not been published, trial (NCT02545868) data have been reported in meetings and adopted in the Summary of Product Characteristics produced as part of the regulatory label [67, 91]. Importantly, the data have been deposited on a trial registration site (www.clinicaltrials.gov) allowing data extraction, as shown here (Fig. 2). It is evident that there is a lower frequency of seroconversion and reduced titre to 23‐valent pneumococcal polysaccharide vaccine (23‐PPV) (Fig. 2a,b) with or without a booster vaccine (Fig. 2c), keyhole limpet haemocyanin (KLH) neoantigen (Fig. 2d), tetanus toxoid vaccine (Fig. 2e,f) and seasonal influenza vaccines (Fig. 2g–i). The percentage of people with MS who gave a positive response (titre ≥ 0·2 IU/ml or fourfold increase in titre is baseline levels ≥ 0·1 IU) to tetanus vaccine 8 weeks after vaccination was 23·9% in the ocrelizumab group compared to 54·5% in the control group (no DMT except interferon beta). The geometric mean anti‐tetanus toxoid‐specific antibody titres at 8 weeks were 3·74 and 9·81 IU/ml, respectively. Although these vaccine booster responses to tetanus toxoid were clearly blunted (Fig. 2c), the titres were generally above protective levels (0·16 IU/ml) [92], even at baseline. The percentage of people with MS on ocrelizumab with seroprotective titres against five influenza strains ranged from 20·0−60·0 and 16·7−43·8% prevaccination, and at 4 weeks post‐vaccination from 55·6−80·0%, in people treated with ocrelizumab and 75·0−97·0% in the control group, respectively (Fig. 2g). However, haemaggulination inhibition titres were reduced (Fig. 2h). Similarly, while there was a positive response to five or more serotypes in polyvalent pneumococcal vaccine (23‐PPV) at 4 weeks after vaccination (71·6% in the ocrelizumab group and 100% in the control group), the frequency of seroconversion and antibody titres were, however, markedly reduced (Fig. 2a). Furthermore, a booster of the 13‐PPV vaccine administered 4 weeks later did not markedly enhance the response to 12 serotypes, in common with 23‐PPV (Fig. 2c), further indicating the blunting of the vaccine responses. This was also seen using KLH (Fig. 2d). It is likely that this would be reduced further following repeated infusion of ocrelizumab, as hypogammaglobulinaemia, notably within IgM production, develops and increases while IgG hypogammaglobulinaemia develops over a longer time‐frame [9, 15].
Fig. 2.
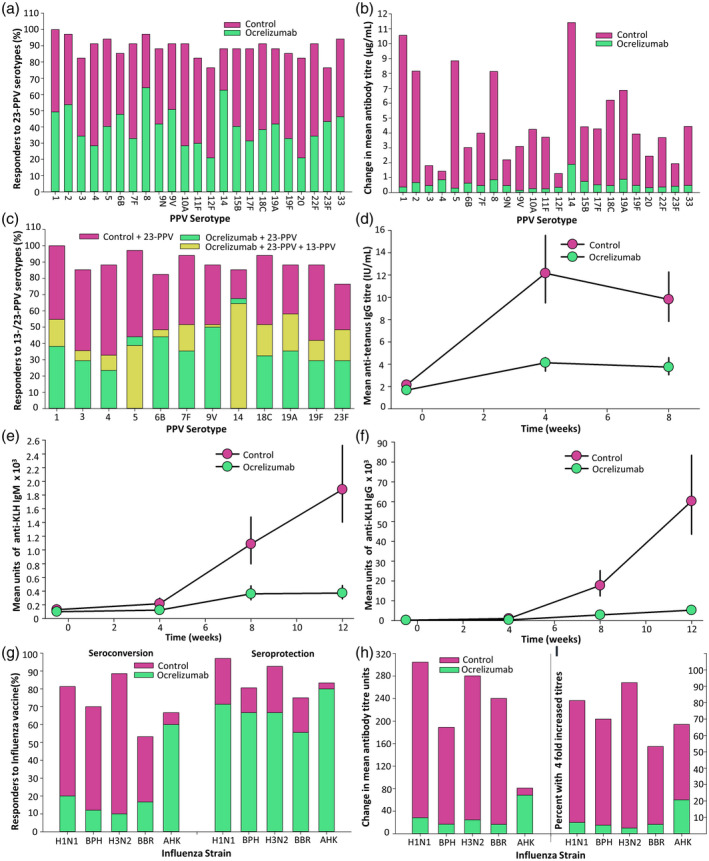
Ocrelizumab inhibits vaccination responses. People with multiple sclerosis who did not receive ocrelizumab (control) or were infused with 300 mg ocrelizumab on days 0 and 15 and were vaccinated from weeks 12–24 after ocrelizumab. The experimental details and results were from www.clinicaltrials.gov NCT02545868 [91]. The results show: (a) The frequency of seroconversion in people treated with ocrelizumab following injection pneumococcal 23‐polyvalent pneumococcal vaccine (PPV) vaccine, 4 weeks after vaccination (n = 66–68). A 23‐PPV vaccine response against a serotype was defined by a twofold increase in anti‐pneumococcal antibody or > 1 µg/ml compared with prevaccination levels, following Food and Drug Administration guidance. (b) The titre of response to the initial challenge with 23‐PPV 4 weeks after vaccination. (c) The frequency of seroconversion in people treated with ocrelizumab following injection of a booster pneumococcal 13‐PPV vaccine 4 weeks after 23‐PPV (n = 33–34). The frequency of responders is shown 8 weeks after 23‐PPV vaccination. (d) The geometric mean and 95% confidence interval (CI) anti‐tetanus toxoid antibody levels measured by enzyme‐linked immunosorbent assay (ELISA) before and following vaccination (n = 34–68). (e,f) The geometric mean and 95% confidence interval titre of (e) immunoglobulin (Ig)M or (f) IgG keyhole limpet haemocyanin (KLH)‐specific antibody after vaccination with keyhole limpet haemocyanin at baseline, weeks 4 and 8 started 12 weeks after ocrelizumab infusion (n = 34–68). (g–i) The response to: A/California/7/2009 (H1N1, n = 33–35); B/Phuket/3073/2013 (BPH, n = 31–33), A/Switzerland/9715293/2013 (H3N2, n = 27–30), B/Brisbane/60/2008 (BBR, n = 16–18), A/Hong Kong/4801/2014 (AHK, n = 5–6) influenza strain vaccination 12 weeks after ocrelizumab infusion was assessed. The results represent (g) the percentage of people with seroconversion, defined either a prevaccination haemagglutination inhibition (HI) titre < 10 and ≥ 40 at 4 weeks or a prevaccination ≥ 10 and at least a fourfold increase in HI titre, and seroprotection defined by titres > 40 at 4 weeks after vaccination. (h) The change in the geometric mean HI titres before and after vaccination (I) The percentage of people with a fourfold increase in strain‐specific > 40) at 4 weeks after vaccination.
The relatively poor vaccine response in people treated with ocrelizumab was predictable, and consistent with that seen following vaccination in people treated with rituximab, suggesting that this is an issue for all classes of anti‐CD20 antibodies used in the treatment of cancer and autoimmune diseases. There was a reduced titre and seroconversion rate (37·5 versus 75·0% healthy controls) of people with NMOSD following vaccination against influenza (H1N1) virus 3–5 weeks after treatment with rituximab [93]. Furthermore, vaccine responses to Streptococcus pneumoniae and influenza were still impaired in people with idiopathic thrombocytopenia and RA 6 months after treatment [94, 95]. This conclusion was also supported by studies in RA following treatment with rituximab, with a more markedly blunted seroconversion and titre when vaccinated during periods of peripheral B cell depletion with influenza [96], hepatitis B vaccines [97], PPV‐23, KLH [94] and a greater, but still blunted, vaccine response 6–10 months after infusion [96]. However, despite a relative lack of memory B cells, CD19‐repopulated individuals could mount a robust recall response, as shown in people with pemphigus vulgaris [98]. This suggests that it is possible to create a time‐window to vaccinate an individual due to the differential kinetics of repopulation with pathogenic memory B cells and naive B cells that will allow immunity to new infections [3, 99, 100]. In addition, ocrelizumab does not appear to impair pre‐existing humoral immunity [101], suggesting that people with MS who receive the SARS‐CoV‐2 vaccine if and when it becomes available will be able to start treatment with ocrelizumab without risking vaccine‐acquired immunity. However, the effect of ocrelizumab‐induced hypogammaglobulinaemia on the levels of protection from prior immunizations is unknown, and warrants further investigation.
Repopulation kinetics of ocrelizumab
If COVID‐19‐related vaccine responses become a key concern among people with MS or other autoimmune diseases choosing treatment options, the selection of B cell‐depleting agents that allow quick repopulation of B cells may be relevant for optimum vaccine readiness. Continuous B cell depletion with ocrelizumab and rituximab will clearly limit naive B cell repopulation; however, memory B cell depletion persists for a significant time after depletion with rituximab and alemtuzumab, consistent with the slow repopulation of this subset [99, 100, 102, 103]. This suggests a possibility for extended interval dosing or dosing interruption to allow immature B cells to recover to facilitate vaccination, while maintaining low levels of pathogenic memory B cells. Data suggest that this is feasible, at least with rituximab [98]. The timing required for this to occur for ocrelizumab is likely to be substantially longer. Repletion with rituximab occurs within approximately 6 months of treatment, and is completed within 12 months due to repopulation of the immature/mature (naive) B cell pool [26, 98]. Monthly subcutaneous treatment with ofatumumab takes a median of 49 weeks (range = 14–102 weeks) for CD19 B cell repletion after six 60‐mg cycles of treatment, and immature (CD19+, CD38+, CD10+) cells repopulate quickly [104]. This may have some merits for ofatumumab if the rapid repopulation of B cells can be confirmed with more prolonged usage, once ofatumumab is licenced to treat MS. Repopulation of B cell subsets following ocrelizumab has not been reported previously, but we report here the influence of ocrelizumab on B cell subsets from the Phase II open‐label extension study (Fig. 3a,b) [105]. It was found that CD4 and CD8 T cell numbers were relatively unaffected (Fig. 3a,b), even during active treatment (Fig. 3b). CD19 B cell subsets, including memory (CD19+, CD27+, CD38low) B cells, are completely depleted during active treatment (Fig. 3b). Even following cessation of treatment, CD19+ B cells remain low for 6–12 months after the last infusion (Fig. 3a). It is evident, however, that the memory B cell pool remained depleted for much longer, at least 18 months (Fig. 3a,b), and probably even longer in many individuals [105]. This is consistent with the durability of relapse inhibition and adds further support to the view that cells within this subset are important in MS disease pathogenesis [2, 9]. However, there appeared to be some recovery of the naive (CD19+, CD21, IgD+, IgM+) B cell pool during this time (Fig. 3a), suggesting the potential to generate new antibody responses which may be crucial to mount an immune response during infections and vaccinations. As found with rituximab, naive/mature B cell repopulation will coincide with CD19 repopulation [26, 97] and would take a median 62–72 weeks after three [95% confidence interval (CI) = 59·7–73·0 weeks,n = 51] or four cycles (95% CI = 59·1–85·4, range = 27–175 weeks, n = 51), respectively [105]. Such levels would require monitoring, as there is marked variability in repopulation kinetics between individuals and is, in part, a product of the ocrelizumab fixed‐dosing schedule, as it is clear that the intensity of B cell depletion and repopulation speed relates to the body mass index of the individual [106, 107]. This suggests that dose‐adjustment for weight may have some benefit, as currently used in the treatment of people with MS with oral cladribine [107]. Cladribine can be considered to be a chemical CD19‐depleting agent that markedly depletes memory B cells while generally maintaining T cells within the lower limit of normal. The compound is rapidly eliminated, allowing CD19 naive B cells to recover within a median of 30 weeks after treatment [108, 109, 110]. Alemtuzumab is administered at a low dose (36–60 mg) per cycle compared to ocrelizumab (600 mg) and rituximab (500–1000 mg), and has a relatively short half‐life compared to ocrelizumab [111]. Alemtuzumab markedly depletes T cells and memory B cells, but naive B cells rapidly repopulate [100, 103], and vaccine‐related antibody responses can be induced within 6 months of infusion [112]. This further supports the concept of a ‘window for vaccination’ for CD20‐depleting antibodies.
Fig. 3.
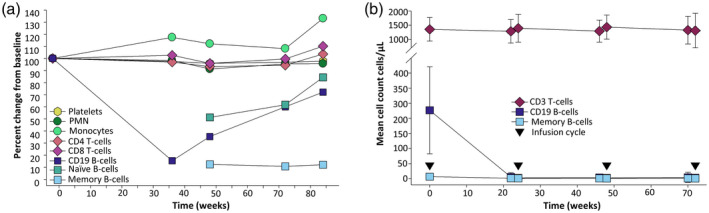
Long‐term depletion of memory B cells induced by ocrelizumab. These data were extracted from the ocrelizumab Phase II clinical study report [105], supplied by the trial sponsor via the www.clinicaltrialdatarequest.com portal. (a) The data represents the mean percentage change from baseline, defined as the last observation up to the first day of ocrelizumab treatment. The subjects received placebo and three cycles of 600 mg ocrelizumab every 24 weeks. This was followed by a treatment‐free period to monitor B cell repletion in the Phase II extension study. The time represents the period from the last ocrelizumab infusion (n = 22–43). Naive [CD19+, CD21+, immunoglobulin (IgD)+, IgM+] and memory B cells (CD19+, CD27+, CD38low) and other immune subsets were assessed (n = 22–43/group). (b) Depletion of memory B cells was maintained during treatment. These data were obtained from people (n = 88) entering the open‐label extension (OLE) study after the treatment‐free period that had received three or four 6‐monthly cycles of at least 600 mg ocrelizumab to week 72, followed by a treatment‐free period to week 144, before entering the OLE phase where 600‐mg cycles of ocrelizumab were maintained at 24‐week intervals. The results represent the mean ± standard deviation of cells/μl (n = 22–69/group weeks 22–72). Although CD19 B cell numbers were consistent with the original levels, the baseline memory B cell levels failed to return to original levels at the start of the OLE. PMN = polymorphonuclear neutrophil.
However, while B cell responses to a variety of different vaccines are clearly inhibited by CD20 depletion despite some inhibition of CD20 T cells [99, 113], inactivated herpes zoster vaccine can at least induce T cell responses [114]. This may be relevant if the CD8 T cell response is a vital part of the coronavirus specific immunity, as reported for SARS‐CoV [27, 115, 116, 117, 118]. This feature may reduce concern about the limited antibody responses that may be generated following infection or after administration of a SARS‐CoV‐2 vaccine, as such asymptomatic people who have cleared SARS‐CoV‐2 and have a detectable anti‐viral T cell response, but may not generate an antibody response [115, 116, 117, 118]. Although adenoviral vaccines have shown some value in generating neutralizing antibodies and cytopathic T cells in early human studies [85], live and attenuated viruses are contraindicated in immunosuppressed people [67]. It remains to be seen if SARS‐CoV‐2 DNA‐RNA vaccines [24], will be useful in people taking immunosuppressive agents. However, it is important that people with autoimmunity continue to be offered the benefit that high‐efficacy immunotherapy can provide. With time, further knowledge will emerge that may help guide treatment selection within the COVID‐19 and post‐COVID‐19 era.
Disclosures
D. B., M. M., K. S. and G. G. have received compensation for either consultancies and presentations and advisory board activities from Genentech/Roche. However, Roche/Genentech were not involved in the decision to write and submit this manuscript. S. A. has received consultancy from Novartis and Roche. C. A. K. K., G. P., A. S. K. and S. R. have nothing to disclose. D. B. has received compensation for activities related to Canbex therapeutics, InMune Biol, Lundbeck, Japan tobacco, Merck and Novartis. M. M. has received speaking honoraria from Sanofi‐Genzyme. K. S. has received compensation for activities related to Biogen, Eisai, Elan, Fiveprime, Lipomed, Merck KGAa, Novartis, Sanofi‐Genzyme and Teva. G. G. has received compensation for activities from AbbVie, Actelion, Atara Bio, Bayer‐Schering Healthcare, Biogen, Celgene, GW Pharma, GSK, Ironwood, Japanese Tobacco, Merck, Merck‐Serono, Mertz, Novartis, Pfizer, Sanofi‐Genzyme, Synthon, Takeda, Teva, UCB Pharma and Vertex Pharmaceuticals.
Acknowledgements
This study received no funding. The authors thank Roche and ClinicalStudyDataRequest.com for providing access to the clinical trial data. We thank Alison Schroeer (Schroeer Scientific Illustration) for advice and assistance concerning figure production and Dr Roger D. Seheult, (MedCram) for invaluable insights relating to pulmonary medicine and COVID‐19.
Contributor Information
D. Baker, Email: david.baker@qmul.ac.uk.
S. Amor, Email: s.amor@amsterdamumc.nl.
References
- 1. Du FH, Mills EA, Mao‐Draayer Y. Next‐generation anti‐CD20 monoclonal antibodies in autoimmune disease treatment. Auto Immun Highlights 2017; 8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baker D, Marta M, Pryce G, Giovannoni G, Schmierer K. Memory B‐cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBioMedicine 2017; 16:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker D, Pryce G, Amor S, Giovannoni G, Schmierer K. Learning from other autoimmunities to understand targeting of B‐cells to control multiple sclerosis. Brain 2018; 141:2824–8. [DOI] [PubMed] [Google Scholar]
- 4. Cang S, Mukhi N, Wang K, Liu D. Novel CD20 monoclonal antibodies for lymphoma therapy. J Hematol Oncol 2012; 5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sabatino JJ, Zamvil SS, Hauser SL. B‐cell therapies in multiple sclerosis. Cold Spring Harb Perspect Med 2019; 9:a032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hauser SL, Bar‐Or A, Cohen J et al Efficacy and safety of ofatumumab versus teriflunomide in relapsing multiple sclerosis: results of the phase 3 ASCLEPIOS I and II trials. Mult Scler 2019; 25(Suppl 2):890–1. [Google Scholar]
- 7. Fox E, Lovett‐Racke AE, Gormley M et al A phase 2 multicenter study of ublituximab, a novel glycoengineered anti‐CD20 monoclonal antibody, in patients with relapsing forms of multiple sclerosis. Mult Scler 2020. 10.1177/1352458520918375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ineichen BV, Moridi T, Granberg T, Piehl F. Rituximab treatment for multiple sclerosis. Mult Scler 2020; 26:137–52. [DOI] [PubMed] [Google Scholar]
- 9. Baker D, Pryce G, James LK, Marta M, Schmierer K. The ocrelizumab phase II extension trial suggests the potential to improve the risk:benefit balance in multiple sclerosis. Mult Scler Relat Disord 2020. 10.1101/2020.01.09.20016774. [DOI] [PubMed] [Google Scholar]
- 10. Juto A, Fink K, Nimer F, Piehl F. Interrupting rituximab treatment in relapsing–remitting multiple sclerosis; no evidence of rebound disease activity. Mult Scler Relat Disord 2020; 37:101468. [DOI] [PubMed] [Google Scholar]
- 11. Trouvin AP, Jacquot S, Grigioni S et al Usefulness of monitoring of B‐cell depletion in rituximab‐treated rheumatoid arthritis patients in order to predict clinical relapse: a prospective observational study. Clin Exp Immunol 2015; 180:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim SH, Kim W, Li XF, Jung IJ, Kim HJ. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B‐cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol 2011; 68:1412–20. [DOI] [PubMed] [Google Scholar]
- 13. Novi G, Fabbri S, Bovis F et al Tailoring B‐cells depleting therapy in MS according to memory B‐cells monitoring: a pilot study. P971. Mult Scler 2019; 25(Suppl 2):509–10. [Google Scholar]
- 14. Marcinnò A, Marnetto F, Valentino P et al Rituximab‐induced hypogammaglobulinemia in patients with neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm 2018; 5:e498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Derfuss T, Weber MS, Hughes R et al Serum immunoglobulin levels and risk of serious infections in the pivotal Phase III trials of ocrelizumab in multiple sclerosis and their open‐label extensions. 65. Mult Scler 2019; 25(Suppl 2):20–1. [Google Scholar]
- 16. Luna G, Alping P, Burman J et al Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol 2019; 77:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baloch S, Baloch MA, Zheng T, Pei X. The coronavirus disease 2019 (COVID‐19) pandemic. Tohoku J Exp Med 2020; 250:271–8. [DOI] [PubMed] [Google Scholar]
- 18. Huang C, Wang Y, Li X et al Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet 2020 30 January]. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brownlee W, Bourdette D, Broadley S, Killestein J, Ciccarelli O. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID‐19 pandemic. Neurology 2020; 94:949–52. [DOI] [PubMed] [Google Scholar]
- 20. Baker D, Amor S, Kang AS, Schmierer K, Giovannoni G. The underpinning biology relating to multiple sclerosis disease modifying treatments during the COVID‐19 pandemic. Mult Scler Relat Disord 2020; 43:102174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu X, Chang XN, Pan HX et al Pathological changes of the spleen in ten patients with coronavirus disease 2019 (COVID‐19) by postmortem needle autopsy. Zhonghua Bing Li Xue Za Zhi 2020; 49:576–82. [DOI] [PubMed] [Google Scholar]
- 22. Henry BM, Vikse J, Benoit S, Favaloro EJ, Lippi G. Hyperinflammation and derangement of renin‐angiotensin‐aldosterone system in COVID‐19: A novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta 2020; 507:167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. AminJafari A, Ghasemi S. The possible of immunotherapy for COVID‐19: a systematic review. Int Immunopharmacol 2020; 83:106455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Le Thanh T, Andreadakis Z, Kumar A et al The COVID‐19 vaccine development landscape. Nat Rev Drug Discov 2020; 19:305–6. [DOI] [PubMed] [Google Scholar]
- 25. Randolph HE, Barreiro LB. Herd immunity: understanding COVID‐19. Immunity 2020; 52:737–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bar‐Or A, Calabresi PA, Arnold D et al Rituximab in relapsing–remitting multiple sclerosis: a 72‐week, open‐label, phase I trial. Ann Neurol 2008;63:395–400. [DOI] [PubMed] [Google Scholar]
- 27. Channappanavar R, Fett C, Zhao J, Meyerholz DK, Perlman S. Virus‐specific memory CD8 T‐cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J Virol 2014; 88:11034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bao L, Deng W, Gao H et al Reinfection could not occur in SARS‐CoV‐2 infected rhesus macaques. bioRxiv 2020. 10.1101/2020.03.13.990226. [DOI] [Google Scholar]
- 29. Bao L, Deng W, Huang B et al The pathogenicity of SARS‐CoV‐2 in hACE2 transgenic mice. Nature 2020. 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 30. Giovannoni G, Hawkes C, Lechner‐Scott J, Levy M, Waubant E, Gold J. The COVID‐19 pandemic and the use of MS disease‐modifying therapies. Mult Scler Relat Disord 2020; 39:102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berger JR, Brandstadter R, Bar‐Or A. COVID‐19 and MS disease‐modifying therapies. Neurol Neuroimmunol Neuroinflamm 2020; 7:e761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Favalli EG, Ingegnoli F, De Lucia O, Cincinelli G, Cimaz R, Caporali R. COVID‐19 infection and rheumatoid arthritis: faraway, so close! Autoimmun Rev 2020; 19:102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang B, Wang L, Kong X et al Long term coexistence of SARS‐CoV‐2 with antibody response in COVID‐19 patients. J Med Virol 2020. 10.1002/jmv.25946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anand P, Slama MCC, Kaku M et al COVID‐19 in patients with myasthenia gravis. Muscle Nerve 2020. 10.1002/mus.26918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dworakowska D, Grossman AB. Thyroid disease in the time of COVID‐19. Endocrine 2020; 68:471–4. 10.1007/s12020-020-02364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salvarani C, Bajocchi G, Mancuso P et al Susceptibility and severity of COVID‐19 in patients treated with bDMARDS and tsDMARDs: a population‐based study. Ann Rheum Dis 2020. 10.1136/annrheumdis-2020-217903. [DOI] [PubMed] [Google Scholar]
- 37. Novi G, Mikulska M, Briano F et al COVID‐19 in a MS patient treated with ocrelizumab: does immunosuppression have a protective role? Mult Scler Relat Disord 2020; 15:102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Louapre C, Maillart E, Roux T et al Patients with MS treated with immunosuppressive agents: across the COVID‐19 spectrum. Rev Neurol 2020; 176:523–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Montero‐Escribano P, Matías‐Guiu J, Gómez‐Iglesias P, Porta‐Etessam J, Pytel V, Matias‐Guiu JA. Anti‐CD20 and COVID‐19 in multiple sclerosis and related disorders: a case series of 60 patients from Madrid, Spain. Mult Scler Relat Disord 2020; 42:102185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Safavi F, Nourbakhsh B, Azimi AR. B‐cell depleting therapies may affect susceptibility to acute respiratory illness among patients with multiple sclerosis during the early COVID‐19 epidemic in Iran. Mult Scler Relat Disord 2020; 43:102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hughes R, Pedotti R, Koendgen H. COVID‐19 in persons with multiple sclerosis treated with ocrelizumab – a pharmacovigilance case series. Mult Scler Relat Disord 2020; 42:102192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suwanwongse K, Shabarek N. Benign course of COVID‐19 in a multiple sclerosis patient treated with ocrelizumab. Mult Scler Relat Disord 2020; 42:102201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ghajarzadeh M, Mirmosayyeb O, Barzegar M et al Favorable outcome after COVID‐19 infection in a multiple sclerosis patient initiated on ocrelizumab during the pandemic. Mult Scler Relat Disord 2020; 43:102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chaudhry F, Bulka H, Rathnam AS et al COVID‐19 in multiple sclerosis patients and risk factors for severe infection. medRxiv 2020. 10.1101/2020.05.27.20114827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sormani MP; Italian Study Group on COVID‐19 Infection in Multiple Sclerosis . An Italian programme for COVID‐19 infection in multiple sclerosis. Lancet Neurol 2020; 19:481–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Salter A. iWiMS MS Covid‐19. 13 May 2020 (https://youtu.be/XAiBJ2sphkU).
- 47. Assmuth Oreja CS.iWiMS MS Covid‐19. 6 May 2020. Available at: https://youtu.be/cp0rtxq_k2Y.
- 48. van der Welt A, Health A.iWiMS MS Covid‐19. 6 May 2020. Available at: https://youtu.be/cp0rtxq_k2Y.
- 49. Louapre C, Collongues N, Stankoff B et al Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol 2020. 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meca‐Lallana V, Aguirre C, del Río B, Cardeñoso L, Alarcon T, Vivancos J. COVID‐19 in 7 multiple sclerosis patients in treatment with anti CD20 therapies. Mult Scler Relat Disord 2020; 44:102306. accessed 20 May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Conte WL. Attenuation of antibody response to SARS‐CoV‐2 in a patient on ocrelizumab with hypogammaglobulinemia. Mult Scler Relat Disord 2020; 44:102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rempe Thornton J, Harel A. Negative SARS‐CoV‐2 antibody testing following COVID‐19 infection in two MS patients treated with ocrelizumab. Mult Scler Rel Disord 2020. 10.1016/j.msard.2020.102341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lucchini M, Bianco A, Del Giacomo P, De Fino C, Nociti V, Mirabella M. Is serological response to SARS‐CoV‐2 preserved in MS patients on ocrelizumab treatment? A case report. Mult Scler Relat Disord 2020. 10.1016/j.msard.2020.102323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barzegar M, Mirmosayyeb O, Ghajarzadeh M et al Characteristics of COVID‐19 disease in multiple sclerosis patients. Mult Scler Relat Disord 2020; 45:102276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hillert J. iWiMS MS Covid‐19. 20 May 2020. Available at: https://youtu.be/z4sRBQEv0yE.
- 56. Parrotta E, Kister I, Charvet L et al COVID‐19 outcomes in MS: early experience from NYU multiple sclerosis comprehensive care center. medRxiv 2020. 10.1101/2020.05.12.20094508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Suthar MS, Zimmerman M, Kauffman R et al Rapid generation of neutralizing antibody responses in COVID‐19 patients. medRxiv 2020. 10.1101/2020.05.03.20084442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bertoglio F, Meier D, Langreder N et al SARS‐CoV‐2 neutralizing human recombinant antibodies selected from pre‐pandemic healthy donors binding at RBD–ACE2 interface. bioRxiv 2020. 10.1101/2020.06.05.135921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zeng QL, Yu ZJ, Gou JJ et al Effect of convalescent plasma therapy on viral shedding and survival in COVID‐19 patients. J Infect Dis 2020:jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ye M, Fu D, Ren Y et al Treatment with convalescent plasma for COVID‐19 patients in Wuhan, China. J Med Virol 2020. 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Soresina A, Moratto D, Chiarini M et al Two X‐linked agammaglobulinemia patients develop pneumonia as COVID‐19 manifestation but recover. Pediatr Allergy Immunol 2020. 10.1111/pai.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Quinti I, Lougaris V, Milito C et al A possible role for B‐cells in COVID‐19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol 2020; 146:211–3.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ackermann M, Verleden SE, Kuehnel M et al Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med 2020. 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tseng YH, Yang RC, Lu TS. Two hits to the renin–angiotensin system may play a key role in severe COVID‐19. Kaohsiung J Med Sci 2020. 10.1002/kjm2.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Monti S, Balduzzi S, Delvino P, Bellis E, Quadrelli VERSUS, Montecucco C. Clinical course of COVID‐19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis 2020; 79:667–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gianfrancesco M, Hyrich KL, Al‐Adely S et al Characteristics associated with hospitalisation for COVID‐19 in people with rheumatic disease: data from the COVID‐19 Global Rheumatology Alliance physician‐reported registry. Ann Rheum Dis 2020. 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ocrevus ® European public assessment report . 7/01/2019 update. Summary of product characteristics. Available at: https://www.ema.europa.eu/en/documents/product‐information/ocrevus‐epar‐product‐information_en.pdf accessed 5 January 2020.
- 68. Fallet B, Kyburz D, Walker UA. Mild course of coronavirus disease 2019 and spontaneous severe acute respiratory syndrome coronavirus 2 clearance in a patient with depleted peripheral blood B‐cells due to treatment with rituximab. Arthritis Rheum 2020; 2020 10.1002/art.41380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xiang F, Wang X, He X et al Antibody detection and dynamic characteristics in patients with COVID‐19. Clin Infect Dis 2020. 10.1093/cid/ciaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shen L, Wang C, Zhao J et al Delayed specific IgM antibody responses observed among COVID‐19 patients with severe progression. Emerg Microbes Infect 2020; 9:1096–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yu HQ, Sun BQ, Fang ZF et al Distinct features of SARS‐CoV‐2‐specific IgA response in COVID‐19 patients. Eur Respir J 2020; 8:2001526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dahlke C, Heidepriem J, Kobbe R et al Distinct early IgA profile may determine severity of COVID‐19 symptoms: an immunological case series. medRxiv 2020. 10.1101/2020.04.14.20059733. [DOI] [Google Scholar]
- 73. Grifoni A, Weiskopf D, Ramirez SI et al Targets of T cell responses to SARS‐CoV‐2 coronavirus in humans with COVID‐19 disease and unexposed individuals. Cell 2020; 181:1489–1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ng K, Faulkner N, Cornish G et al Pre‐existing and de novo humoral immunity to SARS‐CoV‐2 in humans. bioRxiv 2020. 10.1101/2020.05.14.095414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Che XY, Qiu LW, Liao ZY et al Antigenic cross‐reactivity between severe acute respiratory syndrome‐associated coronavirus and human coronaviruses 229E and OC43. J Infect Dis 2005; 191:2033–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Negro F. Is antibody‐dependent enhancement playing a role in COVID‐19 pathogenesis? Swiss Med Wkly 2020; 150:w20249. [DOI] [PubMed] [Google Scholar]
- 77. Magro C, Mulvey JJ, Berlin D et al Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res 2020; 5244:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Xiao T, Wang Y, Yuan J et al Early viral clearance and antibody kinetics of COVID‐19 among asymptomatic carriers. medRxiv 2020. 10.1101/2020.04.28.20083139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Brochot E, Demey B, Touze A et al Anti‐spike, anti‐nucleocapsid and neutralizing antibodies in SARS‐CoV‐2 inpatients and asymptomatic carriers. medRxiv 2020. 10.1101/2020.05.12.20098236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu T, Wu S, Tao H et al. Prevalence of IgG antibodies to SARS‐CoV‐2 in Wuhan – implications for the ability to produce long‐lasting protective antibodies against SARS‐CoV‐2. medRxiv 2020. 10.1101/2020.06.13.20130252. [DOI] [Google Scholar]
- 81. Galanti M, Shaman J. Seasonal cold‐inducing coronavirus can be repeatedly detected in some individuals. medRxiv 2020. 10.1101/2020.04.27.20082032. [DOI] [Google Scholar]
- 82. Ravioli S, Ochsner H, Lindner G. Reactivation of COVID‐19 pneumonia: a report of two cases. J Infect 2020. 10.1016/j.jinf.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang QX, Huang KC, Qi L, Zeng XH, Zheng SL. No infectious risk of COVID‐19 patients with long‐term fecal 2019‐nCoV nucleic acid positive. Eur Rev Med Pharmacol Sci 2020; 24:5772–7. [DOI] [PubMed] [Google Scholar]
- 84. Korean Centre for Disease Control and Prevention . Findings from investigation and analysis of re‐positive cases. Notice 2020–05‐19~2020‐12‐31. accessed 19 May 2020. Available at: http://www.https.com/is.cdc.gov.kr/upload_comm/syview/doc.html?fn=159118745823700.pdf%26rs=/upload_comm/docu/0030.
- 85. Chandrashekar A, Liu J, Martinot AJ et al SARS‐CoV‐2 infection protects against rechallenge in rhesus macaques. Science 2020. 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yu J, Tostanoski LH, Peter L et al DNA vaccine protection against SARS‐CoV‐2 in rhesus macaques. Science 2020. 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhu FC, Li YH, Guan XH et al Safety, tolerability, and immunogenicity of a recombinant adenovirus type‐5 vectored COVID‐19 vaccine: a dose‐escalation, open‐label, non‐randomised, first‐in‐human trial. Lancet 2020; 395:1845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wu LP, Wang NC, Chang YH et al Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis 2007; 13:1562–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cioc AM, Vanderwerf SM, Peterson BA, Robu VG, Forster CL, Pambuccian SE. Rituximab‐induced changes in hematolymphoid tissues found at autopsy. Am J Clin Pathol 2008; 130:604–12. [DOI] [PubMed] [Google Scholar]
- 90. Ramwadhdoebe TH, van Baarsen LGM, Boumans MJH et al Effect of rituximab treatment on T and B‐cell subsets in lymph node biopsies of patients with rheumatoid arthritis. Rheumatology 2019; 58:1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Stokmaier D, Winthrop K, Chognot C et al Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis (S36.002). Neurology 2018; 90(15 Suppl):S36.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Simonsen O, Bentzon MW, Heron I. ELISA for the routine determination of antitoxic immunity to tetanus. J Biol Standard 1986; 14:231–9. [DOI] [PubMed] [Google Scholar]
- 93. Kim W, Kim SH, Huh SY et al Reduced antibody formation after influenza vaccination in patients with neuromyelitis optica spectrum disorder treated with rituximab. Eur J Neurol 2013;20:975–80. [DOI] [PubMed] [Google Scholar]
- 94. Bingham CO, Looney RJ, Deodhar A et al Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum 2010; 62:64–74. [DOI] [PubMed] [Google Scholar]
- 95. Nazi I, Kelton JG, Larché M et al The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood 2013; 122:1946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. van Assen S, Holvast A, Telgt DS et al Patients with humoral primary immunodeficiency do not develop protective anti‐influenza antibody titers after vaccination with trivalent subunit influenza vaccine. Clin Immunol 2010; 136:228–35. [DOI] [PubMed] [Google Scholar]
- 97. Richi P, Alonso O, Martín MD et al Evaluation of the immune response to hepatitis B vaccine in patients on biological therapy: results of the RIER cohort study. Clin Rheumatol 2020. 10.1007/s10067-020-05042-2. [DOI] [PubMed] [Google Scholar]
- 98. Cho A, Bradley B, Kauffman R et al Robust memory responses against influenza vaccination in pemphigus patients previously treated with rituximab. JCI Insight 2017; 2:e93222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Palanichamy A, Jahn S, Nickles D et al Rituximab efficiently depletes increased CD20‐expressing T‐cells in multiple sclerosis patients. J Immunol 2014; 193:580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Baker D, Herrod SS, Alvarez‐Gonzalez C, Giovannoni G, Schmierer K. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of alemtuzumab. JAMA Neurol 2017; 74:961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ziemssen T, Bar‐Or A, Arnold DL et al P 2 Effect of ocrelizumab on humoral immunity markers in the phase iii, double‐blind, double‐dummy, IFN β ‐1a–controlled OPERA I and OPERA II studies. Clin Neurophysiol 2017; 128:e326–e327. [Google Scholar]
- 102. Roll P, Palanichamy A, Kneitz C, Dorner T, Tony HP. Regeneration of B‐cell subsets after transient B‐cell depletion using anti‐CD20 antibodies in rheumatoid arthritis. Arthritis Rheum 2006; 54:2377–86. [DOI] [PubMed] [Google Scholar]
- 103. Akgün K, Blankenburg J, Marggraf M, Haase R, Ziemssen T. Event‐driven immunoprofiling predicts return of disease activity in alemtuzumab‐treated multiple sclerosis. Front Immunol 2020; 11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bar‐Or A, Grove RA, Austin DJ et al Subcutaneous ofatumumab in patients with relapsing‐remitting multiple sclerosis: the MIRROR study. Neurology 2018; 90:e1805–e1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. WA21493 Clinical study report 2016 . WA21493‐Phase II, multicenter, randomized parallel‐group, partially blinded, placebo, Avonex® controlled dose finding study to evaluate the efficacy as measured by brain MRI lesions and safety of 2 dose regimens of ocrelizumab in patients with RRMS. Report no. 1062910. March 2016. https://clinicaltrials.gov/ct2/show/NCT00676715
- 106. Signoriello E, Bonavita S, Di Pietro A et al BMI influences CD20 kinetics in multiple sclerosis patients treated with ocrelizumab. Mult Scler Relat Disord 2020; 43:102186. [DOI] [PubMed] [Google Scholar]
- 107. Kletzl H, Gibiansky E, Petry C et al Pharmacokinetics, pharmacodynamics and exposure‐response analyses of ocrelizumab in patients with multiple sclerosis. Neurol 2019; 92(Suppl 15):N4.001. [Google Scholar]
- 108. Comi G, Cook S, Giovannoni G et al Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult Scler Relat Disord 2019; 29:168–74. [DOI] [PubMed] [Google Scholar]
- 109. Hermann R, Karlsson MO, Novakovic AM, Terranova N, Fluck M, Munafo A. The clinical pharmacology of cladribine tablets for the treatment of relapsing multiple sclerosis. Clin Pharmokinet 2019; 58:283–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Baker D, Pryce G, Herrod SS, Schmierer K. Potential mechanisms of action related to the efficacy and safety of cladribine. Mult Scler Relat Disord 2019; 30:176–86. [DOI] [PubMed] [Google Scholar]
- 111. Li Z, Richards S, Surks HK, Jacobs A, Panzara MA. Clinical pharmacology of alemtuzumab, an anti‐CD52 immunomodulator, in multiple sclerosis. Clin Exp Immunol 2018; 194:295–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. McCarthy CL, Tuohy O, Compston DA, Kumararatne DS, Coles AJ, Jones JL. Immune competence after alemtuzumab treatment of multiple sclerosis. Neurology 2013; 81:872–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gingele S, Jacobus TL, Konen FF et al Ocrelizumab depletes CD20⁺ T cells in multiple sclerosis patients. Cells 2018; 8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Parrino J, McNeil SA, Lawrence SJ et al Safety and immunogenicity of inactivated varicella‐zoster virus vaccine in adults with hematologic malignancies receiving treatment with anti‐CD20 monoclonal antibodies. Vaccine 2017; 35:1764–9. [DOI] [PubMed] [Google Scholar]
- 115. Ng OW, Chia A, Tan AT et al Memory T‐cell responses targeting the SARS coronavirus persist up to 11 years post‐infection. Vaccine 2016; 34:2008–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Robbiani DF, Gaebler C, Muecksch F et al Convergent antibody responses to SARS‐CoV‐2 in convalescent individuals. Nature 2020. 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gallais F, Velay A, Wendling MJ, Nazon C, Partisani M, Sibilia J, Candon S, Fafi‐Kremer S. Intrafamilial exposure to SARS‐CoV‐2 induces cellular immune response without seroconversion. medRxiv 2020. 10.1101/2020.06.21.20132449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sekine T, Perez‐Potti A, Rivera‐Ballesteros O, et al Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID‐19. bioRxiv 2020. 10.1101/2020.06.29.174888. [DOI] [PMC free article] [PubMed] [Google Scholar]


