Abstract
Purpose
To investigate the morphologic features of crystalline lens in primary angle closure disease (PACD) by the swept-source anterior segment optical coherence tomography.
Methods
This cross-sectional observational study included 125 consecutive eyes from 125 patients who underwent anterior segment optical coherence tomography (CASIA 2, Tomey, Nagoya, Japan) examination, including 38 eyes of normal controls, 57 eyes of PAC suspects (PACS), and 30 eyes with PAC or PAC glaucoma (PACG). Crystalline lens parameters were compared among the three groups. Spearman correlation analysis and multiple linear regression models were performed to evaluate the relationships between the lens parameters and related factors.
Results
Significant differences were found for anterior chamber depth, axial length, iridotrabecular contact index, lens vault, lens thickness (all P < 0.001), the anterior radius curvature of lens (normal vs PACS vs PAC/PACG: 9.35 ± 1.29 mm vs 8.40 ± 0.62 mm vs 8.12 ± 0.54 mm; P < 0.001), lens decentration (normal vs PACS vs PAC/PACG: 0.14 ± 0.07 mm vs 0.19 ± 0.09 mm vs 0.22 ± 0.12 mm; P = 0.004), and tilt (normal vs PACS vs PAC/PACG: 4.9 ± 1.0° vs 5.3 ± 1.2° vs 5.8 ± 1.8°; P = 0.033) among the three groups. The multivariate regression analysis found that both iridotrabecular contact index and the stage of the PACD were negatively correlated with the anterior radius curvature of lens, positively correlated with lens thickness and decentration after adjustment for age, sex, and axial length (all P < 0.05).
Conclusions
Steep anterior curvature and decentration of the crystalline lens may be another anatomic characteristic of eyes with PACD. These findings support that the crystalline lens morphologic features may have great contribution to the development of PACD.
Keywords: primary angle closure disease, anterior segment optical coherence tomography, crystalline lens, CASIA 2
Glaucoma is the second leading cause of vision loss in the world with an estimated 67 million people affected. Nearly one-half of these cases are primary angle closure glaucoma (PACG),1 which has the highest prevalence in Asian.2,3 Although PACG can cause severe visual impairment if untreated, it can be cured or well controlled when in early stage such as primary angle closure suspect (PACS), as well as primary angle closure (PAC). PACG, PAC and PACS can be classified into primary angle closure disease (PACD).4
Biometric studies have shown that PACD has a shallow anterior chamber depth (ACD) and a short axial length (AL).5–7 Pupillary block and nonpupillary block have been proposed as two main mechanisms underlying the pathogenesis of PACD.8–10 Furthermore, previous studies have also demonstrated that the crystalline lens plays an important role in the development of PACG, such as the lens thickness (LT) and relative lens position.5,11–16 However, the distribution and change of lens morphologic features during the progression of PACD remains unclear.
In recent years, a new generation of swept-source anterior segment optical coherence tomography (AS-OCT, CASIA 2, Tomev Corporation, Nagoya, Japan) has come into use. It has a wide scanning range of 16 mm, which allows an entire cross-section of the anterior and posterior lens surface to be captured simultaneously. It can calculate the detailed parameters of lens, including the anterior and posterior curvatures, the decentration and the tilt of the lens. In the current study we aimed to explore the lens morphologic features in different stages of PACD by using the CASIA 2.
Methods
Participants
All participants in the study were enrolled from the glaucoma clinic of Eye, Ear, Nose and Throat Hospital of Fudan University (Shanghai, China). This cross-sectional, comparative study was approved by the Ethical Review Committee of the Eye, Ear, Nose and Throat Hospital and it adhered to the Declaration of Helsinki for Research Involving Human Participants. Written informed consent was obtained from each participant involved in the study. All participants were from the Chinese Han population.
Participants diagnosed as PACS, PAC, and PACG were included.4,17 PACS was defined as an eye with pigmented trabecular meshwork invisible for 180° or greater under static gonioscopy but without peripheral anterior synechiae, IOP, or glaucomatous optic neuropathy. Peripheral anterior synechiae was defined as a region of iridotrabecular contact that could not be opened by indentation gonioscopy.18 PAC was defined as an eye with presence of peripheral anterior synechiae or elevated IOP, but without glaucomatous optic neuropathy. PACG was defined as an eye with PAC and glaucomatous optic neuropathy. When both eyes of one patient met the criteria, the eye in the more advanced stage or with better image quality was included for analysis.
The inclusion criteria for the normal subjects were as follows: open angle, normal-appearing optic nerve head; intact neuroretinal rim and retinal nerve fiber layer; normal standard automated perimetry; and no records of elevated IOP of more than 21 mm Hg.
Patients who (1) had incisional surgery or laser peripheral iridotomy (LPI) in either eye (including cataract surgery), (2) had histories of eye diseases (such as uveitis, trauma), or (3) under the use of pilocarpine were excluded.
Ophthalmic Examination and AS-OCT Imaging
Participants underwent a complete ophthalmic examination, including a review of their medical history, measurements of best-corrected visual acuity (BCVA), refractive status, slit-lamp biomicroscopy, IOP (Goldmann applanation tonometry), fundoscopy (TRC-NW200, Topcon Medical Systems, Oakland, NJ), ultrasound biomicroscopy, gonioscopy, central corneal thickness (CCT) and AL (Lenstar, Carl Ziess Inc., Jena, Germany), retinal nerve fiber layer, ganglion cell complex thickness (RTvue OCT; Optovue Inc, Toledo, OH), and visual field (Humphrey Field Analyzer, 24-2; Carl Zeiss Meditec, Dublin, CA). The mean spherical equivalence (MSE) was calculated as the spherical diopter plus one-half of the cylindrical dioptric power. The lens opacity of each eye was graded using the Lens Opacities Classification System III standards. The nuclear opalescence (NO), nuclear color (NC), cortical (C), and posterior subcapsular (P) cataract were compared with the standard grading color photographs. NO and NC were graded on a scale of 1 to 6, C and P were graded on a scale of 1 to 5.19
All subjects underwent AS-OCT (CASIA 2, Tomey Corporation, Nagoya, Japan) imaging before any contact procedure. The CASIA 2 uses a 1310 nm wavelength swept-source laser at a frequency of 0.3 seconds, producing 16 AS-OCT images from 16 different angles. Each two-dimensional image exhibits the anterior and posterior curvature of lens (yellow lines), which are extended to intersect at two symmetrical points and thus the outline of the lens is obtained (Fig. 1). The 16 different two-dimensional angle images are then analyzed to obtain the three-dimensional crystalline lens morphology and lens parameters: front Rs (the anterior radius of steep curvature of the lens), front Rf (the anterior radius of flat curvature of the lens), front R (mean value of anterior Rs and Rf of the lens), back Rs (the posterior radius of steep curvature of the lens), back Rf (the posterior radius of flat curvature of the lens), back R (mean value of posterior Rs and Rf of the lens), thickness (LT along vertex normal), diameter (diameter of equator part of the lens), and lens vault (LV, vertical distance from the anterior lens surface to the horizontal line connecting the two scleral spurs). The methods of decentration and tilt of lens had been reported previously.20 The decentration was the vertical distance from lens center to vertex normal, and the tilt was the angle of lens axis against vertex normal (Fig. 2). The vertex normal was the line between the fixation point and the vertex of corneal topographic map. The decentration data were presented as γ (mm), Δ (degree), where γ represents the lens decentration from the corneal topographic axis, and Δ represents its azimuth. The tilt data are presented as α (degree), β (degree), where α represents the lens tilt from the corneal topographic axis, and β represents its azimuth. The irido-trabecular contact (ITC) index was the rate of contact between the iris and angle wall anterior to the scleral spur over 360°. The best quality image among three captures was chosen for analysis. All images were processed using inbuilt semiautomated software by a single experienced observer who was masked to clinical data. The scleral spurs were determined by two glaucoma specialists (XW and YC).
Figure 1.
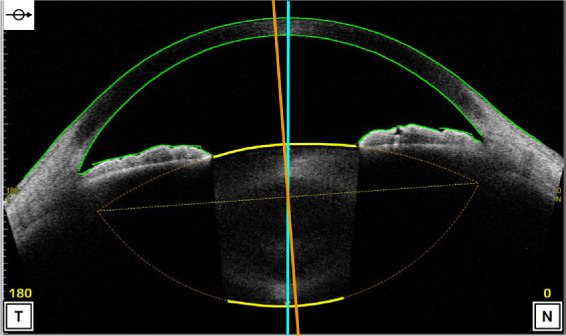
The image of anterior segment captured by CASIA 2. The two-dimensional image exhibits the anterior and posterior curvature of lens (yellow lines), which are extended to intersect at two symmetrical points and thus the outline of the lens is obtained (lens axis, orange line; vertex normal, blue line).
Figure 2.
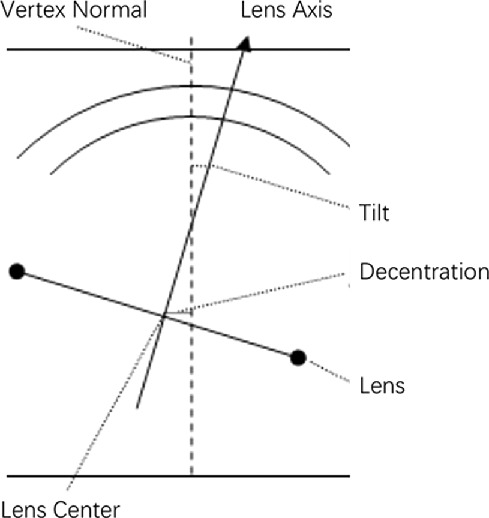
The diagram showing the method of decentration and tilt of the lens. Decentration was the vertical distance from lens center to vertex normal. Tilt was the angle of lens axis against vertex normal. The vertex normal was the line between the fixation point and the vertex of corneal topographic map.
Statistical Analysis
All analyses were performed using an open source R statistical software (version 3.4.3; The R Foundation, Vienna, Austria). Numerical variables were shown as mean ± standard deviation. The ANOVA test and the Kruskal-Wallis test were used to compare the differences among the three groups according to the data distribution. The Spearman correlation analysis and multiple linear regression models were performed to evaluate the relationships between the lens parameters and related factors. Tests for trend were performed by entering the categorical variables as continuous parameters in the models. Restricted cubic spline was used to estimate the dose–response relation of ITC index and lens parameters. The significance level was set at P < 0.05.
Results
A total of 135 eyes of 135 subjects were enrolled in the study. Ten subjects had to be excluded because of poor image quality for both eyes, so 125 eyes were analyzed: 38 eyes were normal controls, 57 eyes were PACS, and 30 eyes were PAC/PACG (Supplementary Table S1). The demographic and basic clinic information of the three groups were shown in Table 1. There were no significant differences in sex, age, and CCT among three groups (all P > 0.05). However, statistically significant differences were found for BCVA (P < 0.001), MSE (P = 0.006), IOP (P < 0.001), ACD (P < 0.001), NO (P = 0.006), NC (P = 0.003), C (P = 0.010), P (P = 0.007), AL (P < 0.001), and ITC index (P < 0.001) among the three groups. The PAC/PACG group had the shallowest ACD, highest IOP, and greatest ITC index (all P < 0.05).
Table 1.
Clinical Characteristics of the Study Participants
| Normal | PACS | PAC/PACG | ||
|---|---|---|---|---|
| Characteristic | (n = 38) | (n = 57) | (n = 30) | P Value* |
| Sex (male:female) | 13:25 | 12:45 | 5:25 | 0.189 |
| Age, years | 58.3 ± 7.2 | 61.4 ± 6.2 | 62.0 ± 10.1 | 0.077 |
| BCVA, LogMAR | 0.16 ± 0.22 | 0.17 ± 0.28 | 0.48 ± 0.51 | <0.001 |
| MSE, diopters | 0.004 ± 0.5 | 0.3 ±0.6 | 0.5 ±0.7 | 0.006 |
| IOP, mmHg | 16.2 ± 3.3 | 15.9 ± 3.9 | 21.7 ± 10.1 | <0.001 |
| ACD, mm | 2.54 ± 0.34 | 2.08 ± 0.22 | 1.93 ± 0.24 | <0.001 |
| CCT, µm | 540.6 ± 35.1 | 533.2 ± 33.7 | 539.7 ± 45.5 | 0.577 |
| NO | 1.1 ± 1.0 | 1.4 ± 1.0 | 1.9 ± 1.3 | 0.006 |
| NC | 1.1 ± 1.0 | 1.4 ± 0.9 | 2.0 ± 1.4 | 0.003 |
| C | 1.6 ± 0.9 | 2.0 ±0.9 | 2.3 ±1.2 | 0.010 |
| P | 1.6 ± 0.8 | 1.9 ± 0.7 | 2.2 ± 1.1 | 0.007 |
| AL, mm | 23.14 ± 0.87 | 22.47 ± 0.86 | 22.34 ± 0.73 | <0.001 |
| ITC Index, % | 11.4 ± 14.7 | 41.1 ± 26.2 | 63.0 ± 24.5 | <0.001 |
Values are mean ± standard deviation.
All calculated by one-way ANOVA, except the sex was calculated by the χ2 test.
Lens parameters of three groups are presented in Table 2. Significant differences were detected for front Rs (normal vs PACS vs PAC/PACG: 8.91 ± 1.10 mm vs 8.07 ± 0.58 mm vs 7.81 ± 0.51 mm; P < 0.001), front Rf (normal vs PACS vs PAC/PACG: 9.80 ± 1.42 mm vs 8.73 ± 0.72 mm vs 8.44 ± 0.61 mm; P < 0.001), front R (normal vs PACS vs PAC/PACG: 9.35 ± 1.29 mm vs 8.40 ± 0.62 mm vs 8.12 ± 0.54 mm; P < 0.001), lens decentration (normal vs PACS vs PAC/PACG: 0.14 ± 0.07 mm vs 0.19 ± 0.09 mm vs 0.22 ± 0.12 mm; P = 0.004), lens tilt (normal vs PACS vs PAC/PACG: 4.9 ± 1.0° vs 5.3 ± 1.2° vs 5.8 ± 1.8°; P = 0.033), LT (normal vs PACS vs PAC/PACG: 4.57 ± 0.28 mm vs 4.87 ± 0.33 mm vs 4.96 ± 0.25 mm; P < 0.001), and LV (normal vs PACS vs PAC/PACG: 0.47 ± 0.30 mm vs 0.80 ± 0.22 mm vs 0.91 ± 0.21 mm; P < 0.001) among the three groups. The PACS and PAC/PACG groups had steeper front Rs, front Rf, front R, thicker LT, greater decentration and LV compared with the normal groups (all P < 0.05). The PAC/PACG groups had greater lens tilt when comparing the normal groups (P = 0.034). However, no statistically significant differences in back Rs (P = 0.474), back Rf (P = 0.152), back R (P = 0.502), or diameter (P = 0.856) of the lens were observed.
Table 2.
The Lens Parameters of Three Groups
| Normal | PACS | PAC/PACG | ||
|---|---|---|---|---|
| Variables | (n = 38) | (n = 57) | (n = 30) | P Value* |
| Front Rs, mm | 8.91 ± 1.10 | 8.07 ± 0.58 | 7.81 ± 0.51 | <0.001 |
| Front Rs Axis, ° | 112.2 ± 38.9 | 106.9 ± 46.7 | 119.6 ± 40.8 | 0.471 |
| Front Rf, mm | 9.80 ± 1.42 | 8.73 ± 0.72 | 8.44± 0.61 | <0.001 |
| Front Rf Axis, ° | 60.1 ± 52.6 | 64.3 ± 48.2 | 59.6 ± 45.3 | 0.808 |
| Front R, mm | 9.35 ± 1.29 | 8.40 ± 0.62 | 8.12 ± 0.54 | <0.001 |
| Back Rs, mm | 5.44 ± 0.38 | 5.38 ± 0.38 | 5.36 ± 0.41 | 0.474 |
| Back Rs Axis, ° | 117.8 ± 57.5 | 99.0 ± 60.4 | 124.4 ± 49.2 | 0.095 |
| Back Rf, mm | 5.83 ± 0.42 | 5.71± 0.38 | 5.78 ± 0.42 | 0.152 |
| Back Rf Axis, ° | 70.4 ± 36.7 | 75.3± 42.0 | 76.4 ± 42.5 | 0.910 |
| Back R, mm | 5.64 ± 0.38 | 5.54± 0.37 | 5.57 ± 0.36 | 0.502 |
| Thickness, mm | 4.57 ± 0.28 | 4.87 ± 0.33 | 4.96 ± 0.25 | <0.001 |
| Diameter, mm | 9.97 ± 0.42 | 9.96 ± 0.36 | 10.0 ± 0.42 | 0.856 |
| Decentration, mm | 0.14 ± 0.07 | 0.19 ± 0.09 | 0.22 ± 0.12 | 0.004 |
| Decentration axis, ° | 173.3 ± 63.2 | 173.6 ± 40.0 | 181.1± 51.3 | 0.814 |
| Tilt, ° | 4.9 ± 1.0 | 5.3 ± 1.2 | 5.8 ± 1.8 | 0.033 |
| Tilt axis, ° | 198.3 ± 16.1 | 194.5 ± 14.9 | 201.2 ± 23.7 | 0.382 |
| LV, mm | 0.47 ± 0.30 | 0.80 ± 0.22 | 0.91 ±0.21 | <0.001 |
Values are mean ± standard deviation.
Differences between groups were tested with the Kruskal-Wallis test.
A Spearman analysis was conducted to determine the correlation of other factors associated with lens parameters (Table 3). Front Rs, front Rf, and front R were significantly associated with the stages of PACD, MSE, ACD, LV, AL, and the ITC index (all P < 0.001). LT was significantly associated with the stage of PACD, age, BCVA, ACD, LV, the grade of lens opacity (NO, NC, C, P), AL, and the ITC index (all P < 0.05). Decentration was significantly associated with the stage of PACD, age, BCVA, ACD, LV, NC, C, AL, and the ITC index (all P < 0.05). Tilt was significantly associated the stage of PACD, BCVA, MSE, ACD, the grade of lens opacity (NO, NC, C, P), AL, and the ITC index (all P < 0.05). No correlation was noted between lens parameters and sex or IOP.
Table 3.
The Association of Lens Parameters by Spearman Analysis
| Front Rs | Front Rf | Front R | Thickness | Decentration | Tilt | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | r | P Value | r | P Value | r | P Value | r | P Value | r | P Value | r | P Value |
| Stage of PACD | −0.454 | <0.001 | −0.444 | <0.001 | −0.464 | <0.001 | 0.447 | <0.001 | 0.285 | 0.001 | 0.232 | 0.009 |
| Age, years | 0.025 | 0.780 | 0.012 | 0.893 | 0.024 | 0.790 | 0.388 | <0.001 | 0.204 | 0.022 | 0.156 | 0.081 |
| Sex (male:female) | −0.056 | 0.538 | −0.023 | 0.893 | −0.032 | 0.726 | 0.064 | 0.477 | −0.114 | 0.207 | 0.012 | 0.893 |
| BCVA, logMAR | −0.140 | 0.119 | −0.157 | 0.081 | −0.147 | 0.103 | 0.201 | 0.025 | 0.195 | 0.029 | 0.301 | 0.001 |
| MSE, diopters | −0.301 | 0.001 | −0.215 | 0.016 | −0.259 | 0.004 | 0.101 | 0.264 | 0.120 | 0.182 | 0.192 | 0.032 |
| IOP, mm Hg | −0.145 | 0.107 | −0.136 | 0.130 | −0.135 | 0.133 | 0.008 | 0.993 | 0.092 | 0.306 | 0.093 | 0.301 |
| ACD, mm | 0.661 | <0.001 | 0.624 | <0.001 | 0.664 | <0.001 | −0.593 | <0.001 | −0.393 | <0.001 | −0.180 | 0.045 |
| CCT, µm | −0.046 | 0.614 | 0.002 | 0.98 | −0.016 | 0.857 | 0.012 | 0.89 | −0.076 | 0.397 | −0.126 | 0.161 |
| LV, mm | −0.681 | <0.001 | −0.635 | <0.001 | −0.671 | <0.001 | 0.583 | <0.001 | 0.318 | <0.001 | 0.148 | 0.099 |
| NO | −0.097 | 0.283 | −0.13 | 0.149 | −1.09 | 0.23 | 0.374 | <0.001 | 0.160 | 0.075 | 0.207 | 0.021 |
| NC | −0.111 | 0.219 | −0.14 | 0.12 | −0.121 | 0.18 | 0.37 | <0.001 | 0.198 | 0.028 | 0.213 | 0.017 |
| C | −0.145 | 0.107 | −0.15 | 0.094 | −0.144 | 0.108 | 0.403 | <0.001 | 0.207 | 0.021 | 0.189 | 0.035 |
| P | −0.151 | 0.093 | −0.166 | 0.064 | −0.157 | 0.081 | 0.387 | <0.001 | 0.138 | 0.125 | 0.195 | 0.030 |
| AL, mm | 0.529 | <0.001 | 0.512 | <0.001 | 0.535 | <0.001 | −0.176 | <0.001 | −0.269 | 0.002 | −0.274 | 0.002 |
| ITC index, % | −0.397 | <0.001 | −0.455 | <0.001 | −0.445 | <0.001 | 0.282 | 0.001 | 0.309 | <0.001 | 0.219 | 0.014 |
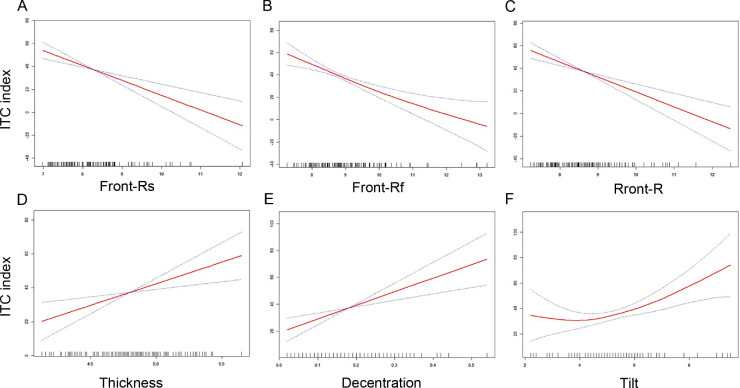
Restricted cubic spline was used to estimate the dose–response relation of the ITC index and lens parameters. We found that the ITC index was negatively correlated with front Rs, front Rf, and front R; and positively correlated with LT, decentration, and tilt after adjustment for age, sex, and AL (Fig. 3), which indicated the ITC index was positively correlated with anterior curvature, LT, decentration, and tilt of lens. Moreover, multivariate regression of lens parameters showed Front Rs, front Rf, front R, LT, and decentration were associated with the stage of PACD in the crude model (Table 4), and nearly all of these associations remained statistically significant after adjusting for age, sex, and AL, expect for the lens tilt. We also performed the tests for trend by entering the stage of the PACD as continuous parameters in the models and found that the stage of the PACD was negatively correlated with front Rs, front Rf, and front R, and positively correlated with LT and decentration even after adjustment for age, sex, and AL (all P < 0.05).
Figure 3.
Restricted cubic spline analysis showed the relationship between ITC index (y-axis) and lens parameters (x-axis) after adjustment for age, sex, and AL, including front Rs (A), front Rf (B), front R (C), thickness (D), decentration (E), and tilt (F).
Table 4.
Multivariate Regression for Lens Parameters
| Crude Model | Multivariate-Adjusted Model 1 | Multivariate-Adjusted Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | β (95% CI) | P Value | P for Trend | β (95% CI) | P Value | P for Trend | β (95% CI) | P Value | P for Trend |
| Front Rs | |||||||||
| Normal | Ref | <0.0001 | Ref | <0.0001 | Ref | <0.0001 | |||
| PACS | −0.83 (−1.14 to −0.52) | <0.0001 | −0.91 (−1.23 to −0.59) | <0.0001 | −0.63 (−0.93 to −0.33) | <0.0001 | |||
| PAC/PACG | −1.09 (−1.46 to −0.73) | <0.0001 | −1.19 (−1.57 to −0.82) | <0.0001 | −0.86 (−1.21 to −0.50) | <0.0001 | |||
| Front Rf | |||||||||
| Normal | Ref | <0.0001 | Ref | <0.0001 | Ref | <0.0001 | |||
| PACS | −1.07 (−1.46 to −0.67) | <0.0001 | −1.17 (−1.58 to −0.77) | <0.0001 | −0.87 (−1.27 to −0.48) | <0.0001 | |||
| PAC/PACG | −1.36 (−1.83 to −0.90) | <0.0001 | −1.49 (−1.97 to −1.02) | <0.0001 | −1.13 (−1.60 to −0.67) | <0.0001 | |||
| Front R | |||||||||
| Normal | Ref | <0.0001 | Ref | <0.0001 | Ref | <0.0001 | |||
| PACS | −0.95 (−1.28 to −0.61) | <0.0001 | −1.04 (−1.38 to −0.70) | <0.0001 | −0.75 (−1.07 to −0.42) | <0.0001 | |||
| PAC/PACG | −1.23 (−1.62 to −0.84) | <0.0001 | −1.34 (−1.74 to −0.94) | <0.0001 | −0.99 (−1.38 to −0.61) | <0.0001 | |||
| Thickness | |||||||||
| Normal | Ref | <0.0001 | Ref | <0.0001 | Ref | <0.0001 | |||
| PACS | 0.30 (0.17 to 0.42) | <0.0001 | 0.27 (0.15 to 0.39) | <0.0001 | 0.25 (0.12 to 0.37) | 0.0002 | |||
| PAC/PACG | 0.39 (0.24 to 0.53) | <0.0001 | 0.35 (0.21 to 0.49) | <0.0001 | 0.33 (0.18 to 0.47) | <0.0001 | |||
| Decentration | |||||||||
| Normal | Ref | 0.001 | Ref | 0.0055 | Ref | 0.0344 | |||
| PACS | 0.05 (0.01 to 0.09) | 0.0144 | 0.04 (0.00 to 0.08) | 0.0464 | 0.03 (−0.01 to 0.07) | 0.1612 | |||
| PAC/PACG | 0.07 (0.03 to 0.12) | 0.0012 | 0.06 (0.02 to 0.11) | 0.0061 | 0.05 (0.00 to 0.10) | 0.035 | |||
| Tilt | |||||||||
| Normal | Ref | 0.0031 | Ref | 0.0097 | Ref | 0.1309 | |||
| PACS | 0.42 (−0.12 to 0.96) | 0.1272 | 0.33 (−0.22 to 0.87) | 0.2409 | 0.03 (−0.52 to 0.58) | 0.9104 | |||
| PAC/PACG | 0.97 (0.34 to 1.60) | 0.0031 | 0.86 (0.22 to 1.49) | 0.0094 | 0.50 (−0.15 to 1.15) | 0.1322 | |||
The crude model adjusted for stage of PACD. Model 1 further adjusted for age and sex. Model 2 further adjusted for AL.
Discussion
Previous studies have demonstrated that crystalline lens contributed to the pathogenesis of PACD.5–7,21 In the present study, we used AS-OCT to further investigate the detailed lens morphologic features in the whole range of PACD by comparing the biometric parameters with healthy control eyes and among different stages. To the best of our knowledge, this is the first time that high-quality imaging has been used to show the lens morphologic features in all stages of PACD by AS-OCT.
This cross-sectional study of 125 participants, which included 38 controls, 57 PACS, and 30 PAC/PACG, showed that the PACD eyes had steeper anterior curvature of lens, thicker LT, higher LV, and greater decentration comparing to normal eyes. As an indicator of the stages of PACD, the ITC index was also found to be negatively correlated with anterior radius curvature of lens and positively correlated with LT, decentration, and tilt after adjustment for age, sex, and AL. Our results suggested that the morphology and position of the crystalline lens were different in the early and late stages of PACD eyes.
Owing to a technical problem, AS-OCT and ultrasound biomicroscopy were not able to simultaneously and quantitatively display the anterior and posterior surface of the lens well. Previous studies of lens biometric parameters in PACD produced inconsistent results. There were studies that found that the PACD eyes had a greater iris–lens contact distance by ultrasound biomicroscopy,22 a more anterior lens position (LP; defined as ACD + 1/2LT) by A-mode applanation ultrasonography,23 and a greater LV by AS-OCT24 compared with normal eyes. There was also one study that they did not find any difference of lens position when comparing angle closure eyes with those open angle eyes. Sihota et al.15 found no differences in relative lens position (defined as LP/AL) when comparing PACG patients with family members. In our study, we demonstrated that the anterior radius curvature of the lens decreased, LV increased, LT increased, and the ACD decreased in the PACD eyes when compared with normal eyes. Because the curvature of the anterior lens surface, thickness of the lens, and relative lens position all partially rely on the tension of zonules, it is possible that the increase of lens curvature, LT, and LV are associated with zonular weakness, a finding that is also suggested by the phenomenon that patients with PACD are found to have high rate of zonular laxity in the cataract surgery.25 Increased curvature of the anterior lens surface and LV likely increases the extent of iridolenticular contact, leading to a more significant pupillary block, and then angle crowding. In this study, we found that, even after adjustment for age, sex, and LV by the multivariate regression model, the stage of PACD was still negatively and significantly associated with the anterior radius curvature of lens (Supplementary Table S2). Therefore, in addition to the shallow ACD, shorter AL, and increased LT and LV, the increased curvature of anterior lens surface might be another anatomic characteristic of PACD eyes.
Another interesting finding in this study was that PACD eyes had a greater decentration of lens compared with normal eyes, which had a trend with the stage of PACD even after adjustment for age, sex, and AL. In this study, PACD eyes showed an average decentration of 0.22 ± 0.12 mm compared with 0.14 ± 0.07 mm in normal eyes. These results in normal eyes were consistent with the report from Kimura et al.,20 which showed that normal eyes had an average decentration of 0.12 mm by using CASIA 2. However, so far, no report has been published about crystalline lens decentration in PACD eyes by AS-OCT. In addition, we observed that the extent of lens decentration is becoming more significant along with the stage of the disease. Because the well-centered position of the lens depends on the equal tension of zonules, the tilt and decentration of lens are probably related with the zonular weakness. The LPI could be effective in the early stage of such patients, because either anterior shift of lens or increasement of lens curvature would increase pupillary block, which can usually be relieved by LPI. However, these patients will eventually need surgery because the effect of LPI gradually wanes with the development of zonular weakness. Our results are also supported by the phenomenon that crystalline lens dislocation was frequently seen in PACG eyes, as reported by Zhang et al.26 and Luo et al.27 Although the tilt and decentration (PACG vs normal: 0.22 ± 0.12 mm vs 0.14 ± 0.07 mm) of the lens in PACG are small in our study, filtering surgery in PACG eyes may have increased risk of complications such as shallow anterior chamber and malignant glaucoma because of zonular weakness. Therefore, such patients should be more closely considered to treat with cataract surgery instead of filtering surgery. Meanwhile, more attention should be paid when cataract surgery is being performed due to the zonular weakness, and the capsular tension ring can be used to provide support to the lens capsule if necessary.
The present study was limited by its cross-sectional design, especially in that the number of participants was small in the PAC/PACG group, so the distribution of the lens morphologic parameters in PACD eyes may not represent the real development process during the progression of PACD. Moreover, the lens morphologic parameters in our study were calculated from certain formula provided by inbuilt semiautomated software, which could potentially introduce bias. Direct measurement from complete imaging of the lens might provide more information.
In conclusion, our study demonstrated that PACD eyes had a steeper anterior curvature and greater decentration of lens when compared with normal eyes. The steep anterior curvature and decentered lens might be another anatomic characteristic in PACD eyes.
Supplementary Material
Acknowledgments
Supported by the State Program of National Natural Science Foundation of China (81570887, 81870692), the State Key Program of National Natural Science Foundation of China (81430007), the subject of major projects of National Natural Science Foundation of China (81790641), the Shanghai Committee of Science and Technology, China (17410712500), the top priority of clinical medicine center of Shanghai (2017ZZ01020). The sponsor or funding organization had no role in the design or conduct of this research.
Disclosure: X. Wang, None; X. Chen, None; Y. Tang, None; J. Wang, None; Y. Chen, None; X. Sun, None
References
- 1. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996; 80: 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Foster PJ, Johnson GJ.. Glaucoma in China: how big is the problem? Br J Ophthalmol. 2001; 85: 1277–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cho HK, Kee C.. Population-based glaucoma prevalence studies in Asians. Surv Ophthalmol. 2014; 59: 434–447. [DOI] [PubMed] [Google Scholar]
- 4. Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002; 86: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lowe RF. Aetiology of the anatomical basis for primary angle-closure glaucoma. Biometrical comparisons between normal eyes and eyes with primary angle-closure glaucoma. Br J Ophthalmol. 1970; 54: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alsbirk PH. Primary angle-closure glaucoma. Oculometry, epidemiology, and genetics in a high risk population. Acta Ophthalmol Suppl. 1976;5–31. [PubMed] [Google Scholar]
- 7. Sihota R, Lakshmaiah NC, Agarwal HC, Pandey RM, Titiyal JS. Ocular parameters in the subgroups of angle closure glaucoma. Clin Exp Ophthalmol. 2000; 28: 253–258. [DOI] [PubMed] [Google Scholar]
- 8. Anderson DR, Jin JC, Wright MM. The physiologic characteristics of relative pupillary block. Am J Ophthalmol. 1991; 111: 344–350. [DOI] [PubMed] [Google Scholar]
- 9. Quigley HA, Friedman DS, Congdon NG. Possible mechanisms of primary angle-closure and malignant glaucoma. J Glaucoma. 2003; 12: 167–180. [DOI] [PubMed] [Google Scholar]
- 10. Sun X, Dai Y, Chen Y, et al.. Primary angle closure glaucoma: what we know and what we don't know. Prog Retin Eye Res. 2017; 57: 26–45. [DOI] [PubMed] [Google Scholar]
- 11. Tomlinson A, Leighton DA.. Ocular dimensions in the heredity of angle-closure glaucoma. Br J Ophthalmol. 1973; 57: 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Markowitz SN, Morin JD.. The ratio of lens thickness to axial length for biometric standardization in angle-closure glaucoma. Am J Ophthalmol. 1985; 99: 400–402. [DOI] [PubMed] [Google Scholar]
- 13. Salmon JF, Swanevelder SA, Donald MA. The dimensions of eyes with chronic angle-closure glaucoma. J Glaucoma. 1994; 3: 237–243. [PubMed] [Google Scholar]
- 14. Marchini G, Pagliarusco A, Toscano A, Tosi R, Brunelli C, Bonomi L. Ultrasound biomicroscopic and conventional ultrasonographic study of ocular dimensions in primary angle-closure glaucoma. Ophthalmology. 1998; 105: 2091–2098. [DOI] [PubMed] [Google Scholar]
- 15. Sihota R, Ghate D, Mohan S, Gupta V, Pandey RM, Dada T. Study of biometric parameters in family members of primary angle closure glaucoma patients. Eye (Lond). 2008; 22: 521–527. [DOI] [PubMed] [Google Scholar]
- 16. Nongpiur ME, He M, Amerasinghe N, et al.. Lens vault, thickness, and position in Chinese subjects with angle closure. Ophthalmology. 2011; 118: 474–479. [DOI] [PubMed] [Google Scholar]
- 17. Ang LP, Aung T, Chew PT. Acute primary angle closure in an Asian population: long-term outcome of the fellow eye after prophylactic laser peripheral iridotomy. Ophthalmology. 2000; 107: 2092–2096. [DOI] [PubMed] [Google Scholar]
- 18. Husain R, Do T, Lai J, et al.. Efficacy of phacoemulsification alone vs phacoemulsification with goniosynechialysis in patients with primary angle-closure disease: a randomized clinical trial. JAMA Ophthalmol. 2019; 137: 1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chylack LT Jr., Wolfe JK, Singer DM, et al.. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993; 111: 831–836. [DOI] [PubMed] [Google Scholar]
- 20. Kimura S, Morizane Y, Shiode Y, et al.. Assessment of tilt and decentration of crystalline lens and intraocular lens relative to the corneal topographic axis using anterior segment optical coherence tomography. PLoS One. 2017; 12: e0184066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li M, Chen Y, Chen X, et al.. Differences between fellow eyes of acute and chronic primary angle closure (glaucoma): an ultrasound biomicroscopy quantitative study. PLoS One. 2018; 13: e0193006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mansoori T, Balakrishna N.. Anterior segment morphology in primary angle closure glaucoma using ultrasound biomicroscopy. J Curr Glaucoma Pract. 2017; 11: 86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lim MC, Lim LS, Gazzard G, et al.. Lens opacity, thickness, and position in subjects with acute primary angle closure. J Glaucoma. 2006; 15: 260–263. [DOI] [PubMed] [Google Scholar]
- 24. Liu L, Liu X, Huang C, et al.. Associated factors of acute primary angle closure glaucoma in a sub-group of Chinese people: comparison between attack eyes and normal controls. Sci Rep. 2017; 7: 14885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kubota T, Touguri I, Onizuka N, Matsuura T. Phacoemulsification and intraocular lens implantation combined with trabeculotomy for open-angle glaucoma and coexisting cataract. Ophthalmologica. 2003; 217: 204–207. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Y, Zong Y, Jiang Y, Jiang C, Lu Y, Zhu X. Clinical features and efficacy of lens surgery in patients with lens subluxation misdiagnosed as primary angle-closure glaucoma. Curr Eye Res. 2019; 44: 393–398. [DOI] [PubMed] [Google Scholar]
- 27. Luo L, Li M, Zhong Y, Cheng B, Liu X. Evaluation of secondary glaucoma associated with subluxated lens misdiagnosed as acute primary angle-closure glaucoma. J Glaucoma. 2013; 22: 307–310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.