Abstract
Purpose
To investigate the effect and mechanism of Agrin on limbal stem cell proliferation and corneal wound healing.
Methods
Limbal stem cells were isolated and treated with different concentrations of Agrin. CCK-8 and cell proliferation markers (Ki67 and pH3) were detected to evaluate cell numbers or proliferative potential of limbal stem cells. The corneal epithelium wound model was induced by debridement of central corneal epithelial, and the effects of Agrin on limbal stem cell proliferation and corneal epithelial wound healing rate were determined.
Results
Agrin promoted the proliferation of cultured limbal stem cells in vitro and increased the expression level of p63α rather than keratin 12. Furthermore, Agrin accelerated the wound healing rate of corneal epithelium through activating limbal stem cell proliferation in vivo. In terms of mechanism, Agrin could facilitate the dephosphorylation of Yap1, which contributed to the nuclear translocation of Yap1 and expression of Cyclin D1, and subsequently promoted proliferation of limbal stem cells.
Conclusions
Agrin promotes the proliferation of limbal stem cells and accelerates the healing rate of corneal wound through Hippo-Yap signaling pathway.
Keywords: limbal stem cells, Hippo-Yap signaling pathway, Agrin, proliferation, wound healing
The cornea, located at the front part of the eyeball, is crucial to maintaining the transparency of the visual pathway; the corneal epithelium layer serves as a surface barrier for the cornea; and limbal stem cells (LSCs) are the source of corneal epithelium regeneration.1 LSCs reside in the Vogt's palisades of the limbus and possess the ability to differentiation as transient amplifying cells (TACs), which contribute to maintaining the homeostasis of corneal epithelium.2,3 Limbal stem cell deficiency (LSCD) is an ocular surface disorder caused by a decrease in the population and function of corneal epithelial stem/progenitor cells, results in vision loss and corneal blindness, which affect 10 million individuals around the world.4–6 LSCD can be classified as acquired or hereditary according to the different causes, including severe chemical or thermal burn, ultraviolet radiation, advanced ocular cicatricial pemphigoid, Stevens-Johnson syndrome, and more.4 The loss and dysfunction of limbal stem cells cause limbus barrier damage and unbalanced homeostasis of the corneal epithelium, leading to deficiency of the corneal epithelium, corneal melting, and infection.7,8 The pathologic changes of LSCD are characterized by conjunctivalization, persistent epithelium defects, pseudopterygium, corneal neovascularization, chronic inflammation, and scarring.9 Allogeneic limbal stem cell transplantation is the mainstay of LSCD treatment but limited by the resource of donors and inevitable immune-rejection response.4 Thus autologous cell–transplanted therapy holds great promise for the treatment of LSCD; however, problems remain in cell culture, purification, and expansion. Recent evidence suggests that activated proliferation of preexisting limbal stem cells is crucial to the treatment of LSCD.10,11 Therefore there is an urgent need to find more effective therapeutic strategies for LSCD through activating the proliferative ability of endogenous limbal stem cells.
Hippo-Yap signaling is a highly conserved pathway that not only maintains cell homeostasis under normal conditions but also regulates tissue regeneration after injury.12,13 Yes-associated protein 1 (Yap1), a key transcriptional coactivator of the Hippo-Yap signaling pathway, plays a vital role in stem cell proliferation, tissue regeneration, and tumor formation in mammalians.12,14 Yap1 is located in the nuclei of limbal stem cells and TACs, which is required for the proliferation and expansion of limbal stem cells.15 Emerging evidence has demonstrated that Yap1 is specifically expressed in limbal stem cells and essential for maintaining their high proliferative potential.12,15 Agrin is a large extracellular heparan sulfate proteoglycan and enriched in the basement membrane at the limbus, which can decrease Yap1 phosphorylation and induce nuclear Yap1 translocation.16,17 Several attempts have been made to investigate the role of Agrin in regulating tissue proliferation and regeneration by triggering the Hippo-Yap signaling pathway, and recent evidence has indicated that Agrin treatment can promote cardiac regeneration after myocardial infarction and promote proliferation of human induced Pluripotent Stem Cell (iPSC)-derived cardiomyocytes.18 In this study, we investigate the effect and mechanism of Agrin on limbal stem cell proliferation and corneal wound healing both in vitro and in vivo. The findings may offer important insights into mechanisms of Agrin on corneal repair regulation, and novel strategies of therapy for LSCD.
Materials and Methods
Limbal Stem Cell Isolation and Culture
Primary limbal stem cells were isolated from eyeballs of 6- to 8-week-old C57BL/6J mice. In brief, eyeballs were washed with phosphate-buffered saline solution (PBS) containing 1 × streptomycin and penicillin antibiotics (SPA) (Gibco 15140-122; Thermo Fisher Scientific, Waltham, MA, USA) three times, and the conjunctiva and Tenon's capsules were removed, then central cornea and corneoscleral tissues were cut and digested in Dispase II (Sigma D4693, 2.4 U/mL in DMEM/F12 medium; Sigma-Aldrich, St. Louis, MO, USA) overnight at 4°C. A trephine (2.5 mm) was used to remove the central cornea and iris tissues, endothelial and posterior stromal layers were mechanically removed. Each remaining tissue was cut into six equal pieces (dimensions, 1.5 mm × 1.5 mm approximately), then cultured in supplemental hormonal epithelial medium (SHEM: HEPES-buffered Dulbecco's modified Eagle medium containing an equal volume of F12, 5% fetal bovine serum, 5 µg/mL insulin, 5 µg/mL transferrin, 5 ng/mL selenium, 0.5 µg/mL hydrocortisone, 2 ng/mL mouse Epidermal Growth Factor (EGF), 30 ng/mL toxin A, 50 µg/mL gentamicin, 1.25 µg/mL amphotericin B and 0.5% dimethyl sulfoxide) at 37°C and 5% CO2.19 Each well of the cell culture plates was precoated with Matrigel (Corning Life Sciences, Corning, NY, USA) overnight at 4°C. Limbal stem cell explants (LSCEs) were carefully laid on the bottom of precoated culture plates and then cultured in SHEM. After 24 hours’ adherence, LSCEs were treated with different concentrations of Agrin (0, 10, and 100 ng/mL in SHEM). The cell morphology and the extension area of LSCEs were captured using a phase contrast microscope (DMIL-PH1; Leica Camera, Wetzlar, Germany).
Cell Count Kit-8 Assay
The number of limbal stem cells was indirectly determined using Cell Count Kit-8 assay (MCE HY-K0301-3000T; MedChem Express, Monmouth Junction, NJ, USA) according to the manufacturer's instructions. A 96-well plate precoated with Matrigel was incubated at 37°C under 5% CO2 overnight. The cultured limbal stem cells were inoculated in precoated 96-well plates (5 × 103cells/well) for 24 hours’ adherence, then treated with different concentrations of Agrin (0, 10, and 100 ng/mL in SHEM). After treatment for 24, 36, or 48 hours, 10 µL of the CCK-8 solution was added to each well. The absorbance at 450 nm was measured by a Microplate Reader (Thermo Fisher Scientific) after 2 hours’ incubation.
Immunofluorescence Staining
The expanded limbal stem cells were trypsinized and inoculated on EZ Slides (PEZGS0816; Millipore, Burlington, MA, USA) for 24 hours’ adherence, then treated with Agrin (0, 10, and 100 ng/mL in SHEM) for 2 days. For immunofluorescence staining, sample slides were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 then blocked with 2.5% normal donkey serum in PBS for 30 minutes at room temperature. Slides were incubated with anti-Ki67 (1:100, Thermo Fisher Scientific, 14-5698-82), anti-pH3 (1:100, 9706; Cell Signaling Technologies, Danvers, MA, USA) or anti-p63α (1:100, 4892, Cell Signaling Technologies) antibodies overnight at 4°C. Then these samples were washed with PBS for 3 times and incubated with Donkey anti-Mouse Alexa Fluor Plus 555 (1:500, A32773, Thermo Fisher Scientific), Goat anti-Rat Alexa Fluor 555 (1:200, A-21434, Thermo Fisher Scientific), Donkey anti-Rabbit Alexa Fluor Plus 488 (1:500, A32790, Thermo Fisher Scientific) or Donkey anti-Rabbit Alexa Fluor Plus 555 (1:500, A32794, Thermo Fisher Scientific) secondary antibodies for 1 hour at 37°C. DAPI (4′,6-diamidino 2-phenylindole) was used for nuclear counterstaining. Images were captured by using an Olympus confocal laser scanning microscope (FluoView 3000; Olympus America, Center Valley, PA, USA) and calculated using the Image Pro Plus 6.0 software. Cells co-expressing p63α and proliferating markers were calculated as targeted limbal stem cells.
Cell Extracts and Western Blot Analysis
The expanded limbal stem cells were trypsinized and inoculated on 6-well plates for 24 hours’ adherence, then treated with Agrin (0 and 10 ng/mL in SHEM) for 2 days. For Western blot analysis, samples were washed with PBS three times and lysed in lysis buffer on ice. Cell lysates were rotated and spun in a centrifuge at 14000g for 40 minutes at 4°C. The supernatant was collected, and the protein concentrations of the cell lysates were determined by BCA Protein Assay Kit (P0010; Beyotime Institute of Biotechnology, Jiangsu, China). Equal amounts of protein samples were electrophoresed in polyacrylamide gel and electrotransferred to nitrocellulose membranes. After that, nitrocellulose membranes were blocked and incubated with anti-Ki67 (1:500, ab16667; Abcam, Cambridge, UK), anti-pH3 (1:500, PA5-17869, Thermo Fisher Scientific), anti-p63α (1:500, 4892; Cell Signaling Technologies), anti-Keratin 12 (1:500, ab185627; Abcam), anti-p-Ser127-Yap1 (1:500, 13008; Cell Signaling Technologies), anti-Yap1 (1:500, 14074; Cell Signaling Technologies), or anti-Cyclin D1 (1:500, 55506; Cell Signaling Technologies) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:500, 10494-1-AP; Proteintech Group, Chicago, IL, USA) antibodies overnight at 4°C. The membranes were washed with Tris Buffered saline Tween (TBST) and incubated with secondary antibody (IRDye 800CW Donkey anti-Rabbit IgG, 925-32213; Li-Cor Biosciences, Lincoln, NE, USA) at a dilution of 1:1000 for 1 hour at room temperature. Chemiluminescent signals were detected by use of a Li-Cor Odyssey Fc instrument. Data were normalized to GAPDH expression.
Corneal Epithelium Debridement Model and Wound Healing Assessment
All animals were kept in a pathogen-free environment and fed as desired. The procedures for care and use of animals were approved by the Ethics Committee and Animal Care and Use Committee of the Animal Center of Academy of Military Medical Sciences and Transformational Medical College of Jilin University. All applicable institutional and governmental regulations concerning the ethical use of animals were followed. C57BL/6J mice (8 weeks) were systemically anesthetized by 2% isoflurane and then topical anesthetized by 0.4% oxybuprocaine hydrochloride eye drops on their right eyes. As previously described,20 the central corneal epithelial cells of the right eyes were demarcated with a 2.5-mm–diameter trephine and removed by gently scraping with a blade. After wounding, the mice were applied with Agrin (10 ng/mL in PBS) or bovine serum albumin (10 ng/mL in PBS) topically four times per day on the right eyes. Each cornea was stained with 1% fluorescein and photographed until the wound was completely healed.
Cornea Histology Immunostaining
Corneal tissues at day 3 after injury were fixed in 4% paraformaldehyde at 4°C overnight; after 3 washes in PBS, the tissues were dehydrated in a 30% sucrose in PBS solution overnight at 4°C, then embedded in optimum cutting tissue (Sakura, Osaka, Japan) and stored at −80 °C until sectioning. Cryosections of 10-µm thickness were collected and stored at −20°C until use. For immunostaining, tissue sections were subjected to PBS to remove optimum cutting tissue. Sections were permeabilized and blocked with 0.1% Triton X-100 and 2.5% normal donkey serum in PBS at room temperature for 30 minutes, then incubated with anti-Ki67 (1:100, 14-5698-82; Thermo Fisher Scientific), anti-pH3 (1:100, 9706; Cell Signaling Technologies) or anti-p63α (1:100, 4892; Cell Signaling Technologies) antibodies overnight at 4°C. Then sections were washed with PBS three times and incubated with Donkey anti-Mouse Alexa Fluor Plus 488 (1:500, A32766; Thermo Fisher Scientific), Goat anti-Rat Alexa Fluor 488 (1:200, A-11006; Thermo Fisher Scientific) or Donkey anti-Rabbit Alexa Fluor Plus 555 (1:500, A32794; Thermo Fisher Scientific) secondary antibodies for 1 hour at 37°C. DAPI was used for nuclear counterstaining.
Images were captured by using an Olympus confocal laser scanning microscope (FluoView 3000) and calculated using the Image Pro Plus 6.0 software. Cells coexpressing p63α and proliferating markers were calculated as targeted limbal stem cells.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 7.0 for Windows. All data are presented as the mean ± standard error (mean ± SE). For comparison of more than two groups, statistical analyses were performed by one-way analysis of variance followed by the Tukey multiple comparison. For comparison of two groups, statistical analyses were performed by Student's t-test. P values <0.05 were considered as statistically significant results.
Results
Agrin Promoted the Outgrowth and Increased the Numbers of Cultured Limbal Stem Cells In Vitro
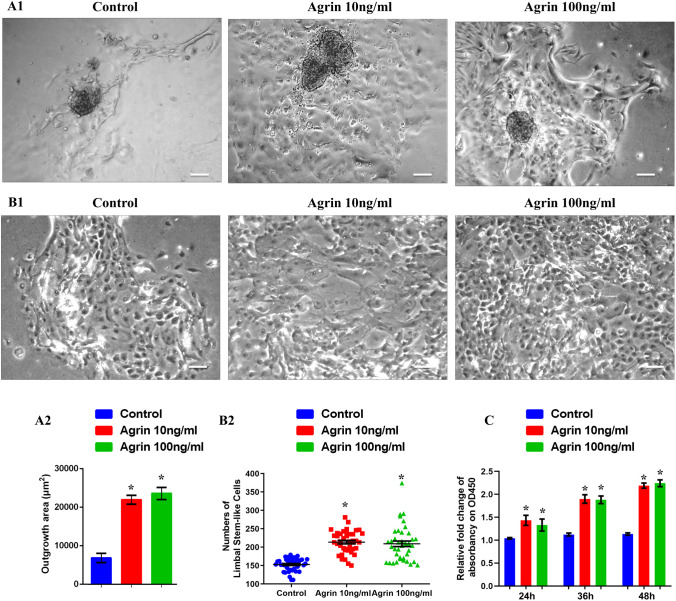
To evaluate the effect of Agrin on LSCEs outgrowth, LSCEs were cultured and treated with different concentrations of Agrin (0, 10, and 100 ng/mL in SHEM) for 5 days. Images of outgrowth area of each LSCEs were daily captured. The results at day 3 indicated that Agrin could promote the outgrowth of LSCEs, although there was no significant difference between 10 and 100 ng/mL Agrin groups (Fig. 1A). In addition, numbers of expanded limbal stem-like cells with prominent nuclei and high nucleus/cytoplasm ratio in outgrowth areas also significantly increased in the presence of Agrin at day 5 (Fig. 1B). Furthermore, the effect of Agrin on proliferation of cultured limbal stem cells was indirectly assessed by a CCK-8 kit. Cultured limbal stem cells were incubated with different concentrations of Agrin (0, 10, and 100 ng/mL in SHEM) on a 96-well-plate for 24, 36, or 48 hours. We found that Agrin could promote limbal stem cells proliferation as determined by the increased cell numbers compared with the control group (Fig. 1C). These results suggested that Agrin could accelerate the outgrowth rate of LSCEs and promoted proliferation of cultured limbal stem cells in vitro.
Figure 1.
Agrin promoted the outgrowth and increased the numbers of cultured limbal stem cells in vitro. (A1) Representative images of limbal stem cell explants treated with different concentrations of Agrin (0, 10 and 100 ng/mL) at day 3, scale bar = 80 µm. (A2) Quantification of the outgrowth area of limbal stem cell explants treated with different concentrations of Agrin (0, 10, and 100 ng/mL) at day 3, n = 6 explants for each group, * P < 0.05 vs. control. (B1) Representative images of expanded limbal stem-like cells with prominent nuclei and high nucleus/cytoplasm ratio in outgrowth areas at day 5, scale bar = 20 µm. (B2) Quantification of limbal stem-like cells numbers at day 5. n = 5 explants for each group (8 microscopic fields for 1 explant), * P < 0.05 vs. control. (C) Quantification of limbal stem cells proliferation indirectly determined by Cell Count Kit-8 after treatment with Agrin (0, 10, and 100 ng/mL) for 24, 36, and 48 hours, the absorbance at 450 nm was measured, n = 5 samples for each group. * P < 0.05 vs. control.
Agrin Promoted the Proliferation of Limbal Stem Cells In Vitro
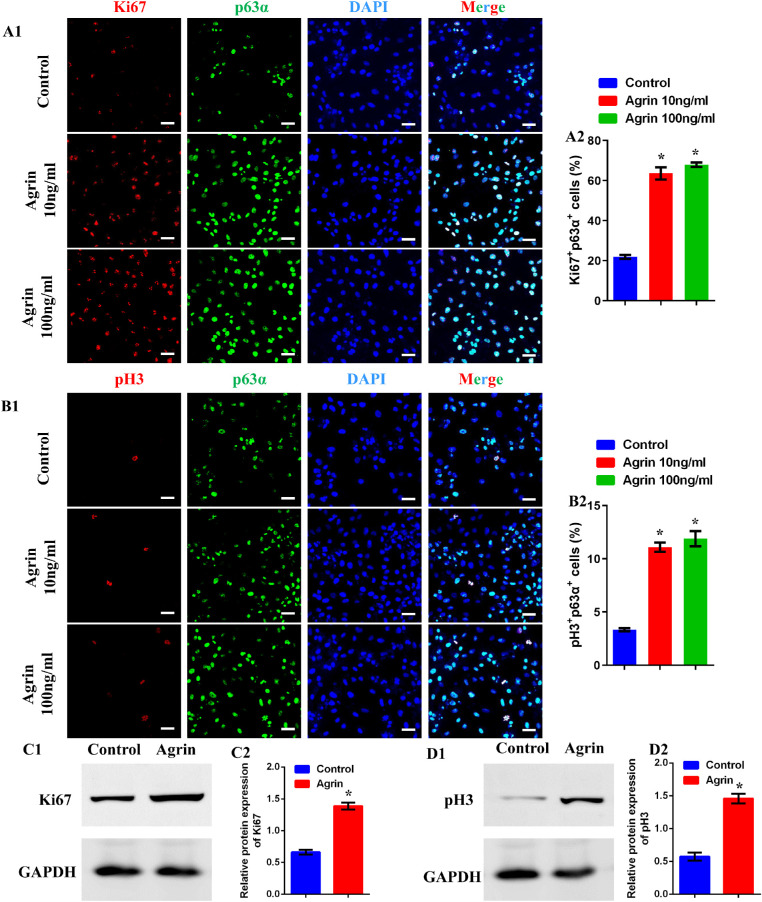
The efficiency of Agrin on limbal stem cell proliferation was assessed through determination of specific proliferative markers, including Ki67 and pH3, and cells co-expressing p63α and proliferating markers were calculated as proliferative limbal stem cells. As shown in Figure 2A and B, there were significantly increased Ki67+ and pH3+ limbal stem cells in Agrin-treated groups, whereas no significant difference was observed between Agrin groups of 10 and 100 ng/mL. Furthermore, the protein levels of Ki67 and pH3 were analyzed by Western blot, indicating significantly increased Ki67 and pH3 expression after Agrin treatment (Fig. 2C and D). The above results suggest the stimulated effect of Agrin on limbal stem cells proliferation in vitro.
Figure 2.
Agrin promoted the proliferation of limbal stem cells in vitro. (A1) Representative images of cultured limbal stem cells stained with DAPI (blue), p63α (green), and Ki67 (red) after treatment with Agrin (0, 10, and 100 ng/mL) for 2 days, scale bar = 20 µm. (A2) Percent of proliferating limbal stem cells (p63α+ Ki67+) in response to 0, 10, and 100 ng/mL Agrin for 2 days, n = 1014, 2696, and 2654 limbal stem cells pooled from five samples (eight microscopic fields for one sample) in 0, 10, and 100 ng/mL Agrin groups. * P < 0.05 vs. control. (B1) Representative images of cultured limbal stem cells stained with DAPI (blue), p63α (green), and pH3 (red) after treatment with Agrin (0, 10, and 100 ng/mL) for 2 days, scale bar = 20 µm. (B2) Percent of proliferating limbal stem cells (p63α+ pH3+) in response to 0, 10, and 100 ng/mL Agrin for 2 days, n = 1022, 2613, and 2640 limbal stem cells pooled from five samples (eight microscopic fields for one sample) in 0, 10, and 100 ng/mL Agrin groups. * P < 0.05 vs. control. (C) Representative images (C1) and quantification (C2) of Ki67 protein expression in limbal stem cells analyzed by Western blotting with or without 10 ng/mL Agrin for 2 days. GAPDH was used as control. n = 6 samples for each group. * P < 0.05 vs. control. (D) Representative images (D1) and quantification (D2) of pH3 protein expression in limbal stem cells analyzed by Western blotting with or without 10 ng/mL Agrin for 2 days. GAPDH was used as control. n = 6 samples for each group. * P < 0.05 vs. control.
Agrin Stimulated a Phenotypic Transition to Proliferation Rather Than Differentiation of Limbal Stem Cells In Vitro
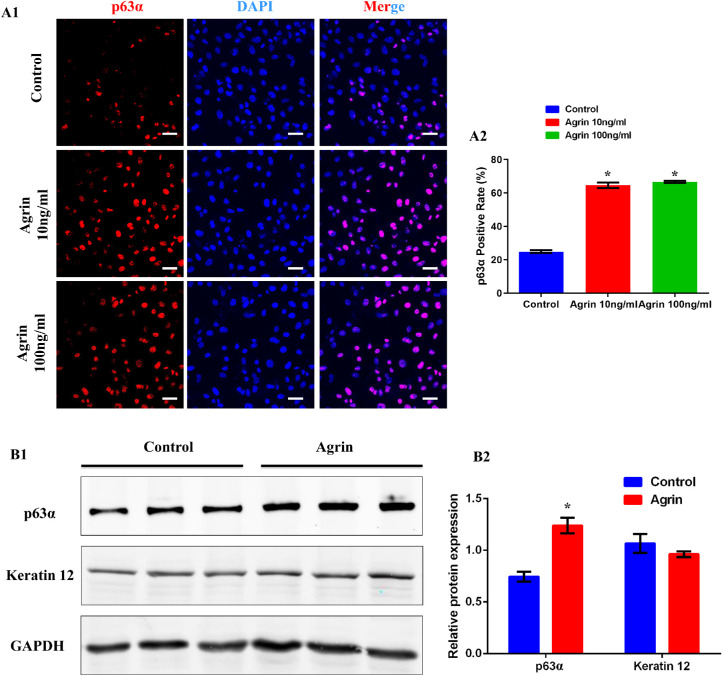
Transcription factor p63, which is present in limbal basal cell layer but absent in central corneal epithelium, is proposed to be a limbal stem cell marker.21,22 Keratin 12 (K12) is a specific marker of corneal epithelial cells.23 According to previous studies, p63 indicates proliferation whereas Keratin 12 indicates differentiation of limbal stem cells during the lineage separation from corneal epithelial progenitor to corneal epithelial cells.23-25 Therefore the effect of Agrin on lineages separation of limbal stem cells was assessed. As shown in Figure 3A, the percentage of p63α+ cells was significantly increased in Agrin-treated groups whereas there was no significant difference between 10 and 100 ng/mL Agrin groups. Further results also confirmed the increased level of p63α protein expression after Agrin treatment, whereas the protein level of K12 showed no significant differences between two groups (Figs. 3B). These results indicated that Agrin stimulated a phenotypic transition to proliferation rather than differentiation of limbal stem cells in vitro.
Figure 3.
Agrin stimulated a phenotypic transition to proliferation rather than differentiation of limbal stem cells. (A1) Representative images of cultured limbal stem cells stained with DAPI (blue) and p63α (red) after treatment with Agrin (0, 10, and 100 ng/mL) for 2 days, scale bar = 20 µm. (A2) Percent of limbal stem cells (p63α+) in response to 0, 10, and 100 ng/mL Agrin for 2 days, n = 4048, 4118, and 4014 cells pooled from five samples (eight microscopic fields for one sample) in 0, 10, and 100 ng/mL Agrin groups. * P < 0.05 vs. control. (B) Representative images (B1) and quantification (B2) of p63α and Keratin 12 protein expression in limbal stem cells analyzed by Western blotting with or without 10 ng/mL Agrin for 2 days. GAPDH was used as control. n = 6 samples for each group. * P < 0.05 vs. control.
Agrin Accelerated Corneal Wound Healing Rate and Promoted the Proliferation of Limbal Stem Cells In Vivo
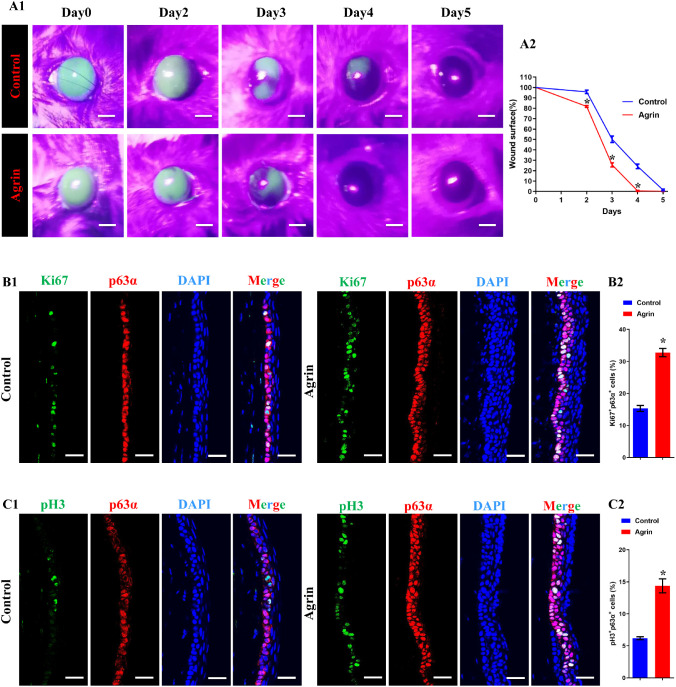
We applied a cornea epithelium wounding model on C57BL/6J mice to identify the effect of Agrin on corneal epithelial wound healing and limbal stem cell proliferation. Central corneal epithelium was carefully removed, and the images of remaining epithelial defects were captured every 24 hours till wound healing completely through fluorescein sodium staining. We found that Agrin treatment could accelerate the healing of corneal epithelial defects (Fig. 4A). In addition, immunostaining of Ki67, pH3 and p63α on corneal sections at day 3 after injury was performed to quantify proliferative limbal stem cells. As shown in Figure 4B and C, there were significantly increased Ki67+ and pH3+ limbal stem cells in Agrin treated groups, suggesting that the therapeutic effect on corneal epithelial defects is based on activating the proliferative ability of endogenous limbal stem cells.
Figure 4.
Agrin accelerated corneal wound healing rate and promoted the proliferation of limbal stem cells in vivo. (A) Representative images (A1) and quantification (A2) of corneal defect areas and wound healing rate, analyzed by fluorescein staining, n = 5 corneas for each group. * P < 0.05 vs. control, scale bar = 1 mm. (B1) Representative images of corneal sections at day 3 after injury stained with DAPI (blue), p63α (red), and Ki67 (green), scale bar = 30 µm. (B2) Percent of proliferating limbal stem cells (p63α+ Ki67+) with or without 10 ng/mL Agrin at day 3 after injury, n = 731 and 3101 limbal stem cells pooled from five samples (eight microscopic fields for one sample from 6–8 sections) in control and Agrin groups. * P < 0.05 vs. control. (C1) Representative images of corneal sections on day 3 after injury stained with DAPI (blue), p63α (red), and pH3 (green), scale bar = 30 µm. (C2) Percent of proliferating limbal stem cells (p63α+ pH3+) with or without 10 ng/mL Agrin at day 3 after injury, n = 758 and 2313 limbal stem cells pooled from five samples (eight microscopic fields for one sample from 6–8 sections) in control and Agrin groups. * P < 0.05 vs. control.
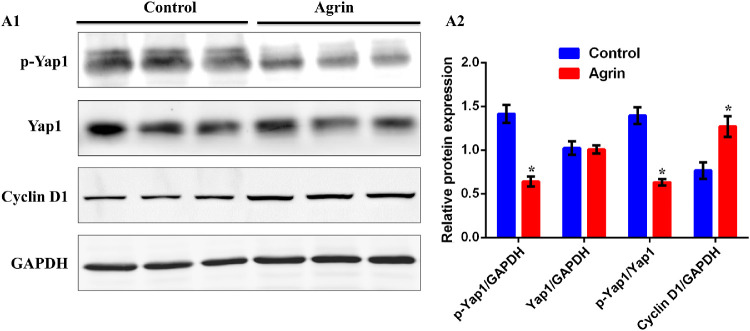
Agrin Could Inhibit the Phosphorylation of Yap1 and Activate Transcription of Cyclin D1
Hippo-Yap pathway is a crucial signaling network implicated in development, oncogenic growth, tissue regeneration and stem cells biology.26 High levels of Hippo signaling will lead to phosphorylation of the downstream factor Yap1 and promote cytoplasmic retention of Yap1.27–30 Although inactive cascade allows dephosphorylated Yap1 to enter the nucleus and bind to members of TEAD family to regulate Cyclin D1 transcription and cell proliferation.31–33 We next determined whether Yap1 activity and localization were involved in Agrin-induced limbal stem cell proliferation. The p-Yap1 (S127) and total Yap1 were detected with Western blot analysis in limbal stem cells. As a result, Agrin could significantly decrease p-Yap1 (S127) expression while not changing total Yap1 expression (Fig. 5). Besides, the expression of Cyclin D1 was significantly increased after Agrin treatment, indicating that the Agrin could upregulate the dephosphorylation of Yap1 and transcription of Cyclin D1, which contributed to limbal stem cell proliferation.
Figure 5.
Agrin could inhibit the phosphorylation of Yap1 and activate transcription of Cyclin D1. (A) Representative images (A1) and quantification (A2) of the protein expression of total Yap1, p-Yap1(S127) and CyclinD1 in limbal stem cells, analyzed by Western blotting with or without 10 ng/mL Agrin for 2 days. n = 6 samples for each group. * P < 0.05 vs. control.
Discussion
Homeostasis and regeneration of corneal epithelium are maintained by limbal stem cells, which are a group of stem cells residing in the Vogt of limbus.9,34–36 Dysfunction or deficiency of limbal stem cells or destruction of niche microenvironment in limbus can lead to LSCD.35 Activated proliferation of endogenous limbal stem cells is crucial to the treatment of LSCD, hence it is necessary to find out an efficient therapeutic strategy to stimulate proliferation of limbal stem cells and regenerate functional limbus after injury. Recent studies have proved that Agrin could promote heart regeneration in mice, maintain functional neuromuscular junctions and regulate oncogenesis, which also locates in ocular surface tissues and enriches in limbus.16,18,37,38 However, the effect of Agrin on corneal repair after LSCD is still unclear. In the present study, we evaluated the role of Agrin on limbal stem cell proliferation and corneal epithelium wound healing both in vitro and in vivo. Agrin exerted a therapeutic effect against LSCD, proved by accelerated corneal wound healing rate and increased proliferation of limbal stem cells.
Over the decades, many efforts had been made to seek for effective ways to promote limbal stem cell proliferation. As previously described, small molecular compounds Y-27632 (a ROCK inhibitor), IIIC3 (an antagonist of the Wnt signaling inhibitor DDK) and IC15 (a Wnt signaling inhibitor), nerve growth factor (NGF), lithium (Li) and Valproic Acid (VA) drugs could regulate limbal epithelial cell proliferation and differentiation through different signaling pathways.39–42 Meanwhile, it has been suggested that human amniotic membrane extract eye drops can increase limbal stem cells proliferation and accelerate corneal epithelium healing.43 However, the current study findings do not yet provide an immediate clinical application; this fact evokes novel strategies in regulating limbal stem cell proliferation and treating LSCD.
Unlike other exogenous compounds, Agrin is an endogenous factor located in the Extracellular Matrices (ECM) of limbal stem cells, which could reduce the potential risk of bringing unexpected effects.16,17 It has been indicated that Agrin reveals a therapeutic effect on myocardial infarction through promoting cardiac regeneration.18 In our present study, we found that Agrin could promote the outgrowth of LSCEs and stimulate the proliferation of limbal stem cells. According to the X+Y=Z theory, maintenance of corneal epithelial cells is sustained by the proliferation and centripetal movement of limbal stem cells.44 It has been widely accepted that limbal stem cells are the source of generated TACs at the limbus; then TACs move to the basal layer of central cornea from peripheral cornea, eventually differentiating into corneal epithelial cells for supplementing corneal epithelial defect and wound healing.45 We performed corneal epithelial debridement and demonstrated the therapeutic effect of Agrin on LSCD via activating the proliferative ability of endogenous limbal stem cells.
During cell division and lineages separation, limbal stem cells can symmetrically divide into two stem cells or two differentiated TACs, or asymmetrically divide into a stem cell and a differentiated TAC.3 TACs can reach the surface of the corneal epithelium layer and differentiate into terminally differentiated cells.3,46 It has been proved that K12 specifically expresses in corneal epithelium instead of limbus epithelial stem cells in adult mouse,23 and K12 can be also observed in corneal epithelium during ocular surface development,44 resulting in regarding K12 as a specific marker of corneal epithelial cells and differentiated limbal stem cells. On the other hand, the nuclear transcription p63α is a specific marker of limbal stem cells and plays a vital role in maintaining stem cell characteristics and high proliferative activity.21,22,24 We found that Agrin could specifically increase p63α instead of K12, suggesting the effect of Agrin on switching differentiation phenotype to proliferation phenotype of limbal stem cells and providing basis sources in treatment of ocular surface disorders.
The Hippo kinase cascade is a potent regulator of cell proliferation and organ size.13 When the Hippo pathway is active, Yap1 is phosphorylated, which prevents its nuclear translocation.47 It has been suggested that Yap1 is essential for maintaining homeostasis of corneal epithelium and nuclear translocation of Yap1 is specifically enriched in corneal limbal stem cells instead of corneal epithelial cells.1 Cyclin D1 is a well-known cell cycle regulator and a transcriptional target of the Hippo-Yap signaling pathway,47 which is also a key factor in regulating limbal stem cell proliferation.48 Our study proved Agrin could inhibit the phosphorylation of Yap1 and modulate the expression of downstream Cyclin D1, which further activating limbal stem cell proliferation.
In conclusion, Agrin promotes the proliferation of limbal stem cells through Hippo-Yap signaling pathway, which is beneficial to the maintenance of normal corneal epithelial homeostasis and the activation of corneal repair. Thus Agrin could be a potential treating target for ocular surface repair and reconstruction in clinical practice.
Acknowledgments
Supported by research grants from the National Key R&D Program of China (2017YFA0103204) and National Natural Science Foundation of China (81770887, 81670830). The authors alone are responsible for the content and writing of the paper.
Disclosure: L. Hou, None; W. Fu, None; Y. Liu, None; Q. Wang, None; L. Wang, None; Y. Huang, None
References
- 1. Kasetti RB, Gaddipati S, Tian S, et al.. Study of corneal epithelial progenitor origin and the Yap1 requirement using keratin 12 lineage tracing transgenic mice. Sci Rep. 2016; 6: 35202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sacchetti M, Rama P, Bruscolini A, et al.. Limbal stem cell transplantation: Clinical results, limits, and perspectives. Stem Cells Int. 2018; 2018: 8086269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Girolamo ND. Moving epithelia: Tracking the fate of mammalian limbal epithelial stem cells. Prog Retin Eye Res. 2015; 48: 203–225 [DOI] [PubMed] [Google Scholar]
- 4. Deng SX, Borderie V, Chan CC, et al.. Global consensus on definition, classification, diagnosis, and staging of limbal stem cell deficiency. Cornea. 2019; 38: 364–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness- A globalperspective. Bull World Health Organ. Bull World Health Organ. 2001; 79: 214–221 [PMC free article] [PubMed] [Google Scholar]
- 6. Kolli S, Ahmad SM, Figueiredo F. Successful clinical implementation of corneal epithelial stem cell therapy for treatment of unilateral limbal stem cell deficiency. Stem Cells. 2010; 28: 597–610 [DOI] [PubMed] [Google Scholar]
- 7. Le Q, Xu J, Deng SX. The diagnosis of limbal stem cell deficiency. Ocul Surf. 2018; 16: 58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dua H, Saini J, Azuarablanco A, et al.. Limbal stem cell deficiency: Concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol. 2000; 48: 83–92. [PubMed] [Google Scholar]
- 9. Ying D, Han P, Lavker RM. Emerging therapeutic strategies for limbal stem cell deficiency. J Ophthalmol. 2018; 2018: 7894647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sati A, Shukla S, Lal I, et al.. Treating limbal stem cell deficiency: Current and emerging therapies. Expert Opin Orphan Drugs. 2015; 3: 619–631. [Google Scholar]
- 11. He H, Yiu S C. Stem cell-based therapy for treating limbal stem cells deficiency: A review of different strategies. Saudi J Ophthalmol. 2014; 28: 188–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hilman D, Gat U.. The evolutionary history of YAP and the Hippo/YAP pathway. Mol Biol Evol. 2011; 28: 2403–2417 [DOI] [PubMed] [Google Scholar]
- 13. Fu V, Plouffe SW, Guan KL. The Hippo pathway in organ development, homeostasis, and regeneration. Curr Opin Cell Biol. 2017; 49: 99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu T, Li Z, Yang Y, et al.. The Hippo/YAP1 pathway interacts with FGFR1 signaling to maintain stemness in lung cancer. Cancer Lett. 2018; 423: 36–46 [DOI] [PubMed] [Google Scholar]
- 15. Li Q, Kasetti RB, Kao W, et al.. Yap1 is required for the corneal epithelial progenitor cell proliferation and expansion. Invest Ophthalmol Vis Sci. 2015; 56: 5609 [Google Scholar]
- 16. Schlötzerschrehardt U, Dietrich T, Saito K, et al.. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res. 2007; 85: 845–860. [DOI] [PubMed] [Google Scholar]
- 17. Chakraborty S, Njah K, Pobbati AV, et al.. Agrin as a mechanotransduction signal regulating YAP through the Hippo pathway. Cell Rep. 2017; 18: 2464–2479 [DOI] [PubMed] [Google Scholar]
- 18. Bassat E, Mutlak YE, Genzelinakh A, et al.. The extracellular matrix protein Agrin promotes heart regeneration in mice. Nature. 2017; 547: 179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Espana EM, Romano AC, Kawakita T, et al.. Novel enzymatic isolation of an entire viable human limbal epithelial sheet. Invest Ophthalmol Vis Sci. 2003; 44: 4275–4281. [DOI] [PubMed] [Google Scholar]
- 20. Stepp MA, Zhu L. Upregulation of alpha 9 integrin and tenascin during epithelial regeneration after debridement in the cornea. J Histochem Cytochem. 1997; 45: 189–201. [DOI] [PubMed] [Google Scholar]
- 21. Pellegrini G, Dellambra E, Golisano O, et al.. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001; 98: 3156–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang A, Schweitzer R, Sun D, et al.. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999; 398: 714–718. [DOI] [PubMed] [Google Scholar]
- 23. Kurpakus MA, Maniaci MT, Esco M. Expression of keratins K12, K4 and K14 during development of ocular surface epithelium. Curr Eye Res. 1994; 13: 805–814. [DOI] [PubMed] [Google Scholar]
- 24. Kawasaki S, Tanioka H, Yamasaki K, et al.. Expression and tissue distribution of p63 isoforms in human ocular surface epithelia. Exp Eye Res. 2006; 82: 293–299. [DOI] [PubMed] [Google Scholar]
- 25. Robertson DM, Su-Inn Ho H., Cavanagh D. Characterization of ΔNp63 isoforms in normal cornea and telomerase-immortalized human corneal epithelial cells. Exp Eye Res. 2008; 86: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fulford A, Tapon N, Ribeiro PS. Upstairs, downstairs: spatial regulation of Hippo signaling. Curr Opin Cell Biol. 2018; 51: 22–32. [DOI] [PubMed] [Google Scholar]
- 27. Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011; 13: 877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dupont S, Morsut L, Aragona M, et al.. Role of YAP/TAZ in mechanotransduction. Nature. 2011; 474: 179–183. [DOI] [PubMed] [Google Scholar]
- 29. Sorrentino G, Ruggeri N, Specchia V, et al.. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014; 16: 357–366. [DOI] [PubMed] [Google Scholar]
- 30. Zhou D, Conrad C, Xia F, et al.. Mst1 and Mst2 Maintain Hepatocyte Quiescence and Suppress Hepatocellular Carcinoma Development through Inactivation of the Yap1 Oncogene. Cancer Cell. 2009; 16: 425–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vassilev A. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001; 15: 1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao B, Wei X, Li X, et al.. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007; 21: 2747–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao B, Ye X, Yu J, et al.. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008; 22: 1962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O'Callaghan AR, Daniels JT. Concise review: Limbal epithelial stem cell therapy: Controversies and challenges. Stem Cells. 2011; 29: 1923–1932. [DOI] [PubMed] [Google Scholar]
- 35. Yin J, Jurkunas U.. Limbal stem cell transplantation and complications. Semin Ophthalmol. 2018; 33: 134–141. [DOI] [PubMed] [Google Scholar]
- 36. Chen D, Qu Y, Hua X, et al.. A hyaluronan hydrogel scaffold-based xeno-free culture system for ex vivo expansion of human corneal epithelial stem cells. Eye (Lond). 2017; 31: 962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma HK, Chen HC, Ma SK, et al.. Preservation of human limbal epithelial progenitor cells on carbodiimide cross-linked amniotic membrane via integrin-linked kinase-mediated Wnt activation. Acta Biomater. 2016; 31: 144–155. [DOI] [PubMed] [Google Scholar]
- 38. Sayan C, Wanjin H.. Linking extracellular matrix agrin to the Hippo pathway in liver cancer and beyond. Cancers (Basel). 2018; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chi-Chin S, Hsiao-Ting C, Yi-Fang L, et al.. Y-27632, a ROCK inhibitor, promoted limbal epithelial cell proliferation and corneal wound healing. PLoS One. 2015; 10: e0144571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. González S, Oh D, Baclagon ER, et al.. Wnt signaling is required for the maintenance of human Limbal stem/progenitor cells In vitro. Invest Ophthalmol Vis Sci. 2019; 60: 107–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kolli S, Bojic S, Ghareeb AE, et al.. The role of nerve growth factor in maintaining proliferative capacity, colony-forming efficiency, and the limbal stem cell phenotype. Stem Cells. 2019; 37: 139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yasemi M, Salouti R, Razmkhah M, et al.. Effect of lithium and valproate on proliferation and migration of limbal epithelial stem/progenitor cells. Curr Eye Res. 2019; 44: 154–161 [DOI] [PubMed] [Google Scholar]
- 43. Asl NS, Nejat F, Mohammadi P, et al.. Amniotic membrane extract eye drop promotes limbal stem cell proliferation and corneal epithelium healing. Cell J. 2019; 20: 459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thoft RA, Friend J.. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983; 24: 1442–1443. [PubMed] [Google Scholar]
- 45. West JD, Dorà NJ, Collinson JM. Evaluating alternative stem cell hypotheses for adult corneal epithelial maintenance. World J Stem Cells. 2015; 7: 281–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Clayton E, Doupé DP, Klein AM, et al.. A single type of progenitor cell maintains normal epidermis. Nature. 2007; 446: 185–189. [DOI] [PubMed] [Google Scholar]
- 47. Li J, Gao E, Vite A, et al.. Alpha-catenins control cardiomyocyte proliferation by regulating Yap activity. Circ Res. 2015; 116: 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fan N-W, Hu T-C, Wu C‐W, et al.. Pigment epithelium-derived factor peptide promotes limbal stem cell proliferation through hedgehog pathway. J Cell Mol Med. 2019; 23: 4759–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]