Abstract
Purpose
Matrix metalloproteinases (MMPs) are involved in extracellular matrix (ECM) maintenance and remodeling. The present study aimed to determine whether transforming growth factor (TGF)-β2 regulates MMP-2 and MMP-9 levels and activities in astrocytes derived from the optic nerve head (ONH) and the role of statins in such modulation.
Methods
Primary astrocytes cultured from the lamina cribrosa of human donor ONHs were incubated with three types of statins (5 µg/mL) for 1 hour followed by recombinant TGF-β2 (5 ng/mL) for various periods to test their effects. Levels and activities of MMP-2 and MMP-9 in astrocytes in vitro were determined by western blotting and zymography, respectively. Levels of phosphorylated myosin phosphatase target subunit 1 (MYPT1) in astrocyte lysates were determined by western blotting, and those of phosphorylated myosin light chain (MLC) were determined by western blotting and immunocytochemistry.
Results
MMP-2 and MMP-9 levels were upregulated by TGF-β2 in human ONH astrocytes. Prior incubation with simvastatin, lovastatin, and atorvastatin inhibited TGF-β2-mediated MMP-2 and MMP-9 expression and activities. Prior incubation with statins downregulated the TGF-β2-induced phosphorylation of MYPT1 and MLC, which are downstream substrates of RhoA and ROCKs.
Conclusions
Statins inhibited the TGF-β2-mediated regulation of MMP-2 and MMP-9 by inhibiting the RhoA/ROCK signaling pathway. Considering the role of MMP in ECM remodeling, the present findings support the notion that statins positively impact ECM remodeling within the ONH.
Keywords: glaucoma, extracellular matrix, statin, matrix metalloproteinase, RhoA, statin
Glaucoma is a neurodegenerative eye disease characterized by damage to the retinal ganglion cell (RGC) axons and subsequent remodeling of the extracellular matrix (ECM) at the lamina cribrosa (LC) of the optic nerve head (ONH) and apoptosis of RGCs at the retina.1–3 RGC axons converge at the ONH to transmit visual signals from the retina to the brain. The LC, which allows the passage of RGC axons, is critical for the health of optic nerves as it provides structural and physiological support. Upon exposure to elevated intraocular pressure (IOP), the LC undergoes ECM remodeling characterized by disorganized elastin deposition along with increased collagen VI, collagen IV, and other basement membrane molecules.4–6 These changes lead to increased stiffness of the LC, which can render this structure more susceptible to mechanical strain.
Remodeling the ECM is a dynamic process that involves the synthesis and degradation of its components. Transforming growth factor (TGF)-β2 is thought to act as a critical regulator of ECM remodeling in the ONH, and it is significantly increased in the ONH and aqueous humor of glaucoma patients.7,8 The source of this increase is thought to be reactive astrocytes in the ONH.7,8 Astrocytes, the major glial cells within the LC, are thought to be responsible for ECM remodeling in response to TGF-β2.9 In ONH astrocyte cultures, TGF-β2 induces the expression of ECM molecules, including collagen I, collagen IV, fibronectin, plasminogen activator inhibitor (PAI-1), and connective tissue growth factor (CTGF).10–12 However, the function of TGF-β2 in ECM production and degradation is complicated. It has been found to enhance the levels and activation of matrix metalloproteinase-2 (MMP-2), tissue inhibitor of metalloproteinase-2, and PAI-1 in human trabecular meshwork (TM) cells, indicating the parallel induction of ECM production and degradation.13 The roles of MMP in fibrotic diseases, metastatic tumors, and glaucoma have been described. The altered expression of several MMPs in patients with glaucoma and in experimental animal models suggests that MMP functions in glaucoma.14–16 Immunoreactivity to MMP-2 is increased in the ONH of patients with glaucoma.15 Cyclic stretching induces MMP-2 expression and activity in glial cells of the ONH in vitro.17,18 Levels of MMP-9 protein are elevated in proliferating glial cells in the optic nerves of patients with angle-closure glaucoma.19
Statins are 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitors that interrupt cholesterol biosynthesis and are therefore widely prescribed for cardiovascular diseases. Statins not only lower cholesterol but also exert pleiotropic effects such as the regulation of fibrosis, inflammation, and immune response. Statins are associated with a low risk of primary open-angle glaucoma and exert beneficial effects on glaucoma.20,21 The progression of glaucoma is delayed in patients medicated with statins compared with controls.22 Lovastatin suppresses the TGF-β2-mediated expression of secreted protein acidic and rich in cysteine (SPARC) in human TM cells.23 We previously reported that statins exert antifibrotic effects by suppressing the TGF-β2-mediated expression of ECM molecules in human astrocytes derived from the LC of the ONH.12 The ability of statins to modulate TGF-β2 can provide insight into the mechanisms of action on ONH astrocytes. The present study aimed to establish whether TGF-β2 regulates MMP-2 and MMP-9 levels and activities in ONH astrocytes. We also aimed to determine whether statins modulate MMP regulation via TGF-β2 and, if so, the underlying mechanism(s).
Methods
Cell Culture
Written informed consent was obtained from family members of Asian donors with no history of ocular disease to harvest eyes within 10 hours of death (28–58 years of age). The Institutional Review Board at Asan Medical Center approved the protocol of the study, which complied with the tenets of the Declaration of Helsinki. Astrocytes were prepared from the ONH as described.12,24 Briefly, the LC was dissected from neighboring tissues and chopped into small explants. Explants were allowed to attach onto 25-cm2 Falcon Tissue Culture Treated Flasks (Thermo Fisher Scientific, Waltham, MA, USA) in the presence of minimal amounts of media. When outgrowth was initiated, the volume of medium was increased to 2 mL. Explants were cultured and maintained in Dulbecco's Modified Eagle's Medium: Nutrient Mixture F-12 supplemented with 10% fetal bovine serum (FBS) (Gibco, Thermo Fisher Scientific), 5 ng/mL human basic fibroblast growth factor (Sigma-Aldrich, Darmstadt, Germany), 5 ng/mL human platelet-derived growth factor A-chain (Sigma-Aldrich), 50 U/mL penicillin (Gibco, Thermo Fisher Scientific), and 50 g/mL streptomycin (Gibco, Thermo Fisher Scientific). The medium was changed every 3 to 4 days. After the cells reached confluence, they were trypsinized and seeded into new plates at a density of 0.8 ∼ 1.5 × 10e5 cells/cm2 in serum-free astrocyte growth medium (AGM) (ScienCell Research Laboratories, Carlsbad, CA, USA) for 24 hours to isolate ONH astrocytes. Floating cells were removed after 24 hours, and adherent ONH astrocytes were maintained in AGM containing 5% FBS.
Western Blot Analysis
For MMP-2 and MMP-9, astrocytes were serum-starved overnight and then incubated with or without statins (5 µg/mL) for 1 hour followed by TGF-β2 (5 ng/mL) for 24 hours. Cell debris was removed from conditioned medium by centrifugation at 300×g for 10 minutes. Thereafter, the amounts of MMP-2 and MMP-9 secreted into the medium were measured with western blotting using 25 µL of supernatant. For western blotting for myosin phosphatase target subunit 1 (MYPT1) or myosin light chain (MLC), serum-starved astrocytes were incubated with or without statins (5 µg/mL) for 1 hour followed by TGF-β2 (5 ng/mL) for 30 minutes (MYPT1) or for 6 hours (MLC). Vehicle (dimethyl sulfoxide [DMSO], 0.1%) was added to cells that were not incubated with statins. Whole cell lysates were prepared from cultured cells using RIPA Lysis and Extraction Buffer (Thermo Fisher Scientific). Protein concentrations were determined in 20- to 25-µg extracts using Bradford reagent (Bio-Rad, Hercules, CA, USA). Extracts were denatured by boiling in SDS sample buffer, separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), blotted onto nitrocellulose membranes, and, finally, incubated with antibodies specific to MMP-2 (ab37150) and MMP-9 (ab38898) (Abcam, Cambridge, UK) or phosphorylated MYPT1 (T696), MYPT1, pMLC (pMLC-S19, pMLC-T18S19), and MLC (D18E2) (Cell Signaling Technology, Danvers, MA, USA) diluted in I-Block (Bio-Rad). Thereafter, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (GeneTex, Hsinchu, Taiwan) and developed using an enhanced chemiluminescence reagent (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Band intensity was measured using ImageJ 1.48 software (National Institutes of Health, Bethesda, MD, USA). Briefly, saved grayscale images of films were inverted to visualize white bands. Regions of interest (ROIs) were selected to cover the largest bands, and these were all measured using the same ROIs. Background intensity was subtracted from band intensity. Proteins on the membranes were washed and stained with Coomassie Brilliant Blue R-250 (VWR, Solon, OH, USA) as a loading control for MMP-2 and MMP-9. The intensities of MMP-2 (72 kDa) and MMP-9 (92 and 82 kDa) bands were normalized with those of the Coomassie Brilliant Blue stain, and relative values are shown with respect to untreated (Fig. 1) or DMSO vehicle only (Fig. 2). The intensities of the pMYPT1(140 kDa) or pMLC (18 kDa) bands were normalized with those of MYTP1 or MLC, and relative values are presented with respect to untreated (Figs. 4B, 5B) or DMSO vehicle only (Figs. 4D, 5D).
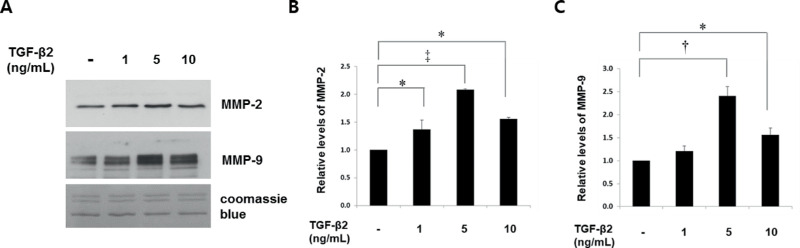
Figure 1.
TGF-β2 induces MMP-2 and MMP-9 expression in astrocytes cultured from human ONH. Levels of MMP-2 and MMP-9 in conditioned media were determined after incubation with TGF-β2 for 24 hours. Representative immunoblots (A) and quantitation of (B) MMP-2 and (C) MMP-9 expression are shown. TGF-β2 began to induce MMP-2 (∼72 kDa) at 1 ng/mL with maximal (twofold) induction at 5 ng/mL; it also maximally induced MMP-9 (∼92 kDa) by 2.4-fold at 5 ng/mL (*P < 0.05, †P < 0.01, ‡P < 0.001 vs. untreated; n = 4).
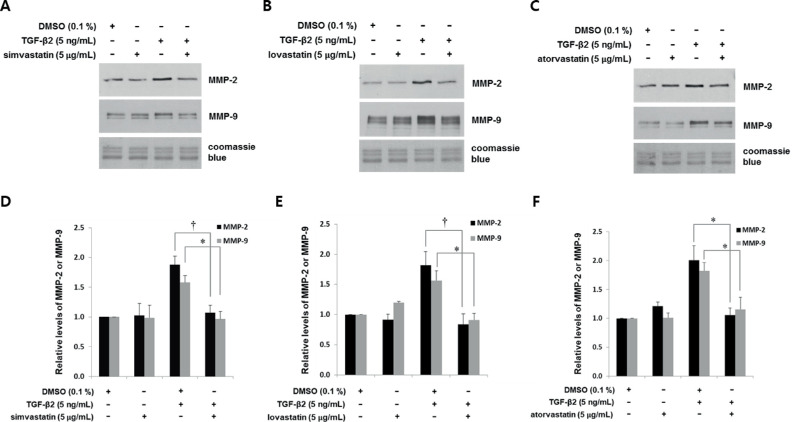
Figure 2.
Statins suppress TGF-β2-mediated induction of MMP-2 and MMP-9 in astrocytes from human ONH. Astrocytes were incubated with simvastatin (A, D), lovastatin (B, E), and atorvastatin (C, F) (5 µg/mL) for 1 hour, followed by TGF-β2 (5 ng/mL) for 24 hours. Proteins in conditioned medium were assessed by western blotting. Representative immunoblots (A–C) and quantitation (D–F) show that TGF-β2 induced MMP-2 and MMP-9 approximately 1.5- to 2-fold. Prior incubation with statins similarly suppressed the TGF-β2-mediated induction of MMP-2 and MMP-9 to basal levels. Equivalent gel loading was determined by Coomassie Brilliant Blue staining. Data are shown as fold changes relative to DMSO controls. Values were compared between TGF-β2 with and without statins (*P < 0.05, †P < 0.01 vs. DMSO; n = 3).
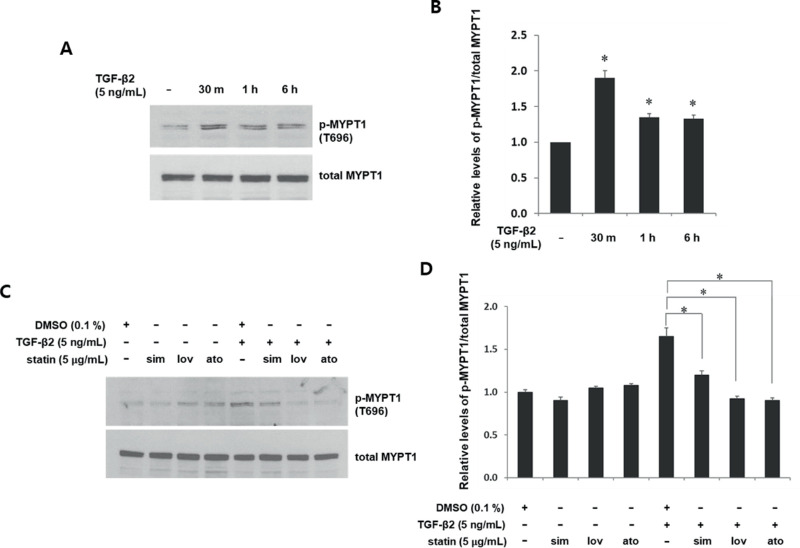
Figure 4.
Statins suppress TGF-β2-induced MYPT1 phosphorylation in astrocytes from human ONH. Serum-starved astrocytes were incubated with TGF-β2 (5 ng/mL) for the indicated periods without (A, B) or with (C, D) prior incubation with simvastatin, lovastatin, and atorvastatin (5 µg/mL) for 1 hour followed by TGF-β2 (5 ng/mL) for 30 minutes. Control cells without statins were incubated with DMSO (0.1%). Phosphorylated MYPT1 at T696 was assessed in cell lysates by western blotting. Data are presented as fold changes relative to DMSO controls. Phosphorylation of MYPT1 induced by TGF-β2 within 30 minutes persisted for 6 hours (B, *P < 0.05 vs. untreated; n = 3). Prior incubation with each statin suppressed TGF-β-mediated MYPT1 phosphorylation (D, *P < 0.05 vs. TGF-β2 + DMSO; n = 3).
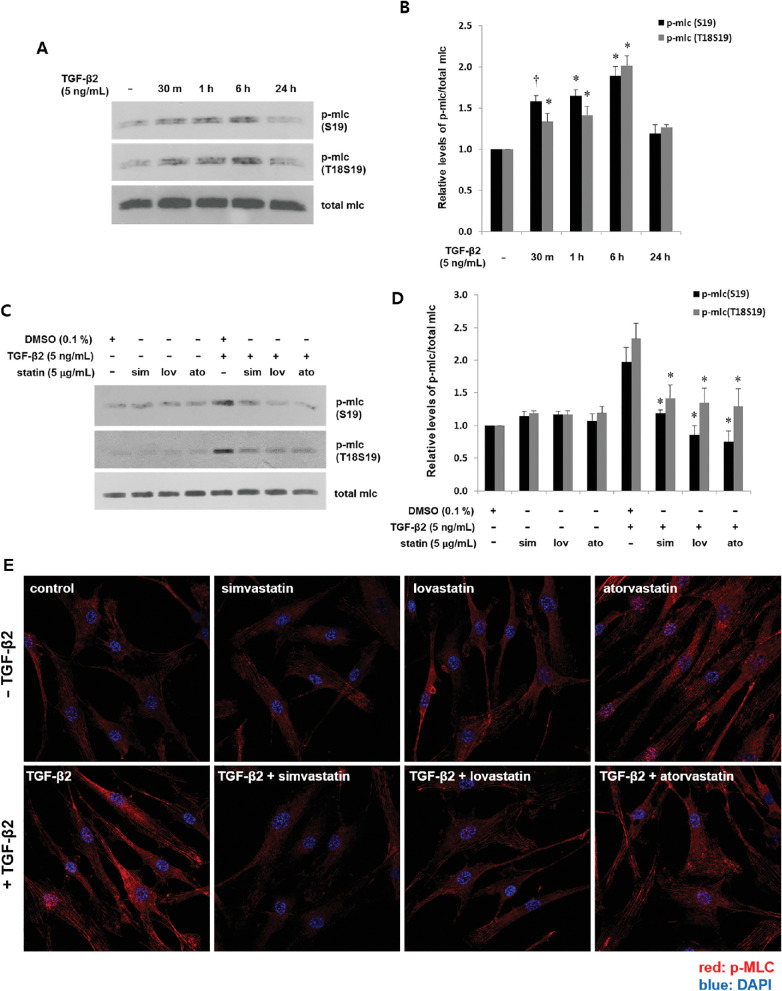
Figure 5.
Statins suppress TGF-β2-induced phosphorylation of MLC in astrocytes from human ONH. Serum-starved astrocytes were incubated with TGF-β2 (5 ng/mL) for the indicated periods without (A, B) or after (C, D) prior incubation with simvastatin, lovastatin, and atorvastatin (5 µg/mL) for 1 hour followed by TGF-β2 (5 ng/mL) for 6 hours. Amounts of pMLC at S19 and at T18S19 were determined by western blotting and are presented as fold changes relative to untreated (B) or DMSO (D). Compared with untreated cells, MLC phosphorylation was induced within 30 minutes and peaked at 6 hours (A, B, *P < 0.001, †P < 0.05 vs. untreated; n = 3). Effects of statins on pMLC were compared between cells incubated with or without statins, followed by TGF-β2. Statins suppressed TGF-β2-mediated phosphorylation of MLC (D, *P < 0.05 vs. TGF-β2 + DMSO; n = 3). (E) Cells were incubated with TGF-β2 (5 ng/mL) without or with prior incubation with statins (5 µg/mL) for 1 hour, then stained with pMLC (S19) antibody. Cell nuclei were counterstained with DAPI. Specific cytoplasmic immunoreactivity for pMLC was increased by TGF-β2 and suppressed by statins.
Gelatin Zymography
Equal amounts (20 µL) of conditioned media collected from cultured astrocytes were mixed with 2× SDS sample buffer and left at room temperature for 5 minutes. Samples were separated with 10% SDS-PAGE using gelatin zymogram gels and buffers (Invitrogen, Carlsbad, CA, USA) containing 0.1% gelatin. Thereafter, the gels were washed in renaturation buffer for 30 minutes with gentle shaking, followed by developing buffer for 30 minutes at room temperature and then with the same buffer at 37°C overnight. The gels were stained with Coomassie Brilliant Blue R-250 (VWR) and subsequently decolorized with 40% methanol in 10% acetic acid. Equal amounts (10 µL) of conditioned media were resolved on separate gels and stained with Coomassie Brilliant Blue as a control. Stained gels were placed between transparent films, and images were acquired using a digital camera.
Immunocytochemistry
Astrocytes were seeded onto 12-mm glass coverslips placed in 24-well plates containing growth medium; then, this medium was removed, and the cells were allowed to incubate in serum-free medium overnight. The astrocytes were then incubated with 0.1% DMSO (vehicle) or with statins (5 µg/mL) for 1 hour followed by incubation with TGF-β2 (5 ng/mL) for 1 hour. The cells were then fixed using 4% paraformaldehyde and permeabilized with PBS containing 0.2% Triton X-100 (Sigma-Aldrich). Cells were incubated overnight at 4°C with a pMLC (S19) antibody (Cell Signaling Technology) in PBS containing 2% BSA. After several washes with PBS, the cells were incubated with cy-3-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) in PBS containing 2% BSA for 1 hour at room temperature. Cell nuclei were counterstained with mounting media containing 1 µg/mL of 4′,6-diamidino-2-phenylinodole (DAPI) (Thermo Fisher Scientific). Images were acquired using an LSM710 confocal microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) and processed using Zeiss ZEN 2012 imaging software.
Statistical Analysis
Unpaired groups were compared using independent sample t-tests, with P < 0.05 being considered statistically significant. All experiments were conducted at least in triplicate, and values are expressed as means ± SEM.
Results
Regulation of MMP-2/MMP-9 Expression by TGF-β2 in Human ONH Astrocytes
MMP-2 (∼72 kDa) and MMP-9 (∼92 kDa) expression levels were significantly and maximally upregulated with 5 ng/mL of TGF-β2 (Fig. 1A). MMP-2 and MMP-9 levels were induced 2-fold (P < 0 .001; Fig. 1B) and 2.4-fold (P < 0.01; Fig. 1C) with 5 ng/mL of TGF-β2.
Statins Regulated TGF-β2-Mediated MMP-2/MMP-9 Expression and Activity
Whereas TGF-β2 enhanced MMP-2 1.5- to 2-fold, simvastatin, lovastatin, and atorvastatin suppressed MMP-2 induction to basal levels (1.9-, 1.8-, and 2.0-fold, respectively; Figs. 2D–2F). Similarly, TGF-β2 enhanced MMP-9 levels, whereas simvastatin, lovastatin, and atorvastatin treatment for 1 hour suppressed the subsequent induction of MMP-9 to basal levels (1.5-, 1.5-, and 2-fold, respectively; Figs. 2D–2F). All three statins had equivalent inhibitory capacity with respect to MMP-2 or MMP-9 suppression.
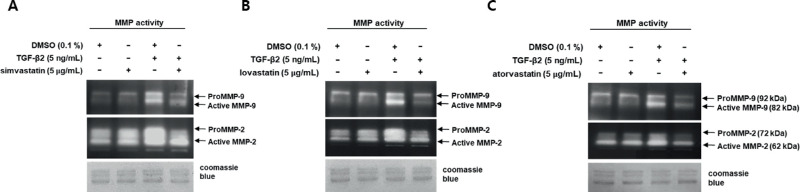
We assessed MMP-2 and MMP-9 protease activities and the ability of simvastatin, lovastatin, and atorvastatin to suppress them (Figs. 3A–3C). Because MMP-2 and MMP-9 belong to the same family of proteases that utilize gelatin as a substrate, we assessed their activities using gelatin zymography. Both MMP-2 and MMP-9 were resolved as two bands each, because MMPs are synthesized as pro-forms that are converted to active forms through proteolytic cleavage of the pro-domains.25 Bands that resolved at 92 and 82 kDa corresponded to pro- and active MMP-9, respectively, and those at 72 and 62 kDa corresponded to pro- and active MMP-2, respectively.
Figure 3.
Statins suppress TGF-β2-mediated MMP-2 and MMP-9 activities in astrocytes from human ONH. Serum-starved astrocytes were incubated with or without statins, following TGF-β2 treatment. Activities of MMP in 20 µL of conditioned media from astrocyte cultures were determined by gelatin zymography. Clear bands on representative gelatin zymograms (n = 3) indicate gelatin degradation by MMP. Bands at 72 and 62 kDa correspond to pro-MMP-2 and active MMP-2, respectively, and those at 92 and 82 kDa correspond to pro-MMP-9 and active MMP-9, respectively. Activities of MMP-2 and MMP-9 were induced by TGF-β2. Prior incubation with statins inhibited TGF-β2-mediated activities of MMP-2 and MMP-9. Gel loading was confirmed by Coomassie Brilliant Blue staining.
The activities of pro-MMP-2 and active MMP-2 were increased by TGF-β2, but prior incubation with statins suppressed these increases (Fig. 3, middle panel). TGF-β2 induced protease activities of active and pro-MMP-9 with more robust induction of active MMP-9, but statins effectively suppressed these activities (Fig. 3, top panel).
TGF-β2-Mediated Activation of Downstream of RhoA/ROCK and Its Regulation by Statins
Statins inhibit RhoA/ROCK pathways by interrupting the synthesis of isoprenoid intermediates, which is necessary for RhoA activation.26 In addition, an association between TGF-β2 and RhoA/ROCK has been suggested. A ROCK inhibitor has prevented TGF-β2-mediated CTGF and collagen I expression.27,28 Thus, we questioned whether statins intercede in TGF-β2-mediated MMP expression and activity by inhibiting RhoA/ROCK pathways. We initially examined RhoA/ROCK activation in human ONH astrocytes incubated with TGF-β2 by assessing pMYPT1, a downstream effector of ROCK.29 We found that TGF-β2 induced MYPT1 phosphorylation approximately twofold within 30 minutes, and this persisted for up to 6 hours (Figs. 4A, 4B). We then assessed the effects of statins on TGF-β2-mediated phosphorylation of MYPT1. Cells were incubated with TGF-β2 alone or with statins followed by TGF-β2. Compared with TGF-β2, statins significantly suppressed the TGF-β2-mediated phosphorylation of MYPT1 (Figs. 4C, 4D). The suppressive effects of the three statins did not differ significantly.
To further confirm RhoA/ROCK activation by TGF-β2, we assessed the phosphorylation of MLC, another substrate of ROCK.30 We found that TGF-β2 induced MLC phosphorylation, which was consistent with that of MYPT. Activation peaked at 6 hours, which was slightly slower than for p-MYPT1 (Figs. 5A, 5B). Next, the cells were incubated with simvastatin, lovastatin, and atorvastatin for 1 hour followed by TGF-β2 for 6 hours. All three statins significantly suppressed the TGF-β2-mediated phosphorylation of MLC, whereas none of them had any significant individual effects on phosphorylation (Figs. 5C, 5D). Finally, we incubated cells with either TGF-β2 or with or without prior incubation with statin, then immunostained the cells for pMLC (S19). Consistent with the western blotting findings, TGF-β2 increased pMLC-specific immunoreactivity throughout the cytoplasm, which was inhibited by prior incubation with statins (Fig. 5E).
Discussion
The matrix protease activities of MMP might be involved in dysregulated ECM remodeling, which is a pathophysiological characteristic of glaucoma. The findings of changed MMP expression and activities in the optic nerve, retina, and aqueous humor of patients with glaucoma, as well as in animal models, support this notion.31 MMP-2 expression is increased in the ONH of patients with glaucoma and in rat glaucoma models.15,32 The present study investigated the regulation of MMP by TGF-β2, which is regarded as a glaucomatous insult. Our findings showed that the expression and activities of MMP-2 and MMP-9 were enhanced by TGF-β2 in astrocytes in the ONH (Figs. 1, 3), indicating another pathway through which TGF-β2 promotes glaucomatous ECM dysregulation in the ONH. The fact that MMP-2 and MMP-9 increase TGF-β bioavailability by releasing it from inhibitory domains or matrix molecules33,34 renders this finding notable. Similar regulation in astrocytes of the ONH would imply that this mechanism amplifies ECM dysregulation via a positive feedback loop. This is an intriguing matter for future exploration.
Here, we showed that three statins suppressed TGF-β2-mediated expression and MMP-2/MMP-9 activities (Figs. 2, 3). TGF-β2-induced phosphorylation of the downstream RhoA/ROCK substrates MYPT1 and MLC (Figs. 4A, 4B; Figs. 5A, 5B), but three statins suppressed it (Figs. 4C, 4D; Figs. 5C–5E). These data suggest that statins modulate TGF-β2 action by suppressing RhoA/ROCK signaling pathways. This notion could be validated by investigating the suppression of TGF-β2-mediated expression of MMPs with statins in the presence of constitutively active RhoA. If statins act on RhoA, expression of the constitutively active RhoA would prevent the statins from interfering with the TGF-β2-mediatd expression of MMP.
We previously reported that statins modulate TGF-β2-mediated expression of ECM components by suppressing the Smad pathway,12 and others have described cross-talk between RhoA and Smad mediated by TGF-β2. Fasudil, a ROCK inhibitor, suppresses TGF-β2-dependent-Smad4 translocation and CTGF gene expression in cultured hyalocytes.27 Thus, statins might modulate cross-talk between RhoA and the TGF-β2-dependent Smad pathway. In contrast, simvastatin and a ROCK inhibitor could inhibit TGF-β2-dependent collagen I expression through both Smad-dependent and -independent pathways in human retinal pigment epithelial cells.28 Therefore, statin-induced RhoA/ROCK inhibition might modulate TGF-β2-mediated MMP-2 and MMP-9 expression independently of Smad pathways. These speculations could be substantiated by revealing whether Smad activation is necessary for TGF-β2-mediated MMP expression using dominant-negative Smad4 that lacks a C-terminal domain.35 If Smad4 is necessary for TGF-β2-mediated MMP expression, then the modulation of Smad4 via the RhoA/ROCK pathway could be confirmed based on whether the ROCK inhibitor blocks TGF-β2-mediated Smad4 activation.
Among glial cells in the ONH, astrocytes and LC cells are responsible for the integrity of LC and ECM remodeling upon exposure to excessive mechanical stress.2 As in astrocytes, TGF-β2 induces expression of the ECM components, fibronectin, and PAI-1 in LC cells.11 Cyclic mechanical stretch induces MMP-2 expression and activity in LC cells.17,18 Considering this functional similarity, the role of statins in TGF-β2-mediated MMP expression and activity in LC cells should be determined in the forthcoming study.
Given that RhoA/ROCK inhibitors are beneficial in the context of glaucoma, they have been considered a potential therapy for this disease. A ROCK inhibitor has reduced IOP and enhanced outflow facility due to changes in stress fiber formation, focal adhesion formation, cellular morphology, and behaviors in TM and Schlemm canal cells.36,37 A ROCK inhibitor has also suppressed the TGF-β2- or lysophosphatidic acid-mediated increase in fibronectin, laminin, and α-SMA in TM cells.38 Here, we showed that RhoA/ROCK is activated by TGF-β2 and that statins can inhibit RhoA activation. These data provide further support for the role of RhoA in ONH remodeling and for statins as a potential glaucoma therapy. Synergistic effects could occur by combining statins and RhoA/ROCK inhibitors.
Taken together, our data provide insights that will help determine the mechanism of TGF-β2-mediated ECM remodeling in astrocytes of the ONH. TGF-β2 orchestrates ECM remodeling by modulating degradation and by influencing the production of ECM components. Our data also support the notion that statins have the potential to treat glaucoma.
Acknowledgments
Supported by the Cheil-Nam Myung Foundation Research Fund and the Basic Science Research Program through the National Research Foundation of Korea, which is funded by the Ministry of Education, Science and Technology (No. NRF-2017R1A2B4007792).
Disclosure: M.-L. Kim, None; K.R. Sung, None; J. Kwon, None; J.A. Shin, None
References
- 1. Weinreb RN, Leung CKS, Crowston JG, et al.. Primary open-angle glaucoma. Br J Ophthalmol Nat Rev. 2016; 2: 1–19. [DOI] [PubMed] [Google Scholar]
- 2. Hernandez MR, Ye H. Glaucoma: changes in extracellular matrix in the optic nerve head. Ann Med. 1993; 25: 309–315. [DOI] [PubMed] [Google Scholar]
- 3. Guo L, Moss SE, Alexander RA, Ali RR, Fitzke FW, Cordeiro MF. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest Ophthalmol Vis Sci. 2005; 46: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pena JD, Netland PA, Vidal I, Dorr DA, Rasky A, Hernandez MR. Elastosis of the lamina cribrosa in glaucomatous optic neuropathy. Exp Eye Res. 1998; 67: 517–524. [DOI] [PubMed] [Google Scholar]
- 5. Quigley HA, Brown A, Dorman-Pease ME. Alterations in elastin of the optic nerve head in human and experimental glaucoma. Br J Ophthalmol. 1991; 75: 552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hernandez MR, Andrzejewska WM, Neufeld AH. Changes in the extracellular matrix of the human optic nerve head in primary open-angle glaucoma. Am J Ophthalmol. 1990; 109: 180–188. [DOI] [PubMed] [Google Scholar]
- 7. Pena JD, Taylor AW, Ricard CS, Vidal I, Hernandez MR. Transforming growth factor beta isoforms in human optic nerve heads. Br J Ophthalmol. 1999a; 83: 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Picht G, Welge-Luessen U, Grehn F, Lutjen-Drecoll E. Transforming growth factor beta 2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefes Arch Clin Exp Ophthal. 2001; 239: 199–207. [DOI] [PubMed] [Google Scholar]
- 9. Hernandez MR, Pena JD.. The optic nerve head in glaucomatous optic neuropathy. Arch Ophthalmol. 1997; 115: 389–395. [DOI] [PubMed] [Google Scholar]
- 10. Fuchshofer R, Birke M, Welge-Lussen U, Kook D, Lutjen-Drecoll E. Transforming growth factor-beta 2 modulated extracellular matrix component expression in cultured human optic nerve astrocytes. Invest Ophthalmol Vis Sci. 2005; 46: 568–578. [DOI] [PubMed] [Google Scholar]
- 11. Zode GS, Sethi A, Brun-Zinkernagel AM, Chang IF, Clark AF, Wordinger RJ. Transforming growth factor-beta 2 increases extracellular matrix proteins in optic nerve cells via activation of the Smad signaling pathway. Mol Vis. 2011; 17: 1745–1758. [PMC free article] [PubMed] [Google Scholar]
- 12. Kim ML, Sung KR, Shin JA, Yoon JY, Jang J. Statins reduce TGF-beta2-modulation of the extracellular matrix in cultured astrocytes of the human optic nerve head. Exp Eye Res. 2017; 164: 55–63. [DOI] [PubMed] [Google Scholar]
- 13. Han H, Kampik D, Grehn F, Schlunck G. TGF-β2-induced invadosomes in Human Trabecular Meshwork Cells. PLoS One. 2013; 8: e70595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agapova OA, Ricard CS, Salvador-Silva M, Hernandez MR. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human optic nerve head astrocytes. Glia. 2001; 33: 205–216. [DOI] [PubMed] [Google Scholar]
- 15. Yan X, Tezel G, Wax MB, Edward DP. Matrix metalloproteinases and tumor necrosis factor alpha in glaucomatous optic nerve head. Arch Ophthalmol. 2000; 118: 666–673. [DOI] [PubMed] [Google Scholar]
- 16. Agapova OA, Kaufman PL, Lucarelli MJ, Gabelt BT, Hernandez MR. Differential expression of matrix metalloproteinases in monkey eyes with experimental glaucoma or optic nerve transection. Brain Res. 2003; 967: 132–143. [DOI] [PubMed] [Google Scholar]
- 17. Kirwan RP, Crean JK, Fenerty CH, Clark AF, O'Brien CJ. Effect of cyclical mechanical stretch and exogenous transforming growth factor-beta1 on matrix metalloproteinase-2 activity in lamina cribrosa cells from the human optic nerve head. J Glaucoma. 2004; 13: 327–334. [DOI] [PubMed] [Google Scholar]
- 18. Quill B, Docherty NG, Clark AF, O'Brien CJ. The effect of graded cyclic stretching on extracellular matrix-related gene expression profiles in cultured primary human lamina cribrosa cells. Invest Ophthalmol Vis Sci. 2011; 52: 1908–1915. [DOI] [PubMed] [Google Scholar]
- 19. Zalewska R, Reszec J, Kisielewski W, Mariak Z. MMP-9 and TIMP-1 expression in retina and optic nerve in absolute angle closure glaucoma. Adv Med Sci. 2016; 61: 6–10. [DOI] [PubMed] [Google Scholar]
- 20. Stein JD, Newman-Casey PA, Talwar N, Nan B, Richards JE, Musch DC. The relationship between statin use and open-angle glaucoma. Ophthalmology. 2012; 119: 2074–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGwin G, McNeal S, Owsley C, Girkin C, Epstein D, Lee PP. Statins and other cholesterol-lowering medications and the presence of glaucoma. Arch Ophthalmol. 2004; 122: 822–826. [DOI] [PubMed] [Google Scholar]
- 22. De Castro DK, Punjabi OS, Bostrom AG, Stamper RL, Lietman TM, Ray K, Lin SC. Effect of statin drugs and aspirin on progression in open-angle glaucoma suspects using confocal scanning laser ophthalmoscopy. Clin Exp Ophthalmol. 2007; 35: 506–513. [DOI] [PubMed] [Google Scholar]
- 23. Villarreal G Jr, Chatterjee A, Oh SS, Oh DJ, Rhee DJ. Pharmacological regulation of SPARC by lovastatin in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2014; 55: 1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shin JA, Kim NW, Kang SS, Kim ML, Sung KR. In vitro Effects of Prostaglandin Analogs on Cultured Astrocytes Obtained from the Lamina Cribrosa. Curr Eye Res. 2016; 41: 676–682. [DOI] [PubMed] [Google Scholar]
- 25. Löffek S, Schilling O, Franzke CW. Biological role of matrix metalloproteinases: a critical balance. Eur Respir J. 2011; 38: 191–208. [DOI] [PubMed] [Google Scholar]
- 26. Pokrovskaya O, Wallace D, O'Brien C. The emerging role of statins in glaucoma pathological mechanisms and therapeutics. Open J Ophthalmol. 2014; 4: 124–138. [Google Scholar]
- 27. Kita T, Hata Y, Kano K, et al.. Transforming growth factor-beta2 and connective tissue growth factor in proliferative vitreoretinal diseases: possible involvement of hyalocytes and therapeutic potential of Rho kinase inhibitor. Diabetes. 2007; 56: 231–238. [DOI] [PubMed] [Google Scholar]
- 28. Itoh Y, Kimoto K, Imaizumi M, Nakatsuka K. Inhibition of RhoA/Rho-kinase pathway suppresses the expression of type I collagen induced by TGF-beta2 in human retinal pigmental epithelial cells. Exp Eye Res. 2007; 84: 464–472. [DOI] [PubMed] [Google Scholar]
- 29. Kimura K, Ito M, Amano M, Chihara K, et al.. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science. 1996; 273: 245–248. [DOI] [PubMed] [Google Scholar]
- 30. Amano M, Ito M, Kimura K, Fukata Y, Chihara K, et al.. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem. 1996; 271: 20246–20249. [DOI] [PubMed] [Google Scholar]
- 31. Groef LD, Hove IV, Dekeyster E, Stalmans I, Moons L. MMPs in the neuroretina and optic nerve: modulators of glaucoma pathogenesis and repair? Invest. Ophthalmol. Vis. Sci. 2014; 55: 1953–1964. [DOI] [PubMed] [Google Scholar]
- 32. Johnson EC, Jia L, Cepurna WO, Doser TA, Morrison JC. Global changes in optic nerve head gene expression after exposure to elevated intraocular pressure in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2007; 48: 3161–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dallas SL, Rosser JL, Mundy GR, Bonewald LF. Proteolysis of latent transforming growth factor-β-binding protein-1 by osteoclasts. J Biol Chem. 2002; 277: 21352–21360. [DOI] [PubMed] [Google Scholar]
- 34. Yu Q, Stmenkovic I. Cell-surface-localized matrix metalloproteinase 9 proteolytically activates TGFβ promotes tumor invasion and angiogenesis. Genes Dev. 2000; 14: 163–176. [PMC free article] [PubMed] [Google Scholar]
- 35. Romero D, Iglesias M, Vary CPH, Quintanilla M. Functional blockade of smad4 leads to a decrease in b-catenin levels and signaling activity in human pancreatic carcinoma cells. Carcinogenesis. 2008; 29;1070–1076. [DOI] [PubMed] [Google Scholar]
- 36. Honjo M, Tanihara H, Inatani M, et al.. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001; 42: 137–144. [PubMed] [Google Scholar]
- 37. Rao PV, Deng P, Maddala R, Epstein DL, Li CY, Shimokawa H. Expression of dominant negative Rho-binding domain of Rho-kinase in organ cultured human eye anterior segments increases aqueous humor outflow. Mol Vis. 2005; 11: 288–297. [PubMed] [Google Scholar]
- 38. Pattabirman PP, Rao PV.. Mechanistic basis of RhoGTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am J Physiol Cell Physiol. 2010; 298: C749–C763. [DOI] [PMC free article] [PubMed] [Google Scholar]