Abstract
Purpose
Glial fibrillary acid protein (GFAP) and vimentin are type III intermediate filament proteins, ubiquitously expressed in retinal glial cells. Under retinal stress, both GFAP and vimentin are well-known sensitive markers for retinal gliosis. However, little is known about whether these proteins are released into the vitreous body in response to retinal gliosis or are related to the severity of retinal gliosis seen in proliferative vitreoretinopathy (PVR).
Methods
Vitreous fluids were collected from 44 patients who underwent pars plana vitrectomy for macular hole (Group 1; n = 8), epiretinal membrane (Group 2; n = 8), or retinal detachment (RD) with various degrees of PVR (Group 3; n = 28). The severity of PVR was determined by cumulative scores using PVR classification. GFAP, vimentin, and total protein levels from the vitreous samples were measured.
Results
Both GFAP and vimentin levels were significantly elevated in vitreous fluid from Group 3 (RD) compared with Groups 1 and 2 (P < 0.01). GFAP levels (ng/mL) were 12.4 ± 9.8, 17.5 ± 17.7, and 572.0 ± 11659.7, and vimentin levels (ng/mL) were 40.8 ± 61.9, 88.6 ± 86.8, and 3952.8 ± 8179.5 in Groups 1, 2, and 3, respectively. Total protein levels were not significantly different among the three groups. Elevated GFAP and vimentin levels in Group 3 were positively correlated with the areas of RD (P < 0.01, r = 0.53 in GFAP and P < 0.05, r = 0.46 in vimentin) and PVR scores (P < 0.05, r = 0.46 in GFAP and P < 0.00001, r = 0.76 in vimentin).
Conclusions
Our data suggest that human vitreous GFAP and vimentin are protein biomarkers for PVR, and reactive gliosis may play a part in PVR formation.
Keywords: GFAP, vimentin, reactive gliosis, retinal detachment, PVR
Glial fibrillary acid protein (GFAP) and vimentin are the most abundant type III intermediate filament (IF) proteins in the retina.1,2 In mammalian retina, GFAP and vimentin are primarily found in the two types of macroglia: Müller cells and astrocytes. Although both GFAP and vimentin are responsible for the cytoskeletal structure of retinal glial cells at the resting state of the retina, vimentin is much more abundant than GFAP. Müller cells, as a principal glial cell comprising 90% of the glial population of the retina, only express vimentin in the physiologic state of the retina. In contrast, astrocytes, making up 10% of the glial cells of the retina, express GFAP in the physiological state of the retina.3
GFAP and vimentin increase dramatically in response to various types of retinal stress, injuries, and pathologic processes and serve as well-known sensitive biomarkers for reactive retinal gliosis. In an experimental mammalian animal model of retinal detachment as a specific form of retinal stress, upregulation of GFAP and vimentin has been well charaterized.4 Increased GFAP expression was also noted in human retinal and preretinal proliferative tissue from proliferative vitreoretinopathy (PVR), the most advanced form of retinal gliosis seen in retinal detachment.5,6 However, increased IFs in retinal detachment or PVR have been regarded as nonspecific findings related to the injured retina from reactive retinal gliosis. Little is known about whether these proteins are released into the vitreous body in response to retinal gliosis or whether they are related to the severity of retinal gliosis seen in PVR. We hypothesized that increased GFAP and vimentin from retinal gliosis are released into the vitreous body, and are correlated with the severity of PVR.
Previous histopathologic study of PVR tissue suggested that PVR is a complex cascade similar to the excessive wound healing process, associated with cellular proliferation, inflammation, and extracellular matrix remodeling.7–10 Retinal pigment epithelial cells have long been recognized as a major cell type in PVR pathogenesis, because they seem to dedifferentiate and proliferate on the retinal surface presumably migrating through the retinal breaks. The role of retinal glial cells, however, has been speculated to be minor in that they may provide a scaffold for membrane formation or release growth factors, even though hypertrophy, proliferation, and migration of Müller glial cells are constant findings in the histopathologic studies of PVR and suggest its involvement in retinal shortening.11–13 Clinically, it is notable that PVR progresses with various clinical findings, including vitreous opacity, surface retinal wrinkling or stiffness, fixed retinal folds, and preretinal or subretinal proliferative fibrous bands further leading to open funnel or closed funnel retinal detachments, that have been used for PVR classification to define its severity.14 The early stages of PVR observed in clinical examinations suggest that a constitutional change in the vitreous body and/or retinal gliosis such as retinal wrinkle, stiffness, or fold may be a key predecessor to the advanced stages of PVR, such as cellular proliferation of retinal pigment epithelial cells. However, little is known about what differs between retinal detachment and retinal detachment leading to mild or severe PVR.
Because the exact mechanism of PVR formation is far from fully understood, and currently there is no treatment for PVR, early recognition and prevention through the modification of the initial cue of the PVR process may be important to prevent further vision loss. The current study explores the diagnostic value of the vitreous GFAP and vimentin levels as a surrogate marker to predict the induction and magnitude of PVR and the potential therapeutic targets for early stages of PVR.
Materials and Methods
Patients
Before study initiation, the study was approved by the Institutional Review Board of the University of Oklahoma Health Sciences Center. The study adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients after verbal and written explanations were provided. Forty-six patients who underwent pars plana vitrectomy for idiopathic full-thickness macular hole (Group 1; n = 8), epiretinal membrane (Group 2; n = 8), and retinal detachment with various degrees of proliferative vitreoretinopathy (Group 3; n = 28) were included. Undiluted vitreous samples (0.3–1.0 mL volume) were obtained from the center of the vitreous cavity at the onset of pars plana vitrectomy under full visualization and closed infusion line by manual aspiration with cutting on through the vitrectomy probe into a 2.5-mL syringe connected along the aspiration line.15 Preoperative demographics and intraoperative clinical findings were reviewed. The clinical information recorded for all patients included sex, age, and preoperative best-available visual acuity (VA). VA was evaluated with a Snellen chart and was converted to a logarithm of the minimum angle of resolution value. For the retinal detachment group (Group 3), areas of retinal detachment (clock hours) and duration of retinal detachment (days; time from reported onset of symptoms to surgical repair) were recorded. PVR severity was recorded using cumulative scores from PVR classification (The Retina Society Terminology Committee, 1983) based on preoperative and intraoperative clinical findings (Table 1).
Table 1.
PVR Scoring System
| Scoring Point | PVR Classification from the Retina Society Terminology Committee (1983) |
|---|---|
| 1 | Grade A (minimal): Presence of vitreous opacity or pigment clumps |
| 1 | Grade B (moderate): Surface retinal wrinkling, rolled edges of the retina, retinal stiffness, and vessel tortuosity |
| 1-4 | Grade C (marked): Fixed retinal folds per quadrant |
| 5 | Grade D-1 and 2 (massive): Open funnel |
| 10 | Grade D-3 (massive): Closed funnel |
| 0–16 | Cumulative total score |
Vitreous Body Preparation
Harvested vitreous was immediately kept on ice and transferred to the laboratory within four hours for centrifugation at 4°C, 12,000 rpm for 3 minutes. Supernatant sample aliquots 100 µL were then stored at −80°C until further analyses.
Sample Analysis
A SimpleStep enzyme-linked immunosorbent assay kit (Abcam, Cambridge, United Kingdom) was used to quantify GFAP and vimentin from vitreous samples following the manufacturer's instructions. In brief, samples were diluted in the provided extraction buffer with the following criteria for macular hole/epiretinal membrane 1/10 and for RD 1/100. Antibody cocktail was prepared in antibody diluent and 50 µLof standards and the blanks were added in duplicate. Sample dilutions 50 µL were added in triplicate, and 50 µL of antibody cocktail was added to every well. The plate was incubated for one hour at room temperature (RT) on a plate shaker at 400 rpm. The wells were then washed three times with 350 µL of wash buffer and blotted, and 100 µL of tetramethylbenzidine (TMB) substrate was added to every well. The plate was then covered with foil and incubated for 10 minutes at RT on a plate shaker at 400 rpm, followed by the addition of 100 µL of stop solution to every well. The plate was then read at 450 nm, a standard curve was generated, and x values for the unknowns were generated from the slope equation and multiplied by the dilution. Both GFAP and vimentin concentrations were determined from triplicate wells in nanograms per milliliter (ng/mL).
Bicinchoninic acid assay reagent (Abcam) was used for total protein determination. Standard and samples were diluted 1/5 in phosphate-buffered saline solution, and additional dilutions were made and tested if the 1/5 dilution was uninterpretable. Standards, samples, and the blank 10 µL were added in duplicate to the plate, and 200 µL of the bicinchoninic acid assay working reagent was added to all of the wells. The plate was sealed and incubated for two hours at RT. The plate was read at 562 nm, a standard curve was generated, and x values for the unknowns were generated from the slope equation and multiplied by the dilution. Total protein was determined from triplicate wells in milligrams per milliliter (mg/mL).
Data Analysis
Data were expressed as mean ± standard deviation. All statistical analyses were performed and graphs were created using Microsoft Excel (version 16.32; Redmond, WA, USA). Mean values were compared using the nonparametric two-sample exact Wilcoxon rank-sum test. The linear relationship between continuous variables was evaluated using the Spearman correlation coefficient.
Results
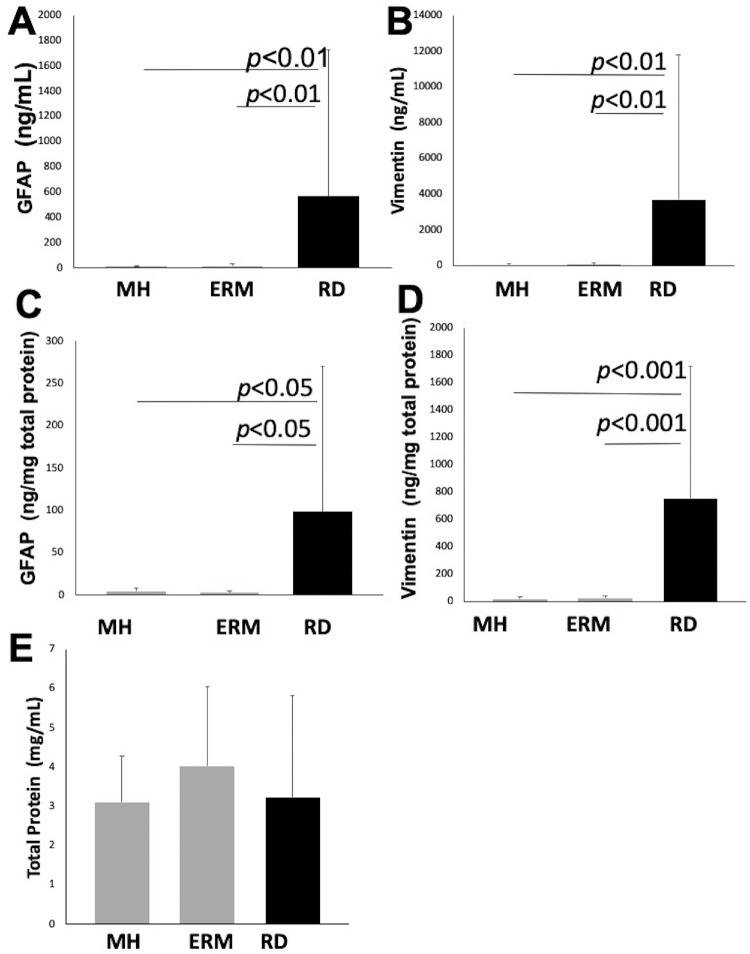
The demographic characteristics of the patients are summarized in Table 2. Vitreous body GFAP levels (ng/mL) were 12.4 ± 9.8, 17.5 ± 17.7, and 572.0 ± 1159.7 in Groups 1, 2, and 3, respectively. Vitreous body vimentin levels (ng/mL) were 40.8 ± 61.9, 88.6 ± 86.8, and 3952.8 ± 8179.5 in Groups 1, 2, and 3, respectively. Vitreous body total protein levels (mg/mL) were 3.1 ± 1.2, 4.0 ± 2.0, and 3.4 ± 2.6 in Groups 1, 2, and 3, respectively. Vitreous body GFAP levels normalized to total protein (ng/mg total protein) were 4.9 ± 3.3, 3.3 ± 1.7, and 202.1 ± 582.2 in Groups 1, 2, and 3, respectively. Vitreous body vimentin levels normalized to total protein (ng/mg total protein) were 16.3 ± 19.3, 23.1 ± 21.0, and 851.0 ± 1055.6 in Groups 1, 2, and 3, respectively. Both GFAP and vimentin levels were significantly elevated in the vitreous fluid from retinal detachment (Group 3) compared with macular hole (Group 1) or epiretinal membrane (Group 2) (P < 0.01). There were no significant differences in vitreous body GFAP and vimentin levels between Groups 1 and 2. Vitreous body total protein levels were not different among Groups 1, 2, or 3 (Fig. 1).
Table 2.
Patient Characteristics
| Macular Hole (Group 1) | Epiretinal Membrane (Group 2) | Retinal Detachment (Group 3) | |
|---|---|---|---|
| Number | 8 | 8 | 28 |
| Age | 71.0 ± 8.3 | 67.5 ± 9.2 | 56.6 ± 15 |
| Gender (M:F) | 6:2 | 5:3 | 20:8 |
| Pre-operative VA (logMAR) | 1.8 ± 0.8 | 1.0 ± 0.7 | 1.2 ± 0.8 |
| Duration of symptoms (days) | 38.9 ± 28.9 | 41.8 ± 49.1 | 39.5 ± 93.4 |
Age, VA, and duration of symptoms were presented as mean ± standard deviation. F, female; logMAR, logarithm of the minimum angle of resolution; M, male.
Figure 1.
Vitreous GFAP, vimentin, and total protein levels in macular hole, epiretinal membrane, and retinal detachment. (A) Vitreous body GFAP level in retinal detachment, 572.0 ± 1159.7 ng/mL, was significantly higher than in macular hole, 12.4 ± 9.8 ng/mL (P < 0.01) and epiretinal membrane 17.5 ± 17.7 ng/mL (P < 0.01). (B) Vitreous body vimentin level in retinal detachment, 3952.8 ± 8179.5 ng/mL, was significantly higher than in macular hole, 40.8 ± 61.9 ng/mL (P < 0.01) and epiretinal membrane 88.6 ± 86.8 ng/mL (P < 0.01). (C) Vitreous body GFAP normalized to total protein level in retinal detachment, 202.1 ± 582.2 ng/mg total protein, was significantly higher than macular hole, 4.9 ± 3.3 ng/mg total protein (P < 0.01) and epiretinal membrane 3.3 ± 1.7 ng/mg total protein (P < 0.01). (D) Vitreous body vimentin normalized to total protein level in retinal detachment, 851.0 ± 1055.6 ng/mg total protein, was significantly higher than in macular hole, 16.3 ± 19.3 ng/mg total protein (P < 0.01) and epiretinal membrane 23.1 ± 21.0 ng/mg total protein (P < 0.01). (E) Vitreous body total protein level was not significantly different among macular hole, 3.1 ± 1.2 mg/mL, epiretinal membrane, 4.0 ± 2.0 mg/mL, and retinal detachment, 3.4 ± 2.6 mg/mL.
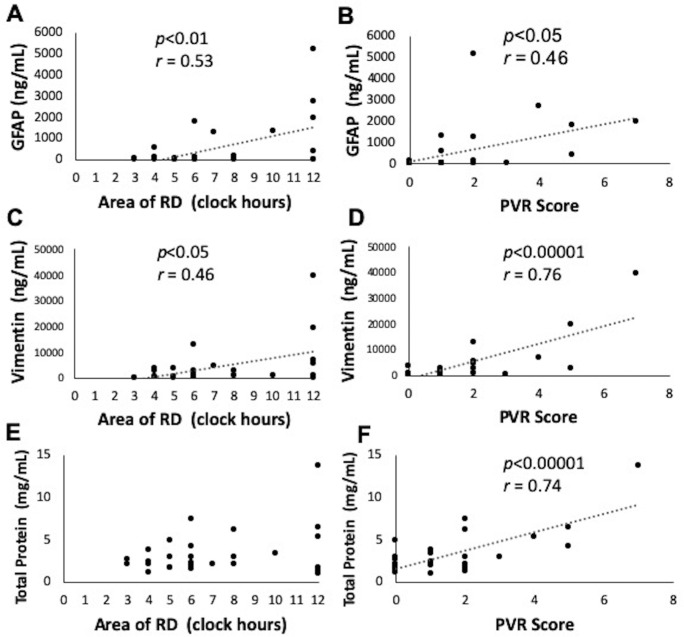
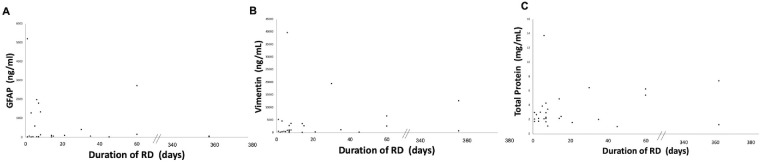
In the retinal detachment group (Group 3), the area of retinal detachment ranged from 3 to 12 o’clock hours, and the cumulative PVR score ranged from 0 to 7 points. The duration of symptomatic vision change ranged from 1 to 365 days (Table 3). Both elevated GFAP and vimentin levels in Group 3 were positively correlated with the areas of retinal detachment (P < 0.01, r = 0.53 in GFAP; P < 0.05, r = 0.46 in vimentin). Elevated GFAP and vimentin levels in Group 3 were more strongly correlated with cumulative PVR scores than they were with areas of retinal detachment (P < 0.05, r = 0.46 in GFAP; P < 0.00001, r = 0.76 in vimentin) (Fig. 2). However, neither GFAP nor vimentin levels were correlated with the duration of retinal detachment (Fig. 3) and age (data not shown). Total protein was correlated with cumulative PVR scores (P < 0.00001, r = 0.74), but not with areas of retinal detachment or duration of retinal detachment (Figs. 2 and 3).
Table 3.
Characteristics of Retinal Detachment
| ID/Age | Duration | RD | Vitreous | Fixed | Open | PVR | GFAP ng/mL | Vimentin ng/mL | Total | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (yrs) | (days) | (clock hours) | Opacity | Wrinkle | folds | funnel | Score | (GFAP/total protein) | (Vim/total protein) | Protein mg/mL | Comments |
| RD1 48 | 14 | 3 | 0 | 0 | 0 | 0 | 0 | 5.6 (2.6) | 46.7 (21.9) | 2.1 | Mild VH |
| RD2 87 | 15 | 4 | 0 | 0 | 1 | 0 | 1 | 42.0 (17.43) | 2607.3 (1082.2) | 2.4 | RD recurred |
| RD3 60 | 30 | 12 | 1 | 0 | 4 | 0 | 5 | 403.9 (62.9) | 19429 (3025.5) | 6.4 | Mild VH |
| RD4 62 | 7 | 6 | 0 | 1 | 0 | 0 | 1 | 14.9 (6.4) | 586.7 (253.2) | 2.3 | Multiple Tears (>2) |
| RD5 47 | 8 | 4 | 0 | 0 | 0 | 0 | 0 | 120.8 (113.6) | 3572 (3359.4) | 1.1 | |
| RD6 75 | 5 | 4 | 1 | 0 | 0 | 0 | 1 | 575.4 (149.4) | 325.5 (84.5) | 3.9 | Two tears |
| RD7 56 | 7 | 6 | 1 | 1 | 3 | 0 | 5 | 1,792.7 (419.6) | 2,719.3 (636.5) | 4.3 | Multiple tears (>2) |
| RD8 56 | 60 | 12 | 1 | 1 | 2 | 0 | 4 | 2,726.3 (507.9) | 6,561 (1222.4) | 5.4 | Multiple tears (>2), Lattice |
| RD9 61 | 7 | 6 | 0 | 1 | 0 | 0 | 1 | 11.7 (5.5) | 987.3 (461.7) | 2.1 | Two tears |
| RD10 54 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 46.1 (17.3) | 80.3 (30.2) | 2.7 | Multiple tears (>2), Recurred RD |
| RD11 50 | 35 | 6 | 0 | 1 | 0 | 0 | 1 | 45.4 (22.6) | 1076.7 (537.0) | 2.0 | |
| RD12 38 | 3 | 7 | 1 | 1 | 0 | 0 | 2 | 1,268.8 (588.8) | 4,546 (2109.5) | 2.2 | |
| RD13 62 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 8.7 (5.0) | 300.7 (174.2) | 1.7 | Multiple tears (>2) |
| RD14 65 | 1 | 5 | 0 | 1 | 2 | 0 | 3 | 10.6 (3.6) | 393 (133.2) | 3.0 | |
| RD15 27 | 6 | 8 | 0 | 0 | 0 | 0 | 0 | 18.4 (8.9) | 861 (416.4) | 2.1 | |
| RD16 53 | 21 | 6 | 0 | 0 | 0 | 0 | 0 | 91 (58.8) | 221 (142.9) | 1.5 | Lattice |
| RD17 45 | 8 | 10 | 0 | 1 | 0 | 0 | 1 | 1,326 (390.4) | 922 (271.4) | 3.4 | Multiple tears (>2) |
| RD18 69 | 45 | 12 | 0 | 0 | 1 | 0 | 1 | 7.6 (7.6) | 70.7 (7.7) | 1.0 | |
| RD19 45 | 365 | 12 | 0 | 0 | 2 | 0 | 2 | 17.9 (14.1) | 600.8 (474.9) | 1.3 | Multiple tears (>2), Lattice |
| RD20 38 | 6 | 12 | 1 | 1 | 0 | 5 | 7 | 1,968 (143.8) | 39,593 (2,892.1) | 13.7 | Multiple tears (>2) |
| RD21 87 | 4 | 6 | 0 | 0 | 0 | 0 | 0 | 12.8 (4.3) | 393.6 (130.8) | 3.0 | |
| RD22 29 | 7 | 5 | 0 | 0 | 0 | 0 | 0 | 5.0 (3.0) | 45.5 (27.3) | 1.7 | |
| RD23 73 | 7 | 8 | 0 | 1 | 1 | 0 | 2 | 19.8 (6.7) | 593.3 (202.0) | 2.9 | Multiple tears (>2) |
| RD24 56 | 60 | 8 | 1 | 1 | 0 | 0 | 2 | 145.2 (23.3) | 2510.5 (403.4) | 6.2 | Two tears |
| RD25 56 | 365 | 6 | 1 | 0 | 1 | 0 | 2 | 39.4 (5.3) | 12,540 (1696.3) | 7.4 | |
| RD26 62 | 1 | 4 | 0 | 1 | 0 | 0 | 1 | 7.0 (3.4) | 422 (206.6) | 2.0 | RD recurred |
| RD27 64 | 14 | 5 | 0 | 0 | 0 | 0 | 0 | 86.6 (17.8) | 3486.2 (716.8) | 4.9 | |
| RD28 59 | 1 | 12 | 1 | 1 | 0 | 0 | 2 | 5,199 (3,049.3) | 5,188.6 (3,043.2) | 1.7 | Failed Pneumopexy, Multiple tears |
RD, retinal detachment; VH, vitreous hemorrhage.
Figure 2.
Correlations between vitreous body GFAP, vimentin, and total proteins in retinal detachment and areas of retinal detachment and PVR scores. (A, B) Elevated vitreous body GFAP levels in retinal detachment were positively correlated with the areas of RD (P < 0.01, r = 0.53) and PVR scores (P < 0.05, r = 0.46). (C, D) Elevated vitreous body vimentin levels in retinal detachment were positively correlated with the areas of RD (P < 0.05, r = 0.46) and PVR scores (P < 0.00001, r = 0.76). (E, F) Vitreous body total protein was positively correlated with PVR scores (P < 0.00001, r = 0.74), but not with areas of retinal detachment.
Figure 3.
Correlations between vitreous body GFAP, vimentin, and total proteins in retinal detachment and duration of retinal detachment. (A, B) Elevated vitreous body GFAP and vimentin levels in retinal detachment were not correlated with the duration of retinal detachment. (C) Vitreous body total protein was not correlated with duration of retinal detachment.
Out of 28 RDs in Group 3, two patients developed recurrent RD after failed primary repair of RD with vitrectomy (RD 10 and 26). Both patients have had low PVR scores at the time of initial presentation, 0 and 1, respectively, with mildly elevated vitreous body GFAP and vimentin levels. Both patients were found to have a new retinal tear during the repeat RD repair.
Discussion
We found that human vitreous GFAP and vimentin were elevated more than 30-fold in retinal detachment with various degrees of PVR (Group 3) compared within idiopathic macular hole (Group 1) or epiretinal membrane (Group 2). Previously, vitreous body GFAP in nondiseased organ donor eyes was reported as 20 pg/mL.16 Neither GFAP nor vimentin was reported as a major protein from the vitreous body of nondiseased organ donor eyes in the study by Wu et al.17 Vimentin, but not GFAP, was expressed in healthy rabbit vitreous.18 For ethical reasons, it is not possible to obtain fresh human vitreous samples from healthy eyes. Therefore we compared elevated vitreous GFAP and vimentin from retinal detachment with vitreous GFAP and vimentin from eyes with macular hole or epiretinal membrane groups. The substantially elevated GFAP and vimentin levels more than 30-fold in retinal detachment compared with those in the macular hole and epiretinal membrane groups suggest that both proteins may have been released into the vitreous body by the reactive retinal gliosis from the retinal detachment. Furthermore, vitreous body total protein levels were not different among Groups 1, 2, or 3, and vitreous body GFAP and vimentin normalized to total protein levels were still significantly increased in retinal detachment (Group 3), indicating that the elevated vitreous GFAP or vimentin levels in Group 3 were not due to a direct increase in total protein in the vitreous from retinal detachment but rather may have been due to the reactive retinal gliosis from the retinal detachment and were related to PVR.
We also found a positive correlation between elevated GFAP and vimentin levels in Group 3 and the areas of retinal detachment (P < 0.01, r = 0.53 in GFAP; P < 0.05, r = 0.46 in vimentin). More importantly, the elevated GFAP and vimentin levels in Group 3 were highly correlated with cumulative PVR scores (P < 0.05, r = 0.46 in GFAP; P < 0.00001, r = 0.76 in vimentin), although GFAP and vimentin are known to be nonspecific markers for retinal gliosis. To the best of our knowledge, this is the first study demonstrating elevated vitreous body GFAP and vimentin correlated with the severity of PVR. Because both GFAP and vimentin are sensitive stress markers for activated Müller cells and astrocytes, increased expression of both GFAP and vimentin in the detached retina in both animal model and human tissue is relatively well known as a stress biomarker for reactive gliosis.2,4–6 However, little is known about the relationship between quantified values of increased GFAP and vimentin and the areas of retinal detachment. It may be plausible to assume a correlation between increased expression of GFAP and vimentin and larger areas of retinal detachment. Our results showed that both GFAP and vimentin proteins were released into the vitreous body, and the increased vitreous body GFAP and vimentin levels were positively correlated with areas of retinal detachment. Furthermore, we demonstrated that the quantified increased levels of vitreous GFAP and vimentin were highly correlated with the severity of PVR. For example, the participant (RD 20) with the highest PVR score, 7, from an open funnel retinal detachment had extremely high vitreous GFAP (1,968 ng/mL) and vimentin (39,593 ng/mL) levels. Our data suggest that retinal gliosis may play a key role in a complex PVR formation, although until now the role of retinal gliosis in PVR formation has been regarded as minor. The participant (RD28) also has had extremely high vitreous GFAP (5199 ng/mL) and vimentin (5188.6 ng/mL) immediately after failed pneumopexy. The PVR score was relatively low (score 2) and total protein was relatively low (1.7 mg/mL) in this case, possibly because of early surgical intervention immediately after failed pneumopexy, suggesting that acute spikes of GFAP and vimentin in the vitreous body is indicative of acute severe reactive gliosis induced by changes in vitreoretinal dynamics, as well as progressed RD. Taken together, elevated GFAP and vimentin in the vitreous body after RD can potentially be useful biomarkers to identify the high-risk PVR patients, who may need more aggressive surgical procedures or may be candidates for potential adjunctive medical therapy trial.
PVR was originally characterized by the growth of membranes on the surface of the detached retina; these membranes contract, causing distortion of the retina and maintaining retinal detachment.14 The pathogenesis of this process was speculated to involve several steps, including migration of cells, mainly retinal pigment epithelial cells; proliferation of the migrating cells; and membrane formation and contraction (epithelial-mesenchymal transition). The detached retina immediately undergoes ischemia, inflammation, and reactive gliosis. However, the differences between retinal detachment and retinal detachment leading to PVR are not fully understood. Because early changes after retinal detachment are difficult to study in human tissues, much of our current understanding of PVR development has been ascertained from experimental models. Although many studies were performed with experimental models of advanced PVR and clinical trials targeting proliferating cells were attempted, it is still unclear how the cellular proliferation is initiated.19 In the present study, we demonstrated that increased GFAP and vimentin in the human vitreous body were strongly correlated with PVR severity from retinal detachments with no PVR change to the various stages of PVR. Further study is needed to determine whether GFAP and vimentin are involved in initiation of cellular migration, proliferation, or epithelial-mesenchymal transition (EMT).
Interestingly, vitreous total protein levels were not different among Groups 1, 2, and 3, while the vitreous total protein in retinal detachment (Group 3) was positively correlated with PVR score, but not with areas of retinal detachment. This finding indicates that total amounts of vitreous protein are relatively unchanged across three different retinal conditions: epiretinal membrane, macular hole, or retinal detachment. Furthermore, the result suggests that vitreous total protein level may not change significantly by reactive gliosis referred by increased areas of retinal detachment. However, the level of total protein in the vitreous does increase by the severity of PVR. A previous study that performed proteomic analyses of the vitreous body from PVR using mass spectrometry revealed 97 to 137 proteins between moderate and severe PVR, confirming that dynamic changes in the vitreous proteins occur during PVR formation.20 The researchers observed that some proteins were elevated or appeared in PVR, whereas the proteins in the control group were decreased or disappeared in PVR. This phenomenon may explain the unchanged total protein levels among different retinal conditions in the present study. However, both GFAP and vimentin were absent from their large-scale proteomic study of the vitreous from PVR. This result is surprising because intermediate filaments, such as GFAP and vimentin, are very stable and are well measured by mass spectrometry. We speculate that the researchers may have excluded soluble protein pools during the process of sample. In contrast, Jünemann's group16 reported elevated GFAP levels in the vitreous of PVR compared with macular hole and epiretinal membrane. However, elevated GFAP levels were not further analyzed for the severity of PVR, nor were the vimentin levels measured in their study.
A major pool of GFAP and vimentin in the retina is known as intracellular cytoskeletal network of retinal glial cells characterized by their insolubility and assembled into IF polymers.21,22 From the observation of the cerebrospinal fluid from acute or chronic neurodegenerative diseases, it has been speculated that degradation of the IF polymer causes the release of more soluble fragments of IF to the adjacent fluid compartments, as seen in multiple sclerosis or traumatic brain injury.22 However, it is not entirely clear whether soluble fragments are primarily derived from degradation of insoluble fragments or whether soluble fragments are secreted into extracellular space independently in reactive gliosis in the central nervous system (CNS). A previous study demonstrated that IFs are confined to the end foot compartment, and vimentin is dominant, in the resting retina. In an animal model of retinal detachment (RD), RD induces up-regulation of both GFAP and vimentin, which first appear in the end foot on days 1 to 3 of retinal detachment. Starting on day 3 of RD, IFs grow outward from the end foot into the cell body and outer nuclear layer, and GFAP is dominant.2 Additional studies are needed to determine whether these overexpressed proteins further undergo degradation or whether soluble IFs are independently overexpressed in PVR processes.
Increased IFs and total protein present in the vitreous body in PVR may have more implications for PVR formation because, clinically, residual vitreous gel from incomplete removal of vitreous during vitrectomy has been postulated to be associated with recurrent retinal detachment with PVR formation.23–25 Further studies are necessary to examine whether altered proteins present in the vitreous body from the retinal detachment may contribute to PVR formation.
In the present study, neither GFAP nor vimentin levels were correlated with the duration of retinal detachment (range, 0 to 365 days), suggesting that elevated GFAP and vimentin are not due to the time lag from the onset of RD to surgery. However, elevated vitreous GFAP and vimentin were noted in the immediate acute phase after RD. Further study is needed to examine whether these proteins are acutely overexpressed and degraded over the time, or consumed for the process of PVR.
The limitations of this study are the relatively small numbers of patients, the lack of normal physiologic value of vitreous GFAP and vimentin, and a cross-sectional design that limits further analyses of the origin of these proteins or a cause and effect relationship of these proteins in retinal detachment and PVR.
In conclusion, we demonstrated that elevated vitreous body GFAP and vimentin levels are correlated with the severity of PVR and may serve as a biomarker for PVR. Further study is needed to examine the functional role of GFAP and vimentin in PVR formation.
Acknowledgments
The authors thank Robert E. Anderson, MD, PhD, for critically reading this manuscript. The authors also thank Kathy J. Kyler, Staff Editor, University of Oklahoma Health Sciences Center, for editing this manuscript.
Supported by Clinician Scientist Development Grant from Presbyterian Health Foundation (PHF), Oklahoma City, Oklahoma, and an unrestricted departmental grant from Research to Prevent Blindness, Inc., to the OUHSC Department of Ophthalmology.
Disclosure: S.Y. Lee, None; J.W. Surbeck, None; M. Drake, None; A. Saunders, None; H.D. Jin, None; V.A. Shah, None; R.V. Rajala, None
References
- 1. Shaw G, Weber K. The intermediate filament complements of the retina: a comparison between different mammalian species. Eur J Cell Biol. 1984; 33: 95–104. [PubMed] [Google Scholar]
- 2. Lewis GP, Fisher SK. Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. Int Rev Cytol. 2003; 230: 263–290. [DOI] [PubMed] [Google Scholar]
- 3. Bringmann A, Pannicke T, Grosche J, et al.. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006; 25: 397–424. [DOI] [PubMed] [Google Scholar]
- 4. Guérin CJ, Anderson DH, Fisher SK. Changes in intermediate filament immunolabeling occur in response to retinal detachment and reattachment in primates. Invest Ophthalmol Vis Sci. 1990; 31: 1474–1482. [PubMed] [Google Scholar]
- 5. Nork TM, Wallow IH, Sramek SJ. Immunocytochemical study of an eye with proliferative vitreoretinopathy and retinal tracks. Retina. 1990; 10: 78–85. [PubMed] [Google Scholar]
- 6. Okada M, Matsumura M, Ogino N, et al.. Müller cells in detached human retina express glial fibrillary acidic protein and vimentin. Graefes Arch Clin Exp Ophthalmol. 1990; 228: 467–474. [DOI] [PubMed] [Google Scholar]
- 7. Campochiaro PA. Pathogenic mechanisms in proliferative vitreoretinopathy. Arch Ophthalmol. 1997; 115: 237–241. [DOI] [PubMed] [Google Scholar]
- 8. Pastor JC, de la Rua ER, Martin F. Proliferative vitreoretinopathy: risk factors and pathobiology. Prog Retinal Eye Res. 2002; 21: 127–144. [DOI] [PubMed] [Google Scholar]
- 9. Weller M, Wiedemann P, Heimann K. Proliferative vitreoretinopathy – is it anything more than wound healing at the wrong place? Int Ophthalmol. 1990; 14: 105–117. [DOI] [PubMed] [Google Scholar]
- 10. Lee S. Pathophysiology of Ocular Trauma., Ryan's Retina, 6th ed.: Elsevier; 2017: 1685–1875. [Google Scholar]
- 11. Garweg JG, Tappeiner C, Halberstadt M. Pathophysiology of Proliferative Vitreoretinopathy in Retinal Detachment. Surv Ophthalm. 2013; 58: 321–329. [DOI] [PubMed] [Google Scholar]
- 12. Pastor JC, Méndez MC, de la Fuente MA, et al.. Intraretinal immunohistochemistry findings in proliferative vitreoretinopathy with retinal shortening. Ophthalmic Res . 2006; 38: 193–200. [DOI] [PubMed] [Google Scholar]
- 13. Charteris DG, Downie J, Aylward GW, et al.. Intraretinal and periretinal pathology in anterior proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol . 2007; 245: 93–100.13. [DOI] [PubMed] [Google Scholar]
- 14. Hilton G, Machemer R, Michels R, et al.. The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology . 1983; 90: 121–125. [DOI] [PubMed] [Google Scholar]
- 15. Angi M, Kalirai H, Coupland SE, et al.. Proteomic analyses of the vitreous humor. Mediators Inflamm. 2012; 2012: 148039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jünemann AG, Rejdak R, Huchzermeyer C, et al.. Elevated vitreous body glial fibrillary acidic protein in retinal diseases. Graefes Arch Clin Exp Ophthalmol. 2015; 253: 2181–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu CW, Sauter JL, Johnson PK, et al.. Identification and localization of major soluble vitreous proteins in human ocular tissue. Am J Ophthalmol. 2004; 137: 655–661. [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Bouhenni RA, Dufresne CP, et al.. Differential Expression of Vitreous Proteins in Young and Mature New Zealand White Rabbits. PLoS One. 2016; 18;11: e0153560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pastor JC, Rojas J, Pastor-Idoate S, et al.. Proliferative vitreoretinopathy: A new concept of disease pathogenesis and practical consequences. Prog Retin Eye Res. 2016 Mar; 51: 125–155. [DOI] [PubMed] [Google Scholar]
- 20. Yu J, Liu F, Cui SJ, et al.. Vitreous proteomic analysis of proliferative vitreoretinopathy. Proteomics. 2008; 8: 3667–3678. [DOI] [PubMed] [Google Scholar]
- 21. Robert A, Hookway C, Gelfand VI. Intermediate filament dynamics: What we can see now and why it matters. Bioessays. 2016; 38: 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petzold A. Glial fibrillary acidic protein is a body fluid biomarker for glial pathology in human disease. Brain Res . 2015; 10;1600: 17–31. [DOI] [PubMed] [Google Scholar]
- 23. Quiram, et al.. Outcomes of Vitrectomy with Inferior Retinectomy in Patients with Recurrent Rhegmatogenous Retinal Detachments and Proliferative Vitreoretinopathy. Ophthalmology. 2006; 113: 2041–2047. [DOI] [PubMed] [Google Scholar]
- 24. Sundar, et al.. Evaluation of hyaloid-retinal relationship during triamcinolone-assisted vitrectomy for primary rhegmatogenous retinal detachment. Eur J Ophthalmol. 2018; 28: 607–613. [DOI] [PubMed] [Google Scholar]
- 25. Chen, et al.. Intravitreal triamcinolone staining observation of residual undetached cortical vitreous after posterior vitreous detachment. Eye (Lond). 2006; 20: 423–427. [DOI] [PubMed] [Google Scholar]