Abstract
Purpose
This study aimed to investigate the role and pathophysiological mechanism of ATP binding cassette transporter A1 (ABCA1) in regulating the IOP and aqueous humor outflow.
Methods
ABCA1 expression was measured in trabecular meshwork samples obtained from patients with POAG and human donor eyes by Western blot. To further evaluate the functional significance of ABCA1, porcine angular aqueous plexus (AAP) cells, which are equivalent to human Schlemm's canal endothelial cells, were either treated with ABCA1 agonist GW3965 or transduced with lentivirus expressing ABCA1-shRNA. Transendothelial electrical resistance, protein expression, and nitric oxide (NO) concentration were measured. GW3965 was administered by intracameral injection. IOP and aqueous humor outflow facility were also measured.
Results
ABCA1 expression was significantly higher in the trabecular meshwork tissue of patients with POAG compared with controls. ABCA1 upregulation in angular aqueous plexus cells decreased the transendothelial electrical resistance in the angular aqueous plexus monolayers accompanied by a 0.56-fold decrease in caveolin-1 expression and a 2.85-fold and 1.17-fold increase in endothelial NO synthase expression and NO concentration, respectively (n = 3, P < 0.05). Conversely, ABCA1 downregulation increased transendothelial electrical resistance and caveolin-1 expression and decreased endothelial NO synthase expression and NO production (n = 3, P < 0.05). GW3965 decreased IOP and significantly increased conventional outflow facility (P < 0.05).
Conclusions
Regulation of aqueous humor outflow via the caveolin-1/endothelial NO synthase/NO pathway is a newly defined function of ABCA1 that is different from its traditional role in mediating cholesterol efflux. ABCA1 is a compelling, novel therapeutic candidate for the treatment of glaucoma and ocular hypertension.
Keywords: ATP binding cassette transporter A1, primary open-angle glaucoma, intraocular pressure
Glaucoma is one of the leading causes of blindness worldwide. It is a neurodegenerative optic neuropathy that leads to irreversible visual field loss.1 POAG is the most common form of glaucoma and is predominantly inherited as a complex trait. Elevated IOP is the most significant risk factor for POAG.2
Recent genome-wide association studies identified ATP binding cassette transporter A1 (ABCA1) as a candidate gene within a POAG susceptibility locus.3–5 ABCA1 belongs to a large superfamily of ABC transmembrane transporters that mediate cholesterol efflux to lipid-free apolipoprotein A–I and apolipoprotein E.6 ABCA1 is expressed in the trabecular meshwork (TM) Schlemm's canal (SC) endothelial cells, optic nerve, and retina.5,7 Previous studies found that ABCA1 was related to retinal ganglion cell death in mouse models of glaucoma.8,9 However, it remains unknown whether ABCA1 plays a role in regulating IOP and aqueous humor outflow.
Based on previous studies, ABCA1 is known to co-localize and interact with caveolin-1 (Cav1) through its scaffolding domain, and this interaction induces the oligomerization of Cav1 and its exit from the Golgi network.10–12 Cav1 is a structural component of caveolae, which is involved in diverse cellular functions, including vesicular transport, cholesterol homeostasis, and signal transduction.13 The scaffolding domain of Cav1 is implicated in cellular mechanoprotection and mechanotransduction. Cav1 transduces flow-mediated vasodilation by endothelial nitric oxide synthase (eNOS), which is also a pressure-dependent regulator of IOP.14,15 Studies have also demonstrated the association of polymorphisms in the NOS3 gene, which encodes eNOS, with the development of glaucoma.16–18 Moreover, our previous study and others have confirmed that eNOS is an important protein in IOP regulation through the conventional outflow pathway.14,15
Therefore, in this study, we aimed to investigate the role and mechanism of ABCA1 in regulating IOP and aqueous humor outflow. We first examined the expression of ABCA1 in TM tissue obtained from human eyes and then explored the functional significance and molecular mechanism of ABCA1 involvement in IOP regulation through aqueous humor outflow.
Methods
TM Tissue Collection
TM tissue was collected from patients with POAG and cadaveric eyes. All procedures were performed in accordance with the Declaration of Helsinki and were approved by the Ethics Committee of the Eye & ENT Hospital of Fudan University. Informed consent for the use of donated human eyes for research was provided by the Shanghai Red Cross Eye Bank. All donors were Han Chinese. Control human TM tissues were provided by the Eye Bank of the Eye & ENT Hospital of Fudan University. Patients with POAG had TM tissues collected from trabeculectomy surgery. Control TM tissues were obtained from cadaveric eyes within 12 hours of death. The tissues obtained from surgery included TM, SC endothelium, and cornea.
Isolation and Culture of Angular Aqueous Plexus (AAP) Cells
Porcine AAP endothelial cells, the equivalent of human SC endothelial cells, were isolated according to an established method developed by our group.19 AAP cells were maintained in Dulbecco's modified Eagle's medium (HyClone, Logan, UT) supplemented with 10% fetal bovine serum (Gibco, Thermal Fisher, Waltham, MA), 100 U/mL penicillin (Invitrogen, Carlsbad, CA), and 100 µg/mL streptomycin (Invitrogen) at 37°C in an incubator equilibrated with 5% CO2. Experimental cells were treated with 1 µM ABCA1 agonist GW3965 (Sigma, St Louis, MO), and control cells were treated with 0.002% dimethylsulfoxide vehicle control (Sigma).
Lentivirus Transduction
ABCA1 was knocked down in AAP cells by introducing short hairpin RNAs (shRNA) against ABCA1 into cells using lentiviral vector transduction. Scrambled shRNA was used as a negative control. ABCA1–shRNA lentiviral vector construction and subsequent lentivirus syntheses were conducted by Ruijiata Co, Ltd (Shanghai, China). The ABCA1-shRNA or scrambled-shRNA lentiviral vectors were diluted in complete medium containing polybrene (8 µg/mL) at a final multiplicity of infection of 10. Quantitative real-time PCR was performed to measure ABCA1 mRNA expression.
Quantitative RT-PCR (qRT-PCR)
ABCA1 mRNA expression was measured using SYBR Green quantitative real-time PCR kit (qRT-PCR, Takara, Osaka, Japan) according to the manufacturer's instructions. qRT-PCR was performed using viiA 7 Real-Time PCR System (Life Technologies, Pleasanton, CA). Relative mRNA expression was normalized with the endogenous mRNA control transcript of β-actin. Results were calculated by the comparative cycle threshold method (2−ΔΔCT) of relative quantification with viiA 7 Software (Life Technologies). Three independent experiments were carried out for statistical analysis. The primer sequences used for amplification were as follows:
ABCA1 primer F: 5′GGTGTTGAAAGTCTCGAACATG3′,
ABCA1 primer R: 5′GGGAAAACCCACCATACCTAA3′,
β-actin primer F: 5′CCCAGAGCAAAAGAGGTATCC3′, and
β-actin primer R: 5′ GGTGTTGAAAGTCTCGAACATG 3′.
Western Blot
Human TM tissue (n = 30) samples and AAP cell (n = 3 cell lines) lysates were prepared using RIPA solution (Beyotime, Shanghai, China), and protein concentration was estimated using the Bradford method. Equal amounts of protein (20 µg protein/lane for human TM tissue, AAP cells, and mouse outflow tissue each) were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. The resolved proteins were electrophoretically transferred to nitrocellulose membranes. The membranes were blocked using 5% nonfat dry milk in Tris-buffered saline with 0.05% Tween-20 (Sigma-Aldrich, Shanghai, China) at 37°C for 2 hours. Membranes were then probed using primary antibodies that specifically recognized ABCA1 (1:1000; Abcam, ab151685, ab18180, Boston, MA), Cav1 (1:1000; Cell Signaling Technology #3238, Danvers, MA), or eNOS (1:1000; Abcam, ab66127). Blots were then incubated with peroxidase-linked secondary antibodies. Glyceraldehyde 3-phosphate dehydrogenase (1:2500; Abcam) was used as a loading control. Signals in the linear range of detection by x-ray film were captured digitally, and densitometry was performed using Kodak Molecular Imaging Software (Kodak, Shinkawa, Japan).
NO Measurements
After AAP cells were treated with GW3965 or infected with ABCA1-shRNA, the NO concentration was measured using the Griess assay kit (Promega Corporation, Madison, WI) according to the manufacturer's instructions. Absorbance was measured using a SpectraMax L Microplate Reader (Molecular Devices, LLC, Sunnyvale, CA) at 540 nm.
Transendothelial Electrical Resistance (TEER)
TEER was measured in AAP cells treated with 1 µΜ ABCA1 agonist GW3965 (Sigma) or vehicle control for 24 hours and in AAP cells transduced with ABCA1–shRNA or scrambled shRNA. TEER across confluent AAP cell monolayers grown on trans-well chambers (COSTAR, Corning, NY) was measured at room temperature with STX-2 Ag/AgCl electrodes (Word Precision Instruments, Sarasota, FL) and an EVOM2 Volt ohm Meter (Word Precision Instruments). All measurements of TEER were corrected for the resistance of the filter membrane.
Animals
All experiments complied with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research. C57BL/6J mice were bred and housed in clear cages covered loosely with air filters and containing white pine shavings for bedding. Mice aged 8 to 10 weeks were used.
IOP Measurements in Mice
Animals were deeply anesthetized with ketamine (100 mg/kg) and xylazine (15 mg/kg). Two microliters of 100 µM GW3965 were slowly injected into the anterior chamber of the eye with a custom-made 33-G needle. The contralateral eye was injected with 0.2% dimethylsulfoxide in PBS as a vehicle control. IOP was measured using rebound tonometry (TonoLab, ICare, Espoo, Finland) immediately after injection and at 6 and 12 hours after anterior chamber injection in live animals. The IOP of both eyes were measured three times at each time point, and the mean was calculated. IOP was compared between experimental and contralateral control eyes at each time point. Expression of ABCA1, Cav1, and eNOS in the outflow tissue of the mice were collected after IOP measurement for Western blot analysis.
Eye Perfusion
Outflow facility was measured by perfusing enucleated mice eyes according to an established method.14 Mice were euthanized by cervical dislocation, and the eyes were enucleated for perfusion. To test the effect of GW3965, eyes were perfused with 100 µM drug solution for 60 minutes to exchange the aqueous humor and ensure that the drug concentration was uniform throughout the experiment. Eyes were then perfused at four different IOPs (8, 12, 16, and 20 mm Hg). Contralateral control eyes were treated in the same way but perfused with 0.2% dimethylsulfoxide in PBS vehicle control.
Total outflow facility (Ctotal) was calculated as:
| (1) |
where F is the total inflow rate. We assumed that at equilibrium, the total inflow rate was equal to the total outflow rate. Conventional outflow facility (Ccon) and unconventional flow rate were calculated according Goldman's equation:
| (2) |
where Fu is the pressure-independent (unconventional) outflow rate, EVP is the episcleral venous pressure, and Ccon is the conventional outflow facility. The EVP was zero because the eyes were enucleated at the time of the experiment.
To characterize the effect of IOP elevation on the expression of ABCA1, eyes were perfused with Dulbecco's PBS at constant pressures of 6 and 15 mm Hg for 30 minutes. At the end of the experiment, conventional outflow tissue was dissected using an established method.20 The outflow tissue may have contained the iris, TM, SC, and possibly some iris root. Expression of ABCA1 in the outflow tissue was measured by Western blot.
Statistical Analysis
One-way ANOVA was used for analysis of TEER, Western blot, qRT-PCR, and NO concentration if data were normally distributed. Mann-Whitney U test was used for analysis on SPSS 22.0 for Windows (IBM-SPSS, Chicago, IL) if data were not normally distributed. IOP and outflow facility data generated from experiments with GW3965 treatment were analyzed by the paired-sample t-test if data were normally distributed. The Mann-Whitney U test was used for analysis if data were not normally distributed. In all cases, differences were considered significant when P < 0.05. Values are presented as mean ± standard deviation.
Results
ABCA1 Is Upregulated in Patients With POAG
We compared ABCA1 expression in the TM tissue of patients with POAG and human donor eyes without glaucoma by Western blot analysis. ABCA1 expression in patients with POAG was 7.78-fold greater than that in the control subjects (n = 30, P < 0.01) (Fig. 1). Although the trabeculectomy surgical samples may have contained cornea tissue in addition to TM tissue, this factor is unlikely to have confounded our robust detection of the difference in ABCA1 expression between TM tissue and donor eyes because it was not expressed in the human cornea.21
Figure 1.
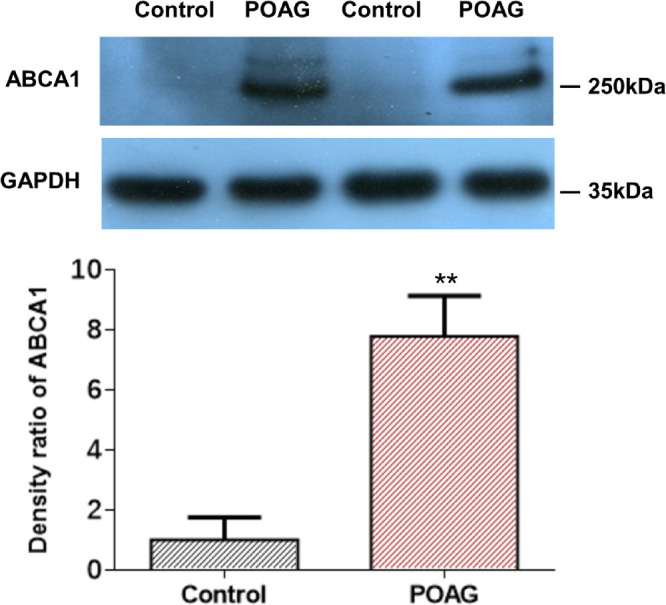
ABCA1 expression in surgical trabecular meshwork specimens from patients with POAG and human donor eyes as measured by Western blot. ABCA1 protein expression is significantly higher in patients with POAG compared with controls (n = 30, **P < 0.01, one-way ANOVA). GADPH, glyceraldehyde 3-phosphate dehydrogenase.
ABCA1 Agonist Encourages NO Production Through Cav1 Inhibition
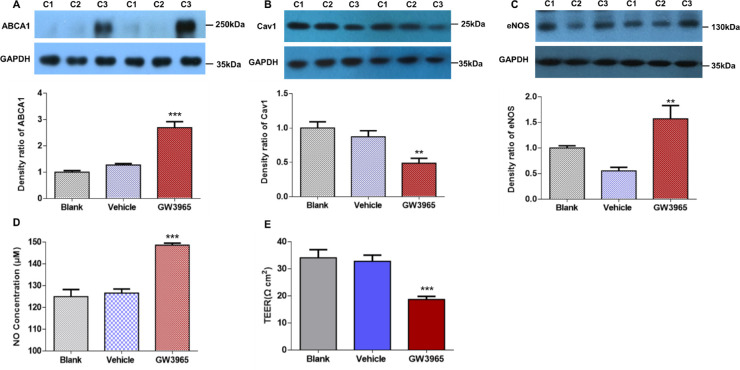
Next, we investigated the functional significance and mechanism of elevated ABCA1 expression in patients with POAG. Owing to the large size of the protein (150 kb), we were not able to construct ABCA1 into a lentiviral overexpression vector. Thus, we used the ABCA1 agonist GW3965 to increase the expression of ABCA1. Twenty-four hours after GW3965 treatment, ABCA1 expression was upregulated by 2.11-fold (n = 3 cell lines, P < 0.001) (Fig. 2A), Cav1 expression was downregulated by 0.56-fold (n = 3 cell lines, P < 0.01) (Fig. 2B), and eNOS expression was upregulated by 2.85-fold (n = 3 cell lines, P < 0.01) (Fig. 2C). The concentration of NO in GW3965-treated AAP cells was 148.55 ± 0.97 µM, which was 1.17-fold higher than that of vehicle control-treated AAP cells (126.61 ± 1.84 µM, n = 3 cell lines, P < 0.001) (Fig. 2D). GW3965 treatment for 24 hours downregulated TEER from 32.75 ± 2.33 Ω*cm2 to 18.67 ± 1.15 Ω*cm2 (n = 4 cell lines, P < 0.001) (Fig. 2E).
Figure 2.
Protein expression and TEER of AAP cells treated with ABCA1 agonist GW3965 for 24 hours. (A) Western blot shows that ABCA1 protein expression was upregulated in the GW3965-treated group compared with the blank and vehicle treated groups (n = 3 cell lines). (B, C) Western blot detected reduced Cav1 and increased eNOS after GW3965 treatment (n = 3 cell lines). (D) Griess assay showed significantly higher NO concentration in the GW3965-treated group than vehicle group control (n = 3 cell lines). (E) TEER of the AAP monolayer declined notably in the GW3965-treated group compared with the vehicle group (n = 4 cell lines). C1, blank; C2, vehicle; C3, GW3965. ***P < 0.001, **P < 0.01, compared with vehicle group, one-way ANOVA. Results are expressed as mean ± standard deviation. GADPH, glyceraldehyde 3-phosphate dehydrogenase.
Lentiviral ABCA1 shRNA Relieves the Inhibitory Effect of Cav1 on eNOS
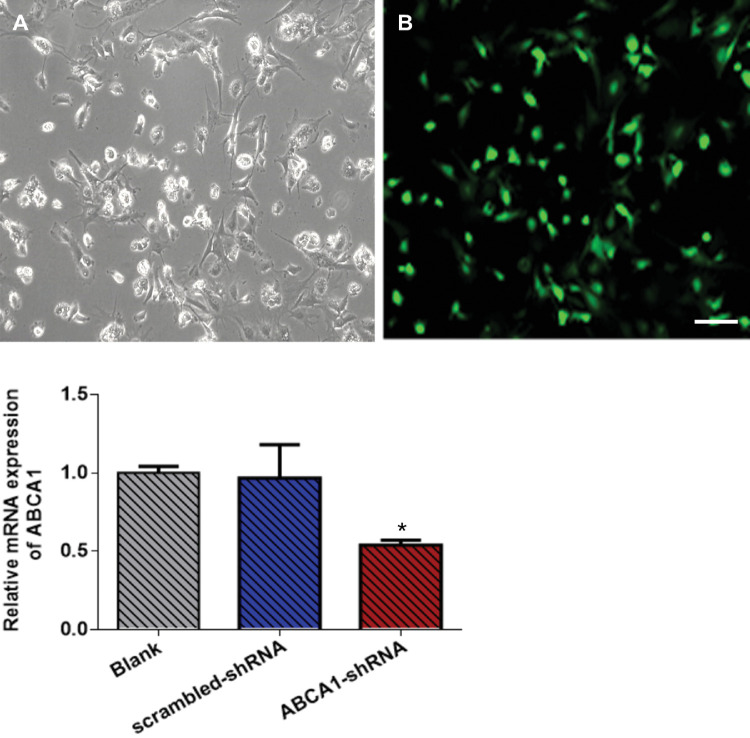
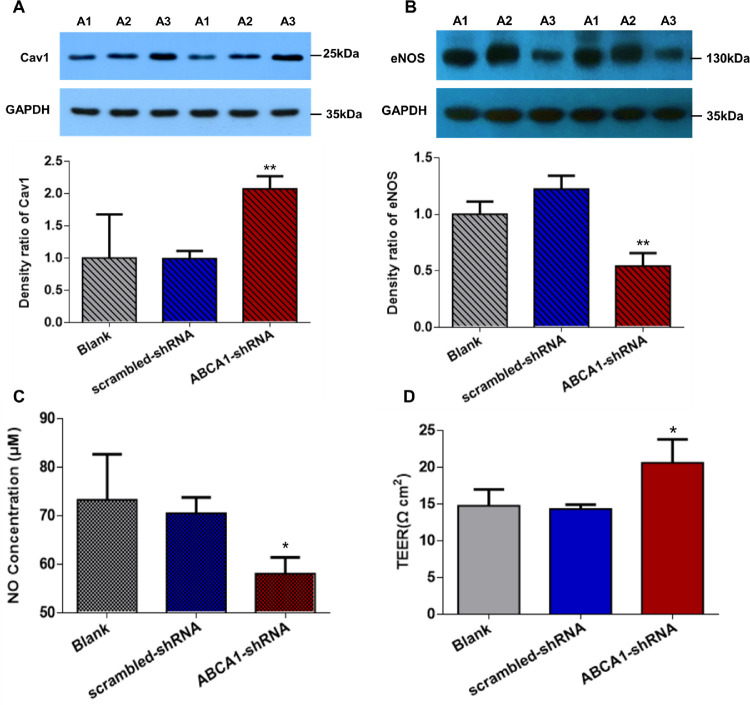
Having demonstrated that upregulation of ABCA1 can increase AAP cell permeability by inhibiting Cav1 and activating eNOS, we downregulated ABCA1 to further confirm this mechanism. Approximately 72.65 ± 0.79% of AAP cells were transduced with lentivirus (Figs. 3A, 3B). ABCA1 mRNA expression was downregulated by 0.56-fold in AAP cells transduced with ABCA1–shRNA than in AAP cells transduced with scrambled shRNA control (n = 3 cell lines, P < 0.05) (Fig. 3C). Cav1 expression was 2.09-fold higher in AAP cells with ABCA1 knockdown than in control cells (n = 3 cell lines, P = 0.01) (Fig. 4A). Expression of eNOS and NO concentration were 0.44-fold (n = 3 cell lines, P < 0.01) and 0.82-fold (n = 3 cell lines, P < 0.05) lower in ABCA1 downregulated cells than in scrambled shRNA cells (Figs. 4B and 4C). The concentration of NO in AAP cells was 58.06 ± 3.36 µM and 70.53 ± 3.25 µM in the ABCA1–shRNA and scrambled shRNA-transduced groups, respectively. TEER in ABCA1–shRNA transduced cells (20.67 ± 3.21 Ω*cm2) was higher than that in scrambled shRNA transduced cells (14.78 ± 2.22 Ω*cm2, n = 3 cell lines, P < 0.05) (Fig. 4D).
Figure 3.
AAP cells transduced with ABCA1–shRNA encoding lentivirus. Bright-field (A) and fluorescence (B) micrographs of AAP cells transduced with shRNA sequences specifically targeting ABCA1 transcript (ABCA1–shRNA). Green fluorescence indicates successful transduction in the cell. (C) ABCA1 transcript accumulation was 0.56-fold lower in response to ABCA1–shRNA than in response to scrambled shRNA transduced controls. **P < 0.05. Data were analyzed by one-way ANOVA. Scale bar = 100 µm.
Figure 4.
Cav1 and eNOS expression in AAP cells transduced with ABCA1–shRNA lentivirus. (A, B) Western blot showed upregulated Cav1 and downregulated eNOS in ABCA1–shRNA transduced AAP cells (n = 3 cell lines). (C) Griess assay showed that NO concentration was significantly lower in the ABCA1–shRNA transduced AAP cells compared with scrambled control transduced AAP cells (n = 3 cell lines). (D) TEER of AAP monolayer was notably higher in the ABCA1–shRNA transduced cells compared with scrambled shRNA transduced cells (n = 3 cell lines). A1, blank; A2, scrambled shRNA; A3, ABCA1–shRNA. *P < 0.05, ** P < 0.01 compared with scrambled shRNA groups. A, B, and C were analyzed using one-way ANOVA. D was analyzed using the Mann-Whitney U test.
ABCA1 Agonist Lowers IOP by Facilitating Conventional Outflow
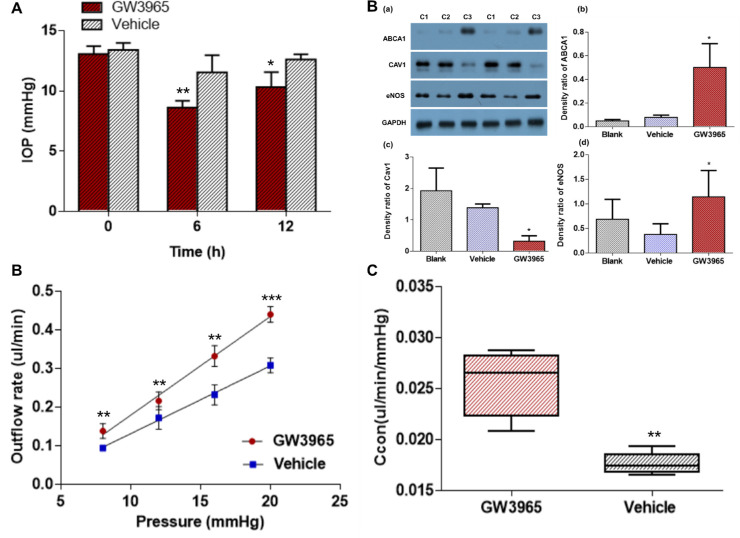
We further investigated the IOP response to ABCA1 agonist GW3965 in mice. IOP was lowered at 6 and 12 hours after anterior chamber injection of GW3965 into mice eyes when compared with vehicle control injection into mice eyes. The magnitude of IOP reduction was 2.9 and 2.3 mm Hg at 6 and 12 hours after injection, respectively (P < 0.01 and P < 0.05, respectively, n = 5) (Fig. 5A; Supplementary Table S1). The LogP of GW3965 is 5.9,22,23 which is much higher than the maximum optimal LogP for cornea permeation, that is, between 1 and 3. Topical application of 100 µM GW3965 did not change the IOP (n = 4, P > 0.05) (Supplementary Fig. S1). After IOP measurements, outflow tissue was collected for Western blot analysis. GW3965 anterior chamber injection caused downregulation of Cav1 expression and upregulation of eNOS expression through activation of ABCA1 in outflow tissue (Fig. 5B).
Figure 5.
Functional consequences and mechanism of ABCA1 agonist GW3965 treatment. (A) IOP was reduced by anterior chamber injection of GW3965 (100 µM, n = 5 mice in each group). (B) Western blot of ABCA1 signaling protein in outflow tissue (n = 4 eyes). (C) The relationship between outflow rate and IOP of GW3965 perfused mouse eyes. (D) A comparison of the Ccon indicates markedly increased in GW3965 treated eyes compared with the vehicle treated eyes. *P < 0.05, **P < 0.01, ***P < 0.001, compared with vehicle-treated eyes. C1, blank; C2, vehicle control; C3, GW3965. A and C were analyzed using the paired-sample t-test. B (a-c) and D were analyzed using the Mann-Whitney U test.
We further investigated the effects of GW3965 on aqueous humor outflow by anterior chamber perfusion in mice. The outflow rates are listed in the Table. The flow rate had a linear relationship with IOP in GW3965-treated eyes. The Ccon in GW3965-treated and vehicle-treated eyes was 0.0256 ± 0.0032 and 0.0177 ± 0.0010 µL/min/mm Hg, respectively. The Ccon was 1.45-fold higher with GW3965 treatment than with vehicle treatment (n = 5 mice in each group; P < 0.01) (Figs. 5C, 5D).
Table.
The Relationship of Outflow Rate and IOP of GW3965 Perfused Mouse Eyes
| Pressure (mm Hg)/Outflow rate (µL/min) | GW3965 | Vehicle | Fold Change |
|---|---|---|---|
| 8 mm Hg | 0.14 ± 0.02 | 0.09 ± 0.01 | 1.56 |
| 12 mm Hg | 0.22 ± 0.02 | 0.17 ± 0.03 | 1.29 |
| 16 mm Hg | 0.33 ± 0.03 | 0.23 ± 0.03 | 1.43 |
| 20 mm Hg | 0.44 ± 0.02 | 0.31 ± 0.02 | 1.42 |
To characterize ABCA1 expression under elevated IOP, we perfused enucleated mouse eyes at constant pressures. After eye perfusion, outflow tissue was collected for Western blot analysis. After perfusion, expression of ABCA1 was 36.01% lower at 15 mm Hg than at 6 mm Hg (n = 3, P < 0.05) (Supplementary Fig. S2).
Discussion
The current work demonstrates that ABCA1 expression in the TM tissue was higher in patients with POAG compared with control subjects. Subsequent cell and animal experiments demonstrated that ABCA1 modulated Cav1 expression and reduced the inhibitory influence of endogenous Cav1 toward eNOS, therefore increasing eNOS-derived NO production, which reduced IOP.
This work follows from our previous genome-wide association studies where we observed significant genome-wide association studies of multiple single nucleotide polymorphisms near ABCA1 on 9q31.1 in 1007 patients with POAG and 1009 controls.5 Hysi et al.3 and Gharahkhani et al.4 also discovered that rs2472493, located near ABCA1, was associated with elevated IOP and POAG in 18 population cohorts. It is possible that increased ABCA1 function in patients with POAG may be a result of malfunctioning or mutant protein arising from single nucleotide polymorphism-linked variants or other kinds of variations in ABCA1. However, no mutations have been found in patients with POAG after extensive sequencing of the entire ABCA1 gene and its neighboring regions (unpublished data).
Upregulation of ABCA1 is consistent with the current literature regarding patients with glaucoma.24 Consistent with our study, ABCA1 was shown to be involved in outflow regulation because inhibition of ABCA1 reduced outflow by 40% (ARVO annual meeting abstract, Investigative Ophthalmology & Visual Science, 2015, Vol. 56, 3282). However, ABCA1 expression was lower in glaucomatous SC cells than in control cells.25 This discrepancy may be due to a difference in experimental models. ABCA1 activity has also been found to influence neuroinflammation and neurodegeneration in neurons and glial cells.26 The alteration of ABCA1 expression in glaucomatous mice and ischemia–reperfusion mice can lead to retinal ganglion cell death.8,9 ABCA1 expression in leukocytes was higher in patients with glaucoma than in controls (who did not have glaucoma). In leukocytes, ABCA1 controls the recruitment of inflammatory cells. Upregulation may indicate the involvement of ABCA1 in glaucomatous vascular pathology.24,27
Using the ABCA1 agonist GW3965 and lentiviral ABCA1–shRNA, we showed that ABCA1 negatively regulates IOP by increasing SC permeability and conventional outflow. Multiple population and basic studies have indicated that the ABCA1 gene plays a crucial role in the development of POAG, but the mechanism was previously unknown. In the current study, the ABCA1 agonist GW3965 inhibited Cav1 expression, which in turn led to eNOS activation, decreased TEER, and increased NO production. Conversely, the downregulation of ABCA1 by lentiviral transduction of ABCA1-shRNA increased Cav1 expression and reduced eNOS expression.
Together, our data suggest that the ABCA1 upregulation in patients with POAG modulates IOP in patients with POAG via the regulation of the Cav1/eNOS/NO pathway. ABCA1, Cav1, and NOS3 are important contributing genes in the pathogenesis of POAG.3–5,28 Recent studies showed that Cav1 co-localizes and inters with ABCA1 and that this interaction is crucial for Cav1 regulation of cholesterol efflux.10,12 Moreover, ABCA1 was found to induce the oligomerization of Cav1 and its exit from the Golgi network. The deletion of the Cav1 oligomerization domain abolishes the co-localization and interaction of Cav1 and ABCA1.12 An earlier study showed that ABCG1 and/or ABCA1 played key roles in preserving eNOS activity in animals fed a high cholesterol diet by promoting efflux of sterols and oxysterols from endothelial cells.29 Cav1 is a scaffolding protein that interacts with many signaling molecules. One of the most important signaling molecules is eNOS, which binds to Cav1 leading to its inactivation.30,31 Our previous studies demonstrated that the interaction of Cav1 and eNOS plays an important role in regulating aqueous outflow resistance.14,15,32
In our study, patients with POAG have elevated ABCA1 expression in TM tissue, which is consistent with a previous report of increased ABCA1 expression in the circulating leucocytes of hypertensive patients with glaucoma.24 Thus, both vascular and local evidence support ABCA1 activation in hypertensive patients with glaucoma. It is interesting that the author showed increased ABCA1 in patients with normal tension glaucoma. Our laboratory is unable to confirm this finding because TM tissue from patients with normal tension glaucoma is hard to obtain.
Ex vivo mouse eye perfusion with elevated pressure led to the downregulation of ABCA1 expression. Together with increased ABCA1 expression in patients with POAG, it suggests that a more complex mechanism may exist in the pathogenesis of ocular hypertension in patients with POAG. Further studies are needed to confirm the relationship between IOP and ABCA1 expression in animal models of ocular hypertension.
In this study, we identified the role of ABCA1 in regulating aqueous humor outflow and IOP, which are important factors in POAG pathogenesis. This finding is different from the conventional role of ABCA1, which is mediating cholesterol efflux. ABCA1 plays a major role in high-density lipoprotein transport and is associated with several human inherited diseases, including Tangier disease, severe premature coronary heart disease, and familial high-density lipoprotein deficiency.33–35 Previous studies identified that a vast majority of cholesterol and glycosphingolipid species were similar between normal and POAG donors. Only a few of these species were found to be different in aqueous humor and TM between those with POAG and controls, which may have been because of the use of multiple drugs in patients with POAG.36
One shortcoming of our study is that the expression of ABCA1 in the control group could be affected by the freshness of the tissue. Cadaveric TM tissue was collected within 12 hours after death from our eye bank. This lag time is longer than for TM samples from patients with POAG, which were collected immediately after trabeculectomy surgery. In addition, potential leakage problems and effects of aneathesia37 may be confounding factors associated with GW3965 anterior chamber injection. However, to minimize the impact of these factors, we used injection of the contralateral eye with the drug vehicle as a control.
Collectively, our data provide evidence of a novel role for ABCA1 in IOP modulation via the regulation of the Cav1/eNOS/NO signaling, which is likely to be an important mechanism of pathogenesis in patients with POAG. Therefore, enhancing the ABCA1 signaling pathway could be of therapeutic value in the treatment of glaucoma and ocular hypertension.
Supplementary Material
Acknowledgments
The authors thank the Biobank and Eye Bank of Eye & ENT Hospital of Fudan University for providing human eyeball tissues. We also thank Xing Chao and Jufang Shi for their excellent assistance with the animal work.
Supported by the following: National Natural Science Foundation of China (81430007, 81570887, 81870692), the Shanghai Committee of Science and Technology, China (17410712500), the subject of major projects of National Natural Science Foundation of China (81790641), the Young Scientist Program of EENT Hospital of Fudan University (XYQ2013083), General Program of National Science Foundation China (81100662, 81371015), 211 Project of Fudan University (EHF158351), Young Scientists Program of EENT Hospital of Fudan University and BrightFocus Foundation (G2018112), and the International Science and Technology Cooperation Program of China (No.2015DFA31340).
Disclosure: C. Hu, None; L. Niu, None; L. Li, None; M. Song None; Y. Zhang None; Y. Lei, None; Y. Chen, None; X. Sun None
References
- 1. Quigley HA, Broman AT.. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolfs RC, Klaver CC, Ramrattan RS, van Duijn CM, Hofman A, de Jong PT. Genetic risk of primary open-angle glaucoma. Population-based familial aggregation study. Arch Ophthalmol. 1998; 116: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 3. Hysi PG, Cheng CY, Springelkamp H, et al.. Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nat Genet. 2014; 46: 1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gharahkhani P, Burdon KP, Fogarty R, et al.. Common variants near ABCA1, AFAP1 and GMDS confer risk of primary open-angle glaucoma. Nat Genet. 2014; 46: 1120–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen Y, Lin Y, Vithana EN, et al.. Common variants near ABCA1 and in PMM2 are associated with primary open-angle glaucoma. Nat Genet. 2014; 46: 1115–1119. [DOI] [PubMed] [Google Scholar]
- 6. Danik M, Champagne D, Petit-Turcotte C, Beffert U, Poirier J. Brain lipoprotein metabolism and its relation to neurodegenerative disease. Crit Rev Neurobiol. 1999; 13: 357–407. [DOI] [PubMed] [Google Scholar]
- 7. Cai J, Perkumas KM, Qin X, Hauser MA, Stamer WD, Liu Y. Expression profiling of human Schlemm's canal endothelial cells from eyes with and without glaucoma. Invest Ophthalmol Vis Sci. 2015; 56: 6747–6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howell GR, Macalinao DG, Sousa GL, et al.. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest. 2011; 121: 1429–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li L, Xu L, Chen W, et al.. Reduced annexin A1 secretion by ABCA1 causes retinal inflammation and ganglion cell apoptosis in a murine glaucoma model. Front Cell Neurosci. 2018; 12: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuo CY, Lin YC, Yang JJ, Yang VC. Interaction abolishment between mutant caveolin-1(Delta62-100) and ABCA1 reduces HDL-mediated cellular cholesterol efflux. Biochem Biophys Res Commun. 2011; 414: 337–343. [DOI] [PubMed] [Google Scholar]
- 11. Chao WT, Tsai SH, Lin YC, Lin WW, Yang VC. Cellular localization and interaction of ABCA1 and caveolin-1 in aortic endothelial cells after HDL incubation. Biochem Biophys Res Commun. 2005; 332: 743–749. [DOI] [PubMed] [Google Scholar]
- 12. Lu R, Tsuboi T, Okumura-Noji K, Iwamoto N, Yokoyama S. Caveolin-1 facilitates internalization and degradation of ABCA1 and probucol oxidative products interfere with this reaction to increase HDL biogenesis. Atherosclerosis. 2016; 253: 54–60. [DOI] [PubMed] [Google Scholar]
- 13. Gu X, Reagan AM, McClellan ME, Elliott MH. Caveolins and caveolae in ocular physiology and pathophysiology. Prog Retin Eye Res. 2017; 56: 84–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stamer WD, Lei Y, Boussommier-Calleja A, Overby DR, Ethier CR. eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011; 52: 9438–9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lei Y, Song M, Wu J, Xing C, Sun X. eNOS activity in CAV1 knockout mouse eyes. Invest Ophthalmol Vis Sci. 2016; 57: 2805–2813. [DOI] [PubMed] [Google Scholar]
- 16. Kang JH, Wiggs JL, Rosner BA, et al.. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with sex and postmenopausal hormone use. Invest Ophthalmol Vis Sci. 2010; 51: 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liao Q, Wang DH, Sun HJ. Association of genetic polymorphisms of eNOS with glaucoma. Mol Vis. 2011; 17: 153–158. [PMC free article] [PubMed] [Google Scholar]
- 18. Xiang Y, Dong Y, Li X, Tang X. Association of common variants in eNOS gene with primary open angle glaucoma: a meta-analysis. J Ophthalmol. 2016; 2016: 1348347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lei Y, Overby DR, Read AT, Stamer WD, Ethier CR. A new method for selection of angular aqueous plexus cells from porcine eyes: a model for Schlemm's canal endothelium. Invest Ophthalmol Vis Sci. 2010; 51: 5744–5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lei Y, Zhang X, Song M, Wu J, Sun X. Aqueous humor outflow physiology in NOS3 knockout mice. Invest Ophthalmol Vis Sci. 2015; 56: 4891–4898. [DOI] [PubMed] [Google Scholar]
- 21. Li XB, Phan K, Acott TS, Kelley MJ. Involvement of ATP binding cassette transporter 1 (ABCA1) in the regulation of outflow facility. Invest Ophth Vis Sci. 2015; 56. [Google Scholar]
- 22. Pescina S, Sala M, Padula C, et al.. Design and synthesis of new cell penetrating peptides: diffusion and distribution inside the cornea. Mol Pharm. 2016; 13: 3876–3883. [DOI] [PubMed] [Google Scholar]
- 23. Shirasaki Y. Molecular design for enhancement of ocular penetration. J Pharm Sci. 2008; 97: 2462–2496. [DOI] [PubMed] [Google Scholar]
- 24. Yeghiazaryan K, Flammer J, Wunderlich K, Schild HH, Orgul S, Golubnitschaja O. An enhanced expression of ABC 1 transporter in circulating leukocytes as a potential molecular marker for the diagnostics of glaucoma. Amino Acids. 2005; 28: 207–211. [DOI] [PubMed] [Google Scholar]
- 25. Cai JW, Perkumas KM, Qin XJ, Hauser MA, Stamer WD, Liu YT. Expression profiling of human Schlemm's canal endothelial cells from eyes with and without glaucoma. Invest Ophth Vis Sci. 2015; 56: 6747–6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karasinska JM, de Haan W, Franciosi S, et al.. ABCA1 influences neuroinflammation and neuronal death. Neurobiol Dis. 2013; 54: 445–455. [DOI] [PubMed] [Google Scholar]
- 27. Efferth T. Adenosine triphosphate-binding cassette transporter genes in ageing and age-related diseases. Ageing Res Rev. 2003; 2: 11–24. [DOI] [PubMed] [Google Scholar]
- 28. Thorleifsson G, Walters GB, Hewitt AW, et al.. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet. 2010; 42: 906–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Terasaka N, Yu S, Yvan-Charvet L, et al.. ABCG1 and HDL protect against endothelial dysfunction in mice fed a high-cholesterol diet. J Clin Invest. 2008; 118: 3701–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jin Y, Lee SJ, Minshall RD, Choi AM. Caveolin-1: a critical regulator of lung injury. Am J Physiol Lung Cell Mol Physiol. 2011; 300: L151–L160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thompson MA, Prakash YS, Pabelick CM. The role of caveolae in the pathophysiology of lung diseases. Expert Rev Respir Med. 2014; 8: 111–122. [DOI] [PubMed] [Google Scholar]
- 32. Song M, Wu J, Lei Y, Sun X. Genetic deletion of the NOS3 gene in CAV1-/- mice restores aqueous humor outflow function. Invest Ophthalmol Vis Sci. 2017; 58: 4976–4987. [DOI] [PubMed] [Google Scholar]
- 33. Bodzioch M, Orso E, Klucken T, et al.. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nature Genetics. 1999; 22: 347–351. [DOI] [PubMed] [Google Scholar]
- 34. Marcil M, Brooks-Wilson A, Clee SM, et al.. Mutations in the ABC1 gene in familial HDL deficiency with defective cholesterol efflux. Lancet. 1999; 354: 1341–1346. [DOI] [PubMed] [Google Scholar]
- 35. Clee SM, Zwinderman AH, Engert JC, et al.. Common genetic variation in ABCA1 is associated with altered lipoprotein levels and a modified risk for coronary artery disease. Circulation. 2001; 103: 1198–1205. [DOI] [PubMed] [Google Scholar]
- 36. Aribindi K, Guerra Y, Piqueras Mdel C, Banta JT, Lee RK, Bhattacharya SK. Cholesterol and glycosphingolipids of human trabecular meshwork and aqueous humor: comparative profiles from control and glaucomatous donors. Curr Eye Res. 2013; 38: 1017–1026. [DOI] [PubMed] [Google Scholar]
- 37. Johnson TV, Fan S, Toris CB. Rebound tonometry in conscious, conditioned mice avoids the acute and profound effects of anesthesia on intraocular pressure. J Ocul Pharmacol Ther. 2008; 24: 175–185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.