Highlight
-
•
It is challenging to identify effective SARS-CoV-2 main protease inhibitor urgently.
-
•
This study involves classification QSAR based data mining of diverse SARS-CoV Mpro inhibitors.
-
•
Important molecular features regulating the Mpro inhibitory properties are identified.
-
•
Prediction of recently reported natural origin based virtual hits is reported.
Keywords: COVID-19, SARS-CoV-2, SARS-CoV Mpro, SPCI analysis, Monte Carlo based optimization, Natural product
Abstract
As the world struggles against current global pandemic of novel coronavirus disease (COVID-19), it is challenging to trigger drug discovery efforts to search broad-spectrum antiviral agents. Thus, there is a need of strong and sustainable global collaborative works especially in terms of new and existing data analysis and sharing which will join the dots of knowledge gap. Our present chemical-informatics based data analysis approach is an attempt of application of previous activity data of SARS-CoV main protease (Mpro) inhibitors to accelerate the search of present SARS-CoV-2 Mpro inhibitors. The study design was composed of three major aspects: (1) classification QSAR based data mining of diverse SARS-CoV Mpro inhibitors, (2) identification of favourable and/or unfavourable molecular features/fingerprints/substructures regulating the Mpro inhibitory properties, (3) data mining based prediction to validate recently reported virtual hits from natural origin against SARS-CoV-2 Mpro enzyme. Our Structural and physico-chemical interpretation (SPCI) analysis suggested that heterocyclic nucleus like diazole, furan and pyridine have clear positive contribution while, thiophen, thiazole and pyrimidine may exhibit negative contribution to the SARS-CoV Mpro inhibition. Several Monte Carlo optimization based QSAR models were developed and the best model was used for screening of some natural product hits from recent publications. The resulted active molecules were analysed further from the aspects of fragment analysis. This approach set a stage for fragment exploration and QSAR based screening of active molecules against putative SARS-CoV-2 Mpro enzyme. We believe the future in vitro and in vivo studies would provide more perspectives for anti-SARS-CoV-2 agents.
1. Introduction
Severe acute respiratory syndrome (SARS) coronavirus-2 (SARS-CoV-2) has been spreading alarmingly by causing tremendous social and economic disruption [1], [2], [3]. This zoonatic infection has spread over 216 countries and territories [4], [5].
SARS-CoV-2 is the 7th human coronavirus (HCoV) and the 3rd HCoV which posted hefty means in the 21st century [2] after SARS-CoV in 2002 and Middle East respiratory syndrome (MERS) coronavirus (MERS-CoV) in 2012. The MERS-CoV infected 1,700 people with a fatality rate of ~36% and the SARS-CoV infected 8422 people with a fatality rate of ~10% [2], [6]. SARS-CoV-2 created an unprecedented health emergency around the world and till date 11 591 595 confirmed cases and 537 859 deaths have been documented [5].
Notably, the genome of SARS-CoV-2 comprises ~30,000 nucleotides with 10 Open Reading Frames (ORFs). The 3′ terminal regions encode viral structural proteins including spike (S), membrane (M), envelope (E) and nucleocapsid (N) proteins. On the other hand, the 5′ terminal ORF1ab encodes two viral replicase polyproteins pp1a and pp1b [1]. A number of 16 non-structural (ns) proteins (nsp1 to nsp16) are raised upon proteolytic cleavage of pp1a and pp1b. The nsp5 (Chymotrypsin-like protease 3CLpro also called Main protease Mpro) is a prerequisite enzyme of the viral replication and maturation. Mpro turns into a charismatic target for anti-SARS-CoV-2 drug discovery and development [7], [8], [9], [10], [11], [12].
The research work in terms of molecular docking and target based virtual screening studies on Mpro have moved at a much faster pace [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28] after releasing of the several covalent and non-covalent inhibitor bound crystal structures. Despite the availability of inhibitor-bound SARS-CoV-2 Mpro crystal structures and lots of proteomic knowledge, the significant fragment/feature which modulates the structure-activity relationships (SARs) pattern is still not known. Therefore, ligand-based molecular modelling approaches are necessary to gather knowledge about the favourable and/or unfavourable molecular features/fingerprints/substructures regulating the Mpro inhibitory properties.
Quantitative structure-activity relationship (QSAR) study is a very significant ligand-based molecular modelling technique that easily recognised the effect of structural and physico-chemical features of ligands on the biological activity [29], [30]. Not only that, it offers prediction of particular compounds to their biological activities of interest.
As the SARS-CoV-2 genome has over 80% identity to SARS-CoV (about 96% sequence similarity for their Mpro), previously reported SARS-CoV Mpro inhibitors may have huge prospect to show their efficacy against SARS-CoV-2 also. In this connection, we design our current research, a part of our rational molecular modelling studies [3], [31], [32], [33], [34], by covering three major characteristics- (1) classification QSAR based data mining of diverse SARS-CoV Mpro inhibitors, (2) identification of favourable and/or unfavourable molecular features/fingerprints/substructures regulating the Mpro inhibitory properties, (3) QSAR based prediction to validate recently reported virtual hits from natural origins.
This study encompasses the effect of structural and physicochemical features required for Mpro inhibition. The study provides useful information to the medicinal chemists for design effective Mpro/3CLpro inhibitors in future. It set the stage for molecule identification and QSAR based screening of active molecules against putative SARS-CoV-2 Mpro enzyme which surely claim a momentous attention to the scientific audiences.
2. Methods and materials
2.1. Dataset collection
The fighting against COVID-19 disease requires strong and sustainable global collaborative works especially in terms of data sharing which will join the dots of knowledge gap [35]. A set of 113 compounds were retrieved from the data as shared by Bobrowski and co-workers [35], [36]. We considered only compounds having half-maximal inhibitory concentration (IC50) and eliminated the compounds with binding affinity (K i) value. Thus, 88 compounds were kept for this current modelling study (Table S1).
2.2. Classification based QSAR
The classification modelling assists to discriminate the Active and Inactive molecules in terms of their investigated biological significance. The ‘activity threshold’ for the current work was set to the IC50 of 10,000 nM. Here, we performed Structural and physico-chemical interpretation (SPCI) analysis [37], [38] and Monte Carlo based Coral QSAR studies [39], [40], [41], [42]. Performing Monte Carlo based Coral QSAR study not only offer a graphical visualization of critical fingerprint or fragments attributed to enhance/decrease the SARS-CoV Mpro inhibitory activity but also it allows the chance of screening external set compounds.
2.3. Structural and physico-chemical interpretation (SPCI) analysis
The SPCI analysis was explored to identify and approximate the contributions of different fragments that are important for Mpro inhibition. Initially, the descriptor calculation was performed with the help of SiRMS tool. Further, these descriptors were used for model development and validation in our study [37], [38].
In SPCI analysis, four diverse classification-based QSAR models were generated by using machine learning approaches like: Gradient boosting classification (GBC), Random Forest (RF), Support Vector Machine (SVM) and k-nearest neighbour (kNN). These models were further evaluated by different statistical parameters like: balanced accuracy, sensitivity, and specificity [38]. Additionally, all the fragments comprising of at most three attachment points were preferred and subsequently, favoured fragments were counted by RDKit in amalgamation with SMARTS pattern [38]. Finally, the overall contribution of the different fragments obtained from four machine learning models are shown in median fragment contribution graphs generated by using rspciR software package [43].
2.4. Monte Carlo optimization based QSAR
Monte Carlo optimization method was used to identify the important structural fingerprints that are solely responsible for endorsing or deterring of activity [3]. Different descriptors that are generally employed in the study are: SMILES-based descriptors, Graph-based descriptors and Hybrid descriptors.
The SMILES-based descriptors are calculated by the following equation:
Where, T and N represent threshold value and number of epoch, respectively. The correlation weights are represented by CW. The different coefficients like a, b, c, d, α, β and γ are used for descriptor modification. NOSP, HALO, BOND and ATOMPAIR represent global SMILES attributes and the local smile attributes are denoted by Sk, SSk and SSSk [40], [41], [42].
Further, different Graph-based descriptors like: GAO (graph of atomic orbital), HSG (hydrogen-suppressed graph) and HFG (hydrogen-filled graph) are calculated by following equation:
Where, 0ECk, 1ECk and 3ECk represent different Morgan's connectivity indices. Ak denotes different chemical atoms like: C, N, O etc. α, β and γ are the coefficients with 0 and 1 value. The coefficients having value 0 and 1 are denoted by α, β and γ [39], [42].
The mixture of SMILES and graph-based descriptors forms hybrid descriptors which are represented as:
Finally, the model development and validation step was performed by using balance of correlation method, where twenty-one classification models were developed from three different splits. The dataset containing 88 Mpro inhibitors were distributed into training (52 compounds), calibration (18 compounds) and test (18 compounds) sets which were considered for the study. Additionally, optimization of T (threshold) and N (epoch) were also accomplished separately for individual model [3], [39]. The sensitivity, specificity, accuracy along with the MCC values was calculated as a measure of internal and external validation [3]. Lastly, the important structural attributes that are exclusively liable for promoting or hindering of Mpro activity were identified.
3. Result and discussions
Compounds having the SARS-CoV Mpro IC50 value less than the ‘activity threshold’ were yielded to lower Mpro inhibitors or inactives (0) and those with Mpro IC50 value higher than the ‘activity threshold’ (IC50 = 10,000 nM) were classified as promising Mpro inhibitors or actives (1). Thus, 27 molecules were identified as actives (1) while, 61 compounds were distinguished as lower SARS-CoV Mpro inhibitors (0) in the classification analysis (Table S1).
At first structural and physico-chemical interpretation (SPCI) analysis was performed [38]. These machine-learning based models were utilised for a fragment/feature analysis to estimate the contributions of different fragments towards Mpro inhibition. It enables an extensive interpretation of the structural and physico-chemical properties responsible for SARS-CoV Mpro inhibitory activities. The Monte Carlo optimization-based QSAR modelling was also employed by the aid of SMILES and graph-based descriptors to justify fragment contributions [39]. The best Monte Carlo optimization-based QSAR model was used for screening the recently reported docking based natural product hits. Together with the fragment/fingerprint analysis results justified the selection of the potential hits retrieved through such QSAR derived prediction.
3.1. Structural and physico-chemical interpretation (SPCI) analysis
With the aim to construct an interpretable QSAR model, gradient boosting machine (GBM), random forest (RF), support vector machine (SVM) and k-nearest neighbor (kNN) were established using structural and physico-chemical interpretation (SPCI) analysis (Table 1 ). The parameter settings used for the individual models (GBM, RF, SVM and kNN) development are given in Table S2.
Table 1.
Five-fold cross-validation performance for classification model built in this study.
| Model | Balanced accuracy | Sensitivity | Specificity |
|---|---|---|---|
| GBM | 0.62 | 0.41 | 0.84 |
| RF | 0.64 | 0.41 | 0.87 |
| SVM | 0.59 | 0.19 | 1.00 |
| kNN | 0.67 | 0.41 | 0.93 |
Table 1 demonstrates that kNN model produced significant five-fold cross-validation performance as far as the values of balanced accuracy and specificity values were concerned. However, the sensitivity value is comparatively poor. Moreover, RF model shared similar statistical parameters.
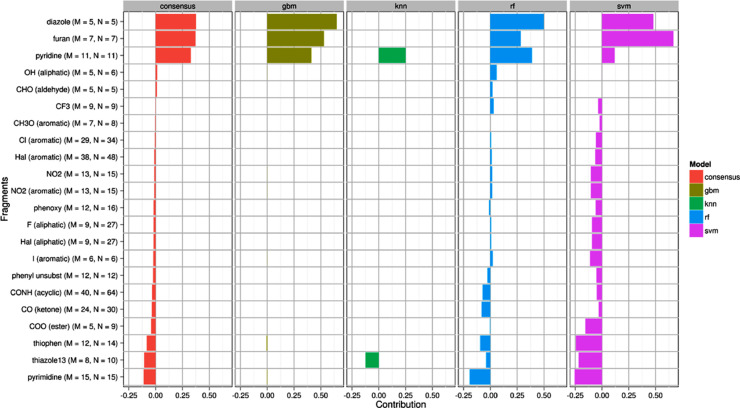
A consensus model was also developed to eliminate biasness of individual models. The fragments obtained from different models are depicted in Fig. 1 . These fragments were found to have different positive and negative contributions towards 3CLpro inhibitory activity.
Fig. 1.
Contribution plot of different fragments (present in at least 5 compounds) identified by using consensus (red), GBM (dark green), kNN (light green), RF (cyan), and SVM (purple) models. The numbers M and N signify the number of compounds containing a fragment and the number of fragments present in the dataset, respectively. (For interepretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
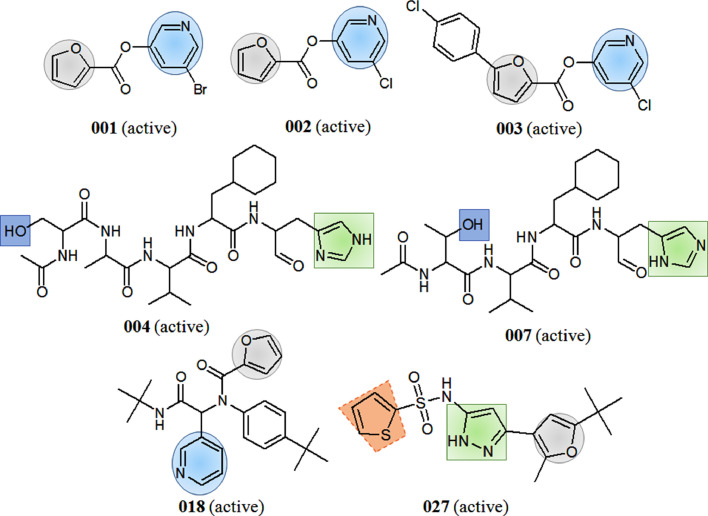
As per the consensus model, heterocyclic like diazole, furan and pyridine have clear positive contribution to the SARS-CoV Mpro inhibition. This can be explained by comparing the order of activities in molecules 001-003 where compounds possessed both furan and pyridine rings in their structure (Fig. 2 ) and showed higher Mpro inhibitory activity (IC50 in between 50 and 63 nM). The importance of five member heterocyclic furan ring was also found in compounds 016, 025, 027and these compounds displayed promising inhibitory activities. In spite of having furan ring in their structures, compounds 036 and 039 (Fig. 2) were found in the category of less active molecules or inactives due to the presence of higher negatively contributing attributes in their structures.
Fig. 2.
Examples of prototype higher active Mpro inhibitors with good and bad molecular fingerprints obtained from SPCI models.
Meanwhile, the positive contribution of diazoles was justified by observing the SARS-CoV Mpro active compounds 004, 007 and 027. Notably, compound 027 (Fig. 2) possessed two positively contributed features including furan, diazole rings and one negatively contributed thiophene ring, therefore, it just crossed the ‘activity threshold’ to be active (IC50 = 10,000 nM). Hence, the contributions of all the groups or moieties present in a structure collectively decide the inhibition potential.
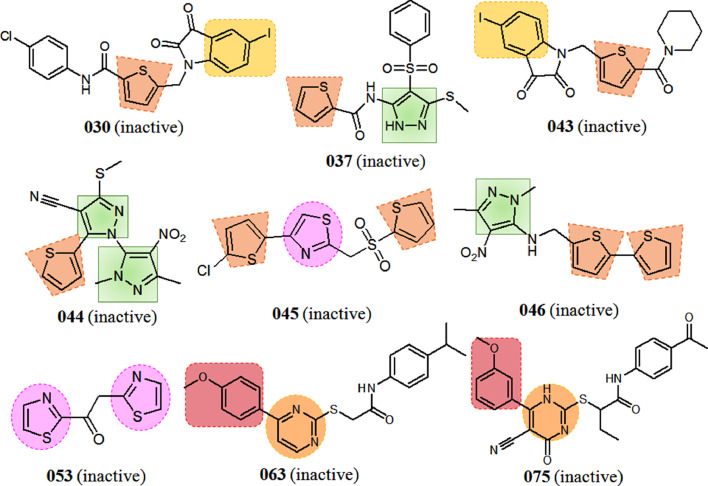
As can be seen from Fig. 1, the aliphatic OH substituent would lead to increase of Mpro IC50 values in the compounds 004, 007, etc. On the other hand, thiophen, thiazole and pyrimidine may exhibit negative contribution. Furthermore, the fragments such as phenoxy, I-aromatic, F-aliphatic, halo-aliphatic, acyclic CONH may negatively contributed to the SARS-CoV Mpro inhibition. For better interpretation, examples of prototype lower active Mpro inhibitors with good and bad molecular fingerprints obtained from SPCI models is depicted in Fig. 3 .
Fig. 3.
Examples of prototype lower active Mpro inhibitors with good and bad molecular fingerprints obtained from SPCI models.
This effect can also be noticed for molecules 061 to 088 where their lower activities can be related to the presence of pyrimidine ring (Fig. 3). Thus, it may be supposed that six member pyridine ring would be effective for the interaction and could be used as replacement of pyrimidine to improve the biological potency. Similarly, five member heterocyclic rings such as thiophene and thiazole should be replaced with furan ring to trigger the Mpro inhibitory activity. Surprisingly, compound 044 (Fig. 3) which possessed two diazole and one thiophene moieties was found as inactive. The probable reason behind this phenomenon will be discussed with the structural attributes predicted by Monte Carlo optimization based classification QSAR.
3.2. Monte Carlo optimization based classification QSAR
The 88 compounds with diverse structural features were also used in Monte Carlo optimization based classification QSAR analysis [3], [42]. Twenty-one different models from three different splits were generated using SMILES and graph-based descriptors with a combination of different connectivity indices were generated for construction of different Monte Carlo optimization based QSAR study [39]. Overall statistical characteristics of twenty-one different models are given in Table 2 .
Table 2.
The statistical characteristics of twenty-one different classification models obtained from Monte Carlo optimization method.
| Parameter | Set | TP | TN | FP | FN | NTotal | Sensitivity | Specificity | Accuracy | MCC |
|---|---|---|---|---|---|---|---|---|---|---|
| Split-1 | ||||||||||
| M1 SMILES | Sub-Training | 15 | 33 | 1 | 3 | 52 | 0.8333 | 0.9706 | 0.9231 | 0.8287 |
| Calibration | 5 | 13 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 3 | 12 | 2 | 1 | 18 | 0.7500 | 0.8571 | 0.8333 | 0.5635 | |
| M2 SMILES, GAO (0ECk) | Sub-Training | 13 | 31 | 3 | 5 | 52 | 0.7222 | 0.9118 | 0.8462 | 0.6535 |
| Calibration | 5 | 12 | 1 | 0 | 18 | 1.0000 | 0.9231 | 0.9444 | 0.8771 | |
| Test | 3 | 12 | 2 | 1 | 18 | 0.7500 | 0.8571 | 0.8333 | 0.5635 | |
| M3 SMILES, GAO (1ECk) | Sub-Training | 15 | 33 | 1 | 3 | 52 | 0.8333 | 0.9706 | 0.9231 | 0.8287 |
| Calibration | 5 | 13 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 3 | 10 | 4 | 1 | 18 | 0.7500 | 0.7143 | 0.7222 | 0.3959 | |
| M4 SMILES, HFG (0ECk) | Sub-Training | 16 | 34 | 0 | 2 | 52 | 0.8889 | 1.0000 | 0.9615 | 0.9162 |
| Calibration | 5 | 13 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 4 | 8 | 6 | 0 | 18 | 1.0000 | 0.5714 | 0.6667 | 0.4781 | |
| M5 SMILES, HFG (1ECk) | Sub-Training | 16 | 34 | 0 | 2 | 52 | 0.8889 | 1.0000 | 0.9615 | 0.9162 |
| Calibration | 5 | 13 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 3 | 9 | 5 | 1 | 18 | 0.7500 | 0.6429 | 0.6667 | 0.3287 | |
| M6 SMILES, HSG (0ECk) | Sub-Training | 17 | 34 | 0 | 1 | 52 | 0.9444 | 1.0000 | 0.9808 | 0.9578 |
| Calibration | 5 | 12 | 1 | 0 | 18 | 1.0000 | 0.9231 | 0.9444 | 0.8771 | |
| Test | 4 | 11 | 3 | 0 | 18 | 1.0000 | 0.7857 | 0.8333 | 0.6701 | |
| M7 SMILES, HSG (1ECk) | Sub-Training | 16 | 33 | 1 | 2 | 52 | 0.8889 | 0.9706 | 0.9423 | 0.8717 |
| Calibration | 5 | 13 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 3 | 11 | 3 | 1 | 18 | 0.7500 | 0.7857 | 0.7778 | 0.4725 | |
| Split-2 | ||||||||||
| M8 SMILES | Sub-Training | 15 | 34 | 2 | 1 | 52 | 0.9375 | 0.9444 | 0.9423 | 0.8677 |
| Calibration | 4 | 14 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 4 | 9 | 2 | 3 | 18 | 0.5714 | 0.8182 | 0.7222 | 0.4029 | |
| M9 SMILES, GAO (0ECk) | Sub-Training | 12 | 34 | 2 | 4 | 52 | 0.7500 | 0.9444 | 0.8846 | 0.7226 |
| Calibration | 4 | 14 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 4 | 10 | 1 | 3 | 18 | 0.5714 | 0.9091 | 0.7778 | 0.5230 | |
| M10 SMILES, GAO (1ECk) | Sub-Training | 16 | 36 | 0 | 0 | 52 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| Calibration | 4 | 14 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 4 | 6 | 5 | 3 | 18 | 0.5714 | 0.5455 | 0.5556 | 0.1140 | |
| M11 SMILES, HFG (0ECk) | Sub-Training | 14 | 35 | 1 | 2 | 52 | 0.8750 | 0.9722 | 0.9423 | 0.8631 |
| Calibration | 4 | 14 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 4 | 11 | 0 | 3 | 18 | 0.5714 | 1.0000 | 0.8333 | 0.6701 | |
| M12 SMILES, HFG (1ECk) | Sub-Training | 11 | 33 | 3 | 5 | 52 | 0.6875 | 0.9167 | 0.8462 | 0.6287 |
| Calibration | 4 | 14 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 5 | 11 | 0 | 2 | 18 | 0.7143 | 1.0000 | 0.8889 | 0.7774 | |
| M13 SMILES, HSG (0ECk) | Sub-Training | 12 | 36 | 0 | 4 | 52 | 0.7500 | 1.0000 | 0.9231 | 0.8216 |
| Calibration | 4 | 14 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 3 | 10 | 1 | 4 | 18 | 0.4286 | 0.9091 | 0.7222 | 0.3959 | |
| M14 SMILES, HSG (1ECk) | Sub-Training | 14 | 33 | 3 | 2 | 52 | 0.8750 | 0.9167 | 0.9038 | 0.7789 |
| Calibration | 4 | 14 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 5 | 10 | 1 | 2 | 18 | 0.7143 | 0.9091 | 0.8333 | 0.6447 | |
| Split-3 | ||||||||||
| M15 SMILES | Sub-Training | 15 | 33 | 1 | 3 | 52 | 0.8333 | 0.9706 | 0.6231 | 0.8287 |
| Calibration | 4 | 14 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 4 | 13 | 0 | 1 | 18 | 0.8000 | 1.0000 | 0.9444 | 0.8619 | |
| M16 SMILES, GAO (0ECk) | Sub-Training | 18 | 34 | 0 | 0 | 52 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| Calibration | 4 | 14 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 4 | 10 | 3 | 1 | 18 | 0.8000 | 0.7692 | 0.7778 | 0.5230 | |
| M17 SMILES, GAO (1ECk) | Sub-Training | 17 | 34 | 0 | 1 | 52 | 0.9444 | 1.0000 | 0.9808 | 0.9578 |
| Calibration | 4 | 14 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 4 | 12 | 1 | 1 | 18 | 0.8000 | 0.9231 | 0.8889 | 0.7231 | |
| M18 SMILES, HFG (0ECk) | Sub-Training | 16 | 33 | 1 | 2 | 52 | 0.8889 | 0.9706 | 0.9423 | 0.8717 |
| Calibration | 2 | 14 | 0 | 2 | 18 | 0.5000 | 1.0000 | 0.8889 | 0.6614 | |
| Test | 5 | 12 | 1 | 0 | 18 | 1.0000 | 0.9231 | 0.9444 | 0.8771 | |
| M19 SMILES, HFG (1ECk) | Sub-Training | 16 | 33 | 1 | 2 | 52 | 0.8889 | 0.9706 | 0.9423 | 0.8717 |
| Calibration | 4 | 14 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 4 | 12 | 1 | 1 | 18 | 0.8000 | 0.9231 | 0.8889 | 0.7231 | |
| M20 SMILES, HSG (0ECk) | Sub-Training | 16 | 33 | 1 | 2 | 52 | 0.8889 | 0.9706 | 0.9423 | 0.8717 |
| Calibration | 4 | 14 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 3 | 11 | 2 | 2 | 18 | 0.6000 | 0.8462 | 0.7778 | 0.4462 | |
| M21 SMILES, HSG (1ECk) | Sub-Training | 16 | 32 | 2 | 2 | 52 | 0.8889 | 0.9412 | 0.9231 | 0.8301 |
| Calibration | 4 | 14 | 0 | 0 | 18 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | |
| Test | 5 | 12 | 1 | 0 | 18 | 1.0000 | 0.9231 | 0.9444 | 0.8771 | |
The selected model is shown in bold face; True positive (TP); False negative (FN); False positive (FP); True negative (TN); Total number of compounds (NTotal).
From the Table 2, it may be observed that the model M21 showed satisfactory statistical property. The sensitivity, specificity and accuracy as well as MCC values obtained for different sets were statistically significant. The values attained for the test set such as sensitivity, specificity, accuracy and MCC of 100%, 92.31%, 94.44% and 87.71%, respectively) justified the acceptable predictability of classification based QSAR model. Therefore, the model M21 (SMILES and HSG with 1ECk) from split-3 was found to be the best among 21 developed models. The model was also successfully passed Y-randomization test (MCCr2 i.e., average randomized CR2p > 0.5). The end point value calculates for M21 is follows:
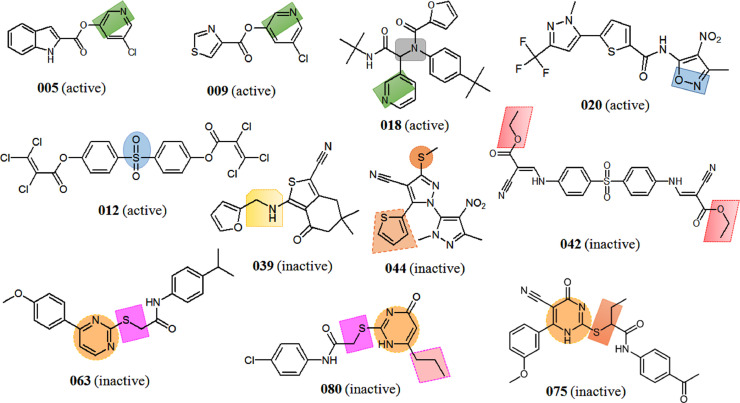
Moreover, different structural attributes of the model M21 (SMILES and HSG with 1ECk) from split-3 is given in Table S3. Positive structural attribute like n...c...c... (representing the presence of nitrogen atom attached to two sp2carbon atoms) was found to have influence on the activity of the Mpro inhibitors. This feature was found in compound 005, 009, 018 (Fig. 4 ) possessing good SARS-CoV Mpro inhibitory activities. As can be observed from Fig. 4, the presence of sulphur atom attached to doubly bonded oxygen atom represented by the structural attribute ++++O—B2==,would lead to moderate increase in Mpro pIC50 values in the compounds 010, 012 and 022 etc.
Fig. 4.
Examples of some prototype SARS-CoV Mpro inhibitors with structural attributes obtained from best model M21.
On the other hand, the presence of N...C...(... (nitrogen attached to an sp3 carbon atom having branching), also found to play a crucial role in promoting the inhibitory activity of compound 018. Similarly, NOSP11000 (existence of nitrogen and oxygen atom) in compound 020 might induce inhibitory activity. Not surprisingly, the compounds containing two or more positive structural attributes were found to have better SARS-CoV Mpro pIC50 values and considered to be higher actives as can be observed from Fig. 4. The analysis of structural attributes from Monte Carlo Optimization results also highlighted similar fragments obtained from our current SPCI analysis. Similar fragments between the two analyses are highlighted in Table 3 .
Table 3.
The fragments/fingerprints modulating the SARS-CoV Mpro inhibitory activities.
| Entry | SPCI analysis | Monte Carlo Optimization based QSAR | Contribution |
|---|---|---|---|
| 1 | Pyridine | n........... | Positive |
| 2 | Furan | o........... | Positive |
| 3 | CHO (aldehyde) | c...(...O... | Positive |
| 4 | CH3O (aromatic) | O...(...C... | Negative |
| 5 | NO2 (aromatic) | ++++N—B3== | Negative |
| 6 | F (aliphatic) | F........... | Negative |
| 7 | Phenyl unsubst | 1........... | Negative |
| 8 | CO (ketone) | O...(...C... | Negative |
| 9 | Thiophene | s...1....... | Negative |
| 10 | Cl (aromatic) | c...(...Cl.. | Negative |
The lower activities of molecules 061-080 (Fig. 4) may be related to the presence of pyrimidine ring in their structure represented by the combined structural attributes n...1...c... and n...c...(.....Thus, it may be anticipated that the replacement of pyrimidine led to improve in the SARS-CoV Mpro inhibitory activities. Further, the structural attribute S...C.... signified the presence of sulphur atom bonded to a sp 3 carbon atom in compound 063 and 080 was also answer able for hindrance of activity. Additionally, the structural attribute S...C...(... found in compound 075 was also responsible for lowering the activity as shown in Fig. 4. In compounds, the structural attribute S........... suggested the presence of sulphur atom outside a ring is also responsible for lowering the activity of Mpro inhibitors. This may be one of the reason for lower inhibition potential of compound 044 (Fig. 4). For instance, compound 044 (Fig. 3) was found as lower active Mpro inhibitors, although having two diazole and one thiophene moieties. In this case a thiophene moiety and the presence of NO2 (aromatic) negatively contributed towards biological activity. Moreover, the compound 044 also possessed –SCH3 which is also negative contributor as suggested by negative structural attribute S...C....... towards the inhibition (Fig. 4).
Meanwhile, structural attributes O...(...C...(oxygen atom having branching attached to sp3 carbon) and C...C....... (two consecutive sp3 carbon actom) exhibited negative contribution in compounds 042 and 080, respectively (Fig. 4). Furthermore, the attribute N...(...C... (nitrogen atom having branching attached to sp3 carbon) shown in many compounds including 035, 039, 058 etc. also negatively contributed to the SARS-CoV Mpro inhibition (Fig. 4). Notably, some good structural attributes such as s...(...c...), ++++O—B2== etc. are also found in some lower active Mpro inhibitors (039, 042, 087 and 067, Fig. 4), but their strong negatively contributing groups further reduces their SARS-CoV Mpro inhibitory activities.
3.3. QSAR derived prediction
Since the SARS-CoV-2 Mpro shares about 96% sequence similarity with SARS-CoV Mpro (while genome has over 80% identity), previously reported SARS-CoV Mpro inhibitors may have huge prospect to show their efficacy against SARS-CoV-2 Mpro also. Thus, considering high statistical significance of the best Monte Carlo optimization based QSAR model, we applied the model M21 (SMILES and HSG with 1ECk) from split-3 to perform QSAR derived prediction of a library of nature product hits from recent publications [7], [8], [9], [13], [16], [17], [18], [19], [20], [23], [24], [26], [27], [28]. The lists of nature product hits are depicted in Table S4.
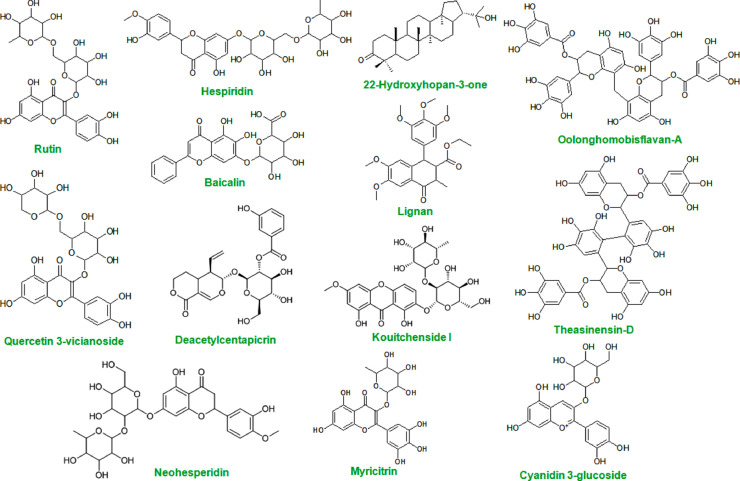
After screening with the model M21, a number of 13 molecules from natural origin were predicted as actives (Table 4 ). These 13 compounds including Rutin, Hespiridine, 22-Hydroxyhopan-3-one, Oolonghomobisflavan-A, Theasinensin-D, Quercetin 3-vicianoside, Deacetylcentapicrin, Kouitchenside I, Neohesperidin, Lignan, Myricitrin, Baicalin, Cyanidin 3-glucoside were considered as the most potent virtual hits for coronavirus Mpro inhibition Fig. 5 .
Table 4.
The name and common sources of the most potent virtual hits for corona virus Mpro inhibition.
| Cpda | Name | Common sources | Pharmacological actions | Reference |
|---|---|---|---|---|
| N1 | Rutin | Passion flower, buckwheat, tea, and apple | Antioxidant, cytoprotective,anticarcinogenic, antiviral, vasoprotective, neuroprotective and cardioprotective activities | [44] |
| N2 | Hespiridine | Citrus fruits including such as oranges, lemons, grapefruits, pomelos and limes | Antimicrobial, cardiovascular function, type II diabetes, anti-inflammation, wound healing, UV protection, antiskin cancer and skin lightening. | [45] |
| N9 | 22-Hydroxyhopan-3-one | Cassia siamea (Fabaceae) | – | [18] |
| N11 | Oolonghomobisflavan-A | It is a constituent of oolong tea. | Antioxidant, antiobesity activity | [46] |
| N13 | Theasinensin-D | Black tea and oolong tea | Antioxidant and antimicrobial effect | [47] |
| N14 | Quercetin 3-vicianoside | – | – | [9] |
| N28 | Deacetylcentapicrin | Swertia macrosperma | Anti-virus, antiinflammatory action | [27] |
| N30 | Kouitchenside I | Swertia kouitchensis | α-Glucosidase inhibitory activity; anti-virus, antiinflammatory action | [27], [48] |
| N31 | Neohesperidin | Citrus fruits including such as oranges, lemons, grapefruits, pomelos and limes | Food supplement | [49] |
| N48 | Lignan | Kiwi fruit, asparagus, pineapple, grapes, lemons, oranges and even in tea and coffee | Antioxidant, antiinflammatory effect | [50] |
| N55 | Myricitrin | Bayberry | Nitric oxide (NO) and protein kinase C (PKC) inhibitor. Also shows antipsychotic-like and anxiolytic-like actions | [51] |
| N57 | Baicalin | Scutellaria baicalensis | Anxiolytic effects without sedative or myorelaxant effects, induces cancer cell apoptosis | [52] |
| N58 | Cyanidin 3-glucoside | Blackberry | Chemopreventive and chemotherapeutic activity | [53] |
Compound number.
Fig. 5.
The chemical structure of the most potent virtual hits for corona virus Mpro inhibition.
From the Fig. 5, it may be conferred that all these actives were structurally similar to each other. Most of these compounds were polyphenols. Maximum of these molecules contain ring fragments while the oxygen atom were common in all these structures. The different structural fragments such as o........... (presence of oxygen in a ring), (........... (presence of branching), ++++O—B2== (presence of double bonded oxygen), O...c...1...(presence of OH/OCH3 attached with ring) etc. are responsible for their predicted Mpro inhibitory activity. The molecular docking interactions analysis also reported that these hits found to potentially bind with active site amino acid residues of SARS-CoV-2 Mpro [8], [13], [16], [18], [24], [27], [28]. The molecular docking study performed by Das and co-workers suggested that rutin (also known as vitamin P) forms non-covalent interactions with the SARS-CoV-2 Mpro active site residues [13]. It interacts with H41, L141, N142, E166, T190 and Q192 by forming hydrogen bonding. Moreover, a π-sulphur and π-alkyl interactions were noticed with C145 and P168, respectively. Hesperidin forms amide-π stacked interaction with T45, π-alkyl interactions with M49 and C145 as well as hydrogen bonding interactions with T24, T25, T45, S46 and C145 [13]. Both these two dietary polyphenols (rutin and hesperidin) having low systemic toxicity indicate promising potential for the treatment of COVID-19.
Apart from hesperidin, another active constituent, neohesperidin, from Citrus aurantium was also found to be active as per our QSAR based prediction. Moreover, kouitchenside I and deacetylcentapicrin from the plants of Swertia genus were also predicted as actives.
A pentacyclic triterpene, 22-hydroxyhopan-3-one from Cassia siamea (Fabaceae) showed AutoDock Vina 4.2 promising binding affinity (-8.6 kcal/mol) against Mpro of SARS-CoV-2 (PDB: 6LU7) [18]. 22-Hydroxyhopan-3-one forms conventional hydrogen bond with K137 along with alkyl and π-alkyl interactions with L275, L287, L286 and Y239, respectively [18].
Oolonghomobisflavan-A is an important polymerized polyphenol present in Tea. The semi-flexible docking tool CDOCKER utility of Discovery Studio suggested that Oolonghomobisflavan-A possesses two π-alkyl (M165, H41), and one π-π T-shaped interaction (H41) as well as forms several hydrogen bonds with T25, N142, H163, E166, R188, and H164 [16]. It showed the binding free energy of -256.875 kJ/mol better than the drug Lopinavir (binding free energy of -250.585kJ/mol) as per MM-PBSA calculations [16].
Quercetin 3-vicianoside interact with catalytic amino acid residues (PDB: 6LU7) by forming hydrogen bonds with L141, G143, S144, H163, E166 and hydrophobic bonds with T25, His41, F140, N142, C145, H164, M165, D187, R188, Q189 [9].
Myricitrin (Plant source: Myrica cerifera) showed a docking score of -15.64 and binding affinity of -22.13 kcal/mol [24]. It forms several hydrogen bonding and other interactions with amino acid residues T24, T25, T26, L27, H41, C44, S46, M49, L141, N142, G143, S144, C145, H163, E166 and Q189 [24].
Baicalin (Plant source: S. baicalensis) is an experimentally manifested antiviral representative against SARS-CoV [54], SARS-CoV-2 [22]. Notably, baicalin exhibited an IC50 of 6.41 µM against SARS-CoV-2 Mpro along with Kd of 11.50 µM [21]. In addition, the docking study of baicalin performed by Islam et al. [8] showed interaction through one hydrophobic, one π-sulfur and six hydrogen bonding interactions with the catalytic residues of SARS-CoV-2 Mpro (AutoDock Vina score of -8.1 kcal/mol and GOLD score of 59.19) [8]. Cyanidin 3-glucoside exhibits numerous hydrogen bonding interactions and hydrophobic interactions (AutoDock Vina score of -8.4 kcal/mol) in which one hydrophobic interaction is noticed with the catalytic C145 [8].
These hits could be tested for their in-vitro and in-vivo inhibition potential against SARS-CoV-2 Mpro. Further, the backbone structure of these molecules could be exploited to develop more potent Mpro inhibitors in future.
4. Conclusion
Quantitative structure-activity relationship (QSAR) study is an efficient technique that extracts crucial information from complex datasets. Recently, QSAR modelling truly recognised the effect of structural and physicochemical features of compounds on the investigated biological activity and also offers simultaneous prediction of virtual libraries.
In this current study, we developed multiple classification QSAR models with a diverse dataset of compounds possessing SARS-CoV Mpro inhibitory properties. On one hand, the Structural and physico-chemical interpretation (SPCI) analysis was accomplished to perform fragment/fingerprint analysis, where the contributions of different molecular features modulating Mpro inhibition were estimated. On the other hand, Monte Carlo optimization based QSAR was constructed and the best model was further used for screening of a library of nature product hits from recent publications. Lastly, the resulted active molecules analysed from the aspects of fragment analysis and highlighted their natural sources. This study surely motivate medicinal chemists to rejuvenate potential chemicals by joining fragments/features together or attaching with other scaffolds in hopes to trigger antiviral potency against Mpro of SARS-CoV-2 and other coronavirus as well as efficacy without accruing much toxicities.
CRediT authorship contribution statement
Kalyan Ghosh: Data curation, Methodology, Software, Investigation. Sk. Abdul Amin: Conceptualization, Visualization, Investigation, Writing - original draft. Shovanlal Gayen: Conceptualization, Writing - review & editing. Tarun Jha: Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors have no conflict of interests.
Acknowledgment
Financial assistance from the Council of Scientific and Industrial Research (CSIR), New Delhi, India in the form of a Senior Research Fellowship (SRF) [FILE NO.: 09/096(0967)/2019-EMR-I, Dated: 01-04-2019] to Sk. Abdul Amin is thankfully acknowledged. Tarun Jha is also thankful for the financial support from RUSA 2.0 of UGC, New Delhi, India to Jadavpur University, Kolkata, India. Authors are thankful to Prof. Alla P. Toropova and Prof. Andrey Toropov of Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Italy for their useful suggestions during preparation of the revised manuscript. We are very much thankful to the Department of Pharmaceutical Technology, Jadavpur University, Kolkata, India and Department of Pharmaceutical Sciences, Dr. Harisingh Gour University, India for providing the research facilities.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.molstruc.2020.129026.
Appendix. Supplementary materials
References
- 1.Ghosh A.K., Brindisi M., Shahabi D., Chapman M.E., Mesecar A.D. Drug development and medicinal chemistry efforts toward SARS-coronavirus and Covid-19 therapeutics. ChemMedChem. 2020 doi: 10.1002/cmdc.202000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pillaiyar T., Meenakshisundaram S., Manickam M. Recent discovery and development of inhibitors targeting coronaviruses. Drug Discov. Today. 2020 doi: 10.1016/j.drudis.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amin S.A., Ghosh K., Gayen S., Jha T. Chemical-informatics approach to COVID-19 drug discovery: Monte Carlo based QSAR, virtual screening and molecular docking study of some in-house molecules as papain-like protease (PLpro) inhibitors. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1780946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020 (as accessed on 7th June 2020).
- 5.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (as accessed on 8th July 2020).
- 6.http://www.who.int/csr/sars/archive/2003_05_07a/en (as accessed on 10th May 2020).
- 7.Enmozhi S.K., Raja K., Sebastine I., Joseph J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in silico approach. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1760136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Islam R., Parves R., Paul A.S., Uddin N., Rahman M.S., Mamun A.A., Hossain M.N., Ali M.A., Halim M.A. A molecular modeling approach to identify effective antiviral phytochemicals against the main protease of SARS-CoV-2. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1761883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshi T., Joshi T., Sharma P., S.Mathpal H.Pundir, Bhatt V., Chandra S. In silico screening of natural compounds against COVID-19 by targeting Mpro and ACE2 using molecular docking. Eur. Rev. Med. Pharmaco. 2020;24 doi: 10.26355/eurrev_202004_21036. [DOI] [PubMed] [Google Scholar]
- 10.Joshi R.S., Jagdale S.S., Bansode S.B., Shankar S.S., Tellis M.B., Pandya V.K., Chugh A., Giri A.P., Kulkarni M.J. Discovery of potential multi-target-directed ligands by targeting host-specific SARS-CoV-2 structurally conserved main protease. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1760137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan M.T., Ali A., Wang Q., Irfan M., Khan A., Zeb M.T., Zhang Y.J., Chinnasamy S., Wei D.Q. Marine natural compounds as potents inhibitors against the main protease of SARS-CoV-2. A molecular dynamic study. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1769733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan R.J., Jha R.K., Amera G.M., Jain M., Singh E., Pathak A., Singh R.P., Muthukumaran J., Singh A.K. Targeting SARS-CoV-2: a systematic drug repurposing approach to identify promising inhibitors against 3C-like proteinase and 2′-O-ribose methyltransferase. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1753577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das S., Sarmah S., Lyndem S., Roy A.S. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1763201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan S.A., Zia K., Ashraf S., Uddin R., Ul-Haq Z. Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1751298. [DOI] [PubMed] [Google Scholar]
- 15.Al-Khafaji K., AL-DuhaidahawiL D., Tok T.T. Using integrated computational approaches to identify safe and rapid treatment for SARS -CoV-2. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1764392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhardwaj V.K., Singh R., Sharma J., Rajendran V., Purohit R., Kumar S. Identification of bioactive molecules from Tea plant as SARS-CoV-2 main protease inhibitors. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1766572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurung A.B., Ali M.A., Lee J., Farah M.A., Al-Anazi K.M. Unravelling lead antiviral phytochemicals for the inhibition of SARS-CoV-2 Mpro enzyme through in silico approach. Life Sci. 2020 doi: 10.1016/j.lfs.2020.117831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gyebi G.A., Ogunro O.B., Adegunloye A.P., Ogunyemi O.M., Afolabi S.O. Potential inhibitors of coronavirus 3-chymotrypsin-like protease (3CLpro): an in-silico screening of Alkaloids and Terpenoids from African medicinal plants. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1764868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar D., Kumari K., Jayaraj A., Kumar V., Kumar R.V., Dass S.K., Chandra R., Singh P. Understanding the binding affinity of noscapines with protease of SARS-CoV-2 for COVID-19 using MD simulations at different temperatures. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1752310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren X., Shao X., Li X.X., Jia X.H., Song T., Zhou W.Y., Wang P., Li Y., Wang X.L., Cui Q.H., Qiu P.J. Identifying potential treatments of COVID-19 from traditional chinese medicine (TCM) by using a data-driven approach. J. Ethnopharmacol. 2020 doi: 10.1016/j.jep.2020.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su H., Yao S., Zhao W., Li M., Liu J., Shang W., H.Xie C.Ke, Gao M., Yu K., Liu H. Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease in vitro. BioRxiv. 2020 doi: 10.1101/2020.04.13.038687. [DOI] [Google Scholar]
- 22.Liu H., Ye F., Sun Q., Liang H., Li C., Lu R., Huang B., Tan W., Lai L. Scutellariabaicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. BioRxiv. 2020 doi: 10.1101/2020.04.10.035824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mittal L., Kumari A., Srivastava M., Singh M., Asthana S. Identification of potential molecules against COVID-19 main protease through structure-guided virtual screening approach. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1768151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ulQamar M.T., Alqahtani S.M., Alamri M.A., Chen L.-L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants†. J. Pharm. Anal. 2020 doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pant S., Singh M., Ravichandiran V., Murty U.S.N., Srivastava H.K. Peptide-like and small-molecule inhibitors against Covid-19. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1757510Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umesh D., Kundu C., Selvaraj, Singh S.K., Dubey V.K. Identification of new anti-nCoV drug chemical compounds from Indian spices exploiting SARS-CoV-2 main protease as target. J. Biomol. Struct. Dyn. 2020:1–9. doi: 10.1080/07391102.2020.1763202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z.J., Wu W.Y., Hou J.J., Zhang L.L., Li F.F., Gao L., Wu X.D., Shi J.Y., Zhang R., Long H.L., Lei M. Active constituents and mechanisms of respiratory detox shot, a traditional Chinese medicine prescription, for COVID-19 control and prevention: network-molecular docking-LC-MSE analysis. Integr. Med. 2020 doi: 10.1016/j.joim.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neves B.J., Braga R.C., Melo-Filho C.C., Moreira-Filho J.T., Muratov E.N., Andrade C.H. QSAR-based virtual screening: advances and applications in drug discovery. Front. Pharmacol. 2018;9:1275. doi: 10.3389/fphar.2018.01275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown N., Ertl P., Lewis R., Luksch T., Reker D., Schneider N. Artificial intelligence in chemistry and drug design. J. Comput. Aided Mol. Des. 2020;34:709–715. doi: 10.1007/s10822-020-00317-x. [DOI] [PubMed] [Google Scholar]
- 31.Adhikari N., Baidya S.K., Saha A., Jha T. In Viral Proteases and their Inhibitors. Academic Press; U.S.A: 2017. Structural insight into the viral 3C-like protease inhibitors: comparative SAR/QSAR approaches; pp. 317–409. [Google Scholar]
- 32.Amin S.A., Adhikari N., Jha T. Design of aminopeptidase N inhibitors as anti-cancer agents. J. Med. Chem. 2018;61:6468–6490. doi: 10.1021/acs.jmedchem.7b00782. [DOI] [PubMed] [Google Scholar]
- 33.Amin S.A., Adhikari N., Jha T. Exploration of histone deacetylase 8 inhibitors through classification QSAR study: Part II*. J. Mol. Struct. 2020;1204 [Google Scholar]
- 34.Amin S.A., Jha T. Fight against novel coronavirus: A perspective of medicinal chemists. Eur. J. Med. Chem. 2020;201 doi: 10.1016/j.ejmech.2020.112559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bobrowski T., Alves V.M., Melo-Filho C.C., Korn D., Auerbach S., Schmitt C., Muratov E.N., Tropsha A. Computational models identify several FDA approved or 1 experimental drugs as putative agents against SARS-CoV-2. ChemRxiv. 2020 doi: 10.26434/chemrxiv.12153594.v1. [DOI] [Google Scholar]
- 36.https://chemrxiv.org/articles/Computational_Models_Identify_Several_FDA_Approved_or_Experimental_Drugs_as_Putative_Agents_Against_SARS-CoV-2/12153594/1 (as accessed on 21st May 2020).
- 37.Polishchuk P. Interpretation of quantitative structure–activity relationship models: past, present, and future. J. Chem. Inf. Model. 2017;57:2618–2639. doi: 10.1021/acs.jcim.7b00274. [DOI] [PubMed] [Google Scholar]
- 38.Polishchuk P., Tinkov O., Khristova T., Ognichenko L., Kosinskaya A., Varnek A., Kuz'min V. Structural and physico-chemical interpretation (SPCI) of QSAR models and its comparison with matched molecular pair analysis. J. Chem. Inf. Model. 2016;56:1455–1469. doi: 10.1021/acs.jcim.6b00371. [DOI] [PubMed] [Google Scholar]
- 39.Toropova A.P., Toropov A.A., Benfenati E. A quasi-QSPR modelling for the photocatalyticdecolourization rate constants and cellular viability (CV%) of nanoparticles by CORAL. SAR QSAR Environ. Res. 2015;26:29–40. doi: 10.1080/1062936X.2014.984327. [DOI] [PubMed] [Google Scholar]
- 40.Toropov A.A., Toropova A.P., Puzyn T., Benfenati E., Gini G., Leszczynska D., Leszczynski J. QSAR as a random event: modeling of nanoparticles uptake in PaCa2 cancer cells. Chemosphere. 2013;92:31–37. doi: 10.1016/j.chemosphere.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Toropov A.A., Toropova A.P., Raitano G., Benfenati E. CORAL: building up QSAR models for the chromosome aberration test. Saudi J. Biol. Sci. 2018 doi: 10.1016/j.sjbs.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Worachartcheewan A., Mandi P., Prachayasittikul V., Toropova A.P., Toropov A.A., Nantasenamat C. Large-scale QSAR study of aromatase inhibitors using SMILES-based descriptors. Chemometr.Intell. Lab. 2014;138:120–126. [Google Scholar]
- 43.https:https://github.com/DrrDom/rspci (as accessed on 21st May 2020).
- 44.Ganeshpurkar A., Saluja A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017;25:149–164. doi: 10.1016/j.jsps.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Man M.Q., Yang B., Elias P.M. Evidence-Based Complementary and Alternative Medicine; 2019. Benefits of hesperidin for cutaneous functions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sukhbold E., Sekimoto S., Watanabe E., Yamazaki A., Yang L., Takasugi M., Yamada K., Hosomi R., Fukunaga K., Arai H. Effects of oolonghomobisflavanA on oxidation of low-density lipoprotein. Biosci. Biotechnol. Biochem. 2017;81:1569–1575. doi: 10.1080/09168451.2017.1314758. [DOI] [PubMed] [Google Scholar]
- 47.Weerawatanakorn M., Hung W.L., Pan M.H., Li S., Li D., Wan X., Ho C.T. Chemistry and health beneficial effects of oolong tea and theasinensins. Food Sci. Hum. Wellness. 2015;4:133–146. [Google Scholar]
- 48.Ruan J., Zheng C., Liu Y., Qu L., Yu H., Han L., Zhang Y., Wang T. Chemical and biological research on herbal medicines rich in xanthones. Molecules. 2017;22:1698. doi: 10.3390/molecules22101698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winnig M., Bufe B., Kratochwil N.A., Slack J.P., Meyerhof W. The binding site for neohesperidindihydrochalcone at the human sweet taste receptor. BMC Struct. Biol. 2007;7:66. doi: 10.1186/1472-6807-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Korkina L., Kostyuk V., De Luca C., Pastore S. Plant phenylpropanoids as emerging anti-inflammatory agents. Mini Rev. Med. Chem. 2011;11:823–835. doi: 10.2174/138955711796575489. [DOI] [PubMed] [Google Scholar]
- 51.Pereira M., Siba I.P., Chioca L.R., Correia D., Vital M.A., Pizzolatti M.G., Santos A.R., Andreatini R. Myricitrin, a nitric oxide and protein kinase C inhibitor, exerts antipsychotic-like effects in animal models. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35:1636–1644. doi: 10.1016/j.pnpbp.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Tao Y., Zhan S., Wang Y., Zhou G., Liang H., Chen X., Shen H. Baicalin, the major component of traditional Chinese medicine Scutellariabaicalensis induces colon cancer cell apoptosis through inhibition of onco miRNAs. Sci. Rep. 2018;8:1. doi: 10.1038/s41598-018-32734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding M., Feng R., Wang S.Y., Bowman L., Lu Y., Y.Qian V.Castranova, Jiang B.H., Shi X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventiveand chemotherapeutic activity. J. Biol. Chem. 2006;281:17359–17368. doi: 10.1074/jbc.M600861200. https://www.jbc.org/content/281/25/17359.full [DOI] [PubMed] [Google Scholar]
- 54.Chen F., Chan K.H., Jiang Y., Kao R.Y., Lu H.T., Fan K.W., Cheng V.C., Tsui W.H., Hung I.F., Lee T.S., Guan Y. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.