Abstract
Purpose
To investigate the differential expression of cytokines and growth factors in the cornea and aqueous humor after small incision lenticule extraction (SMILE) compared with femtosecond LASIK (FS-LASIK) using rabbit model.
Methods
Sixteen eyes of 16 rabbits in each group underwent SMILE or FS-LASIK with refractive correction of −6.00 DS/−1.00 DC. Eight additional rabbits served as controls. Pre- and 24 hours, 1 week, 1 month, and 3 months postoperatively, slit-lamp and anterior segment optical coherence tomography were performed, followed by cornea and aqueous humor collection. Apoptosis and proliferation were evaluated with TUNEL assay and Ki-67 immunostaining, respectively. The mRNA and protein expression of cytokines and growth factors was determined by RT-qPCR and Western blotting, respectively. Cytokine levels in the aqueous humor were detected with ELISA.
Results
Compared with FS-LASIK, SMILE induced less apoptosis and proliferation in the cornea within 1 week postoperatively. Levels of IL-1β, TNF-α, and EGFR in the cornea were significantly increased after FS-LASIK compared with SMILE within 24 hours. Levels of IL-8 in the aqueous humor remained elevated until 1 week after FS-LASIK but not SMILE. TGF-β1 level was elevated up to 1 month after both procedures, while BFGF level was kept high within 1 month after SMILE but not FS-LASIK.
Conclusions
SMILE could induce significantly less acute inflammation than FS-LASIK in the cornea and aqueous humor. The differential expression of TGF-β1 and BFGF between two procedures until 1 month might contribute to the post-SMILE delayed recovery and underline the importance of continued treatment postoperatively.
Keywords: SMILE, FS-LASIK, cytokine, growth factors, delayed recovery
Rapid advances in technology and innovation have increased refractive surgical options available. Femtosecond LASIK (FS-LASIK) is the most popular laser refractive surgery with established safety and efficacy.1 Recently, small incision lenticule extraction (SMILE) was approved by the US Food and Drug Administration for myopic correction up to –10.0 diopters and myopic astigmatism up to –3.0 diopters. Compared with FS-LASIK, SMILE protects from traumatic flap displacement risk using a 2-mm stromal incision.2 By preserving corneal sensitivity,3,4 reducing postoperative dry eye symptoms, and improving biomechanical stability,5,6 SMILE offers comparable long-term visual outcomes.7,8
However, studies have reported slower recovery of visual acuity after SMILE than that after FS-LASIK, even with laser-scanning pattern optimization.1,2,9 Ang et al.10 reported that postoperative symptoms, such as fluctuation and occasional blurring of vision, were more significant at 1-month postoperation for SMILE than for FS-LASIK. As an elective surgical procedure to restore uncorrected visual acuity and improve quality of life, delayed recovery after SMILE may affect patient satisfaction.2 Therefore, it is necessary for refractive surgeons to better understand initial fluctuation in vision after SMILE,11–14 which is still not fully elucidated.
Corneal wound healing is a complex process involving cytokine-mediated interactions between epithelial cells, keratocytes, tear film, and cells of the immune system.15,16 Understanding the molecular interactions of stromal remodeling and healing of the epithelium, which contributes to postoperative vision regression, surface irregularity, and stromal scarring,17,18 may improve our understanding of delayed recovery after SMILE. Studies using tear samples19 or extracted lenticules20 could not capture healing kinetics after surgery. However, using a rabbit model allowed follow-up sample collection; thus, this model can be used to study the continuing molecular healing response in treated cornea. The current study compared changes in the expression of cytokines and growth factors (GFs) associated with inflammatory reactions and wound-healing response in the cornea and aqueous humor (AqH) of rabbit eyes over the course of 3 months after SMILE compared with those after FS-LASIK.
Methods
Animals
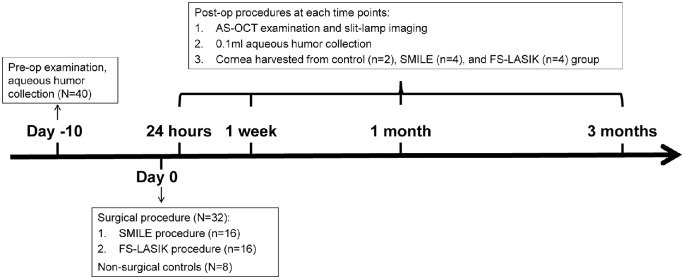
Sixteen-week-old female New Zealand white rabbits were obtained from Tianjin Laboratory Animal Center (Tianjin, China). Rabbits were housed individually under a 12-hour light/dark cycle with food and water ad libitum. Unilateral eyes randomly selected for the surgery underwent SMILE or FS-LASIK independently (n = 16/group). Untreated eyes, from age-matched groups (n = 8), served as controls. Four eyes from each group were randomly collected at 24 hours, 1 week, 1 month, and 3 months postoperatively for further analysis (Fig. 1).
Figure 1.
Experimental approach showing the timeline for pre- and postoperative examinations and sample collection. n = number of rabbits in each group.
Rabbits were anesthetized with chloral hydrate (50 mg/kg body weight, intravenous; Tianjin Medical University General Hospital, Tianjin, China) and xylazine (2 mg/kg body weight, intramuscular; Shengda Co, Jilin, China) for surgery and clinical examinations, respectively. Topical anesthesia was induced by 0.4% oxybuprocaine (Benoxil; Santen, Inc., Osaka, Japan) before AqH collection and surgical procedures. All animal procedures were approved by the Ethics Committee of the Tianjin Eye Hospital (Tianjin Medical University) and complied with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Surgical Procedure
All surgical eyes were reexamined using slit-lamp microscopy by both the observer and surgeon before the procedure to exclude any pathologic issues.
SMILE and FS-LASIK were performed by a single experienced surgeon (YW), and all eyes were treated for the same refractive correction (–6.00 DS/–1.00 DC).
SMILE procedures were performed on rabbits using a 500-kHz VisuMax femtosecond laser (Carl Zeiss, Meditec AG, Oberkochen, Germany). In all cases, a refractive lenticule and one incision at the superior cornea with a 2.0-mm circumferential length was created. The SMILE parameters were as follow: 7.0-mm cap diameter, 110-μm cap depth from the corneal surface, 6-mm refractive lenticule diameter, and 120-µm lenticule central thickness.
In the FS-LASIK group, after the flap was created with the same laser system, refractive ablation was performed with an Allegretto excimer laser system (WaveLight Laser Technologie AG, Erlangen, Germany). A 6.0-mm optical zone, surrounded by a transition zone of 1.0 mm, was applied to all eyes. The flap (8.0-mm diameter and 110-μm thickness) had a nasal hinge. The ablation depth was 101.25 μm. The flap was repositioned immediately after completing the excimer ablation without any extra operation to fix the flap. All rabbits were restrained by means of special collars during the first week postoperatively to prevent them from opening the flap.
Postoperative medications included the antibiotic eyedrop (Tarivid; Santen, Inc.), applied four times a day for 3 days, and anti-inflammatory eyedrop (Flumetholon; Santen, Inc.), applied with a taper four times a day for 14 days.
Ocular Clinical Examinations
Slit-lamp photographs (TOPCON, Tokyo, Japan) and anterior segment spectral-domain optical coherence tomography (AS-OCT, Optovue, Inc., Fremont, CA, USA) were measured before surgery and at each follow-up time point.
Aqueous Humor Collection
After clinical evaluation, AqH was collected from all 16 eyes from each group at 10 days before surgery and at each follow-up time point (Fig. 1). A sterile 27-gauge needle was inserted into the anterior chamber through the clear cornea of the paralimbal area, under aseptic operation. Approximately 0.1 mL AqH was collected before the cornea harvest. Samples were stored at −80°C until use.
Tissue Fixing and Sectioning
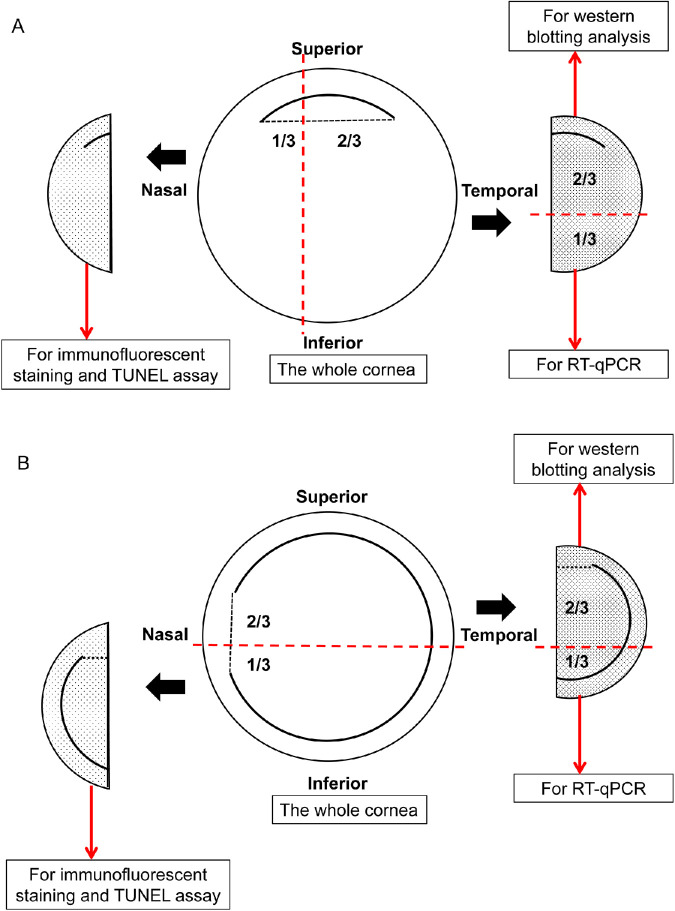
Corneas harvested from the surgical group were perpendicularly cuts to the surgical incision or flap hinge (Fig. 2). Control corneas were dissected vertically with one-third used for TUNEL assay and immunofluorescence staining; the remaining were further cut horizontally for Western blotting and real-time quantitative RT-PCR (RT-qPCR). Samples were collected on dry ice and stored in liquid nitrogen until use.
Figure 2.
Schematic diagram illustrating the cornea tissue collection for immunofluorescence assay, Western blotting, and RT-qPCR in SMILE group (A) and FS-LASIK group (B).
Frozen tissues embedded in optimal cutting temperature compound (Leica Microsystems, Wetzlar, Germany) were sectioned to 8-μm thickness using a cryostat (Leica CM 1900; Leica Microsystems). Sections were adhered to poly-L-lysine–coated glass slides followed by fixing with ice-cold acetone for 10 minutes and air drying before storage and use.
TUNEL Assay
To detect fragmentation of DNA as a marker of apoptosis, a fluorescence-based TUNEL assay (In Situ Cell Death Detection Kit, Fluorescein; Roche Applied Science, Indianapolis, IN, USA) was used according to the manufacturer's instructions. Digital images were captured with an inverted fluorescence microscope (Leica DM 4000B; Leica Microsystems). TUNEL-positive cells in the corneal section were counted in five randomly independent fields at 200× magnification. The results were expressed as the mean values for each group.
Immunofluorescent Staining
Cornea tissue sections were blocked and incubated overnight with mouse monoclonal antibody against Ki67 (1:200; Zhong-Shan Golden-bridge Biotechnology, Beijing, China). Secondary antibody (1:100; EarthOx Life Sciences, Millbrae, CA, USA) was added for 1 hour. After washing, slides were mounted with medium containing 4′,6 diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific, Waltham, MA, USA, USA) to counterstain nuclei. Images were captured using an inverted fluorescence microscope. Ki67-positive cells per total corneal cells at 400× magnification were defined as the percentage of Ki67-positive cells and were counted in five randomly independent fields for each cornea (expressed as the mean for each group).
RNA Extraction and RT-qPCR
Total RNA (0.5 μg) was prepared using TRIzol reagent (Invitrogen, CA, Carlsbad, USA), and cDNA synthesis was performed using M-MLV Reverse Transcriptase (Takara, Tokyo, Japan). Genes of interest were amplified by RT-qPCR using a TransStart Green Q-PCR SuperMix Kit (TransGen, Beijing, China). Relative gene expression was measured using the comparative 2ΔΔCq method. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as a normalizing control. Primer sequence information is listed in the Table.
Table.
Primer List
| Gene and Sequence | ||
|---|---|---|
| GAPDH | Forward | 5′-TCGGAGTGAACGGATTTG-3′ |
| Reverse | 5′-CTCGCTCCTGGAAGATGG-3′ | |
| IL-1β | Forward | 5′-GTAGACCCCAACCGTTACCC-3′ |
| Reverse | 5′-AGACGGGCATGTACTCTGTC-3′ | |
| TNF-α | Forward | 5′-CCTCATCTACTCCCAGGTTCTC-3′ |
| Reverse | 5′-GGCAAGGTCCAGGTACTCA-3′ | |
| TGF-β1 | Forward | 5′-GGAGGAGGAACCAACCAT-3′ |
| Reverse | 5′-GCTGTGCCCGCAATCTTT-3′ | |
| BFGF | Forward | 5′-CACTTCAAGGACCCCAAGCG-3′ |
| Reverse | 5′-TTTGATGTGTGGGTCGCTCT-3′ | |
| EGF | Forward | 5′-GCAGATGCTGGGCACTTTTC-3′ |
| Reverse | 5′-GAGTCGAGTGGTCTTGCTCC-3′ | |
| EGFR | Forward | 5′-GTGCCCTGATGGACGAAGAA-3′ |
| Reverse | 5′-GACAGCTCCCGTTCCTATCC-3′ | |
ELISA
IL-1 and IL-8 concentrations in AqH samples were measured by ELISA, using monoclonal antibodies following the manufacturer's instructions (CUSABIO TECHNOLOGY LLC, Wuhan, China).
Western Blot
Equal amounts of proteins were separated using SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). Membranes were blocked and incubated with primary antibodies against IL-1β (1:1000; Proteintech Group, Inc., Rosemont, IL, USA), TNF-α (1:1000, Abcam, Inc., MA, Cambridge, USA), TGF-β1 (1:1000; Santa Cruz Biotechnology, Inc., Texas, Dallas, USA), BFGF (1:1000; ABclonal Technology, Wuhan, China), and EGF (1:1000; ABclonal Technology). After washing, secondary antibodies (1:5000; Promega, Madison, WI, USA) were applied and blots were developed using an enhanced chemiluminescence detection kit (Millipore). β-Actin (1:1000; Santa Cruz Biotechnology) was used to normalize protein levels. Densitometry quantitation was plotted using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Statistical Analysis
Statistical analyses were performed using SPSS 20.0 software (SPSS, Chicago, IL, USA). Data were presented as the mean ± SEM from three independent experiments. Statistical comparisons among groups were performed using ANOVA followed by Bonferroni-Dunn post hoc test. P values less than 0.05 were considered significant.
Results
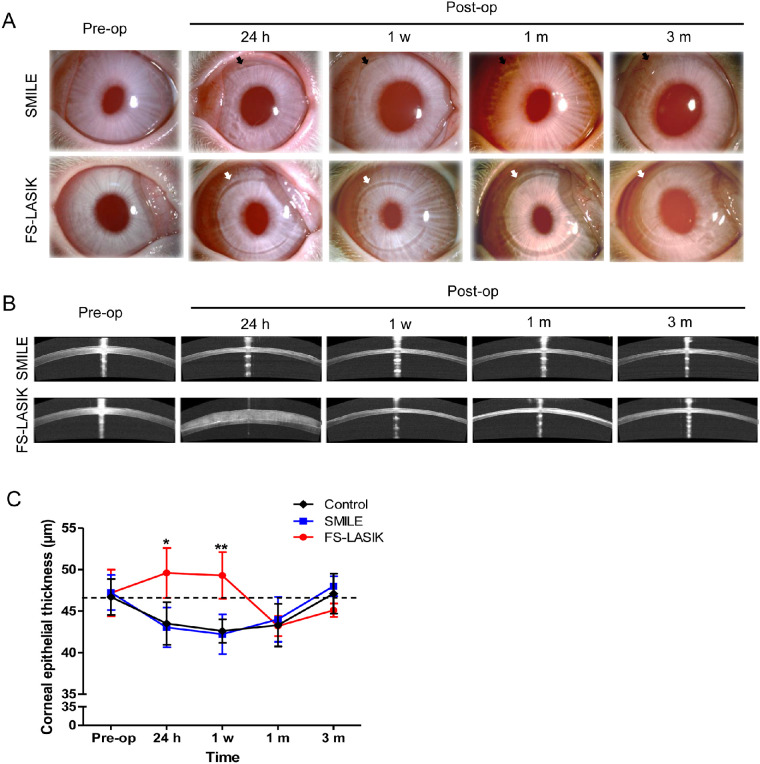
Rabbit corneas that underwent SMILE or FS-LASIK remained transparent and without adverse events at all observation time points. Under the slit-lamp examination (Fig. 3A), the 2-mm incision line in post-SMILE corneas became less visible over a period of 3 months, whereas the flap in post-LASIK corneas exhibited mild shrinkage, such that a gap could still be observed up to 3 months postoperatively. However, cross-section images from AS-OCT examination revealed increased reflection at the ablation interface in both groups that persisted until 3 months postoperatively (Fig. 3B). Hematoxylin-eosin staining was performed to examine cornea histology at 24 hours postoperatively (Supplementary Data S1). Quantitative analysis of the central corneal epithelial thickness (CET) showed a significant increase in 24 hours and 1 week for the FS-LASIK group, in comparison to the SMILE group (P < 0.05, Fig. 3C), suggestive of postoperative edema. In contrast, SMILE-treated corneas were similar to the control group. Both surgical groups had levels of CET similar to the control group at 1 and 3 months postoperatively.
Figure 3.
Slit-lamp microscopy and AS-OCT examination at 24 hours, 1 week, 1 month, and 3 months after SMILE and FS-LASIK. (A) Slit-lamp photographs represent the change of the cornea underwent a –6.00 DS/–1.00 DC correction by different procedures. The top panel shows the pre- and post-SMILE cornea (incisions shown by black arrows), and the bottom panel shows the cornea before and after FS-LASIK (flap outlines shown by white arrows). (B) Cross-sectional visualization of postoperative corneas using AS-OCT. (C) Bar chart showing the changes of central corneal epithelial thickness before and after both procedures. *P < 0.05. **P < 0.01.
Cell Apoptosis and Proliferation
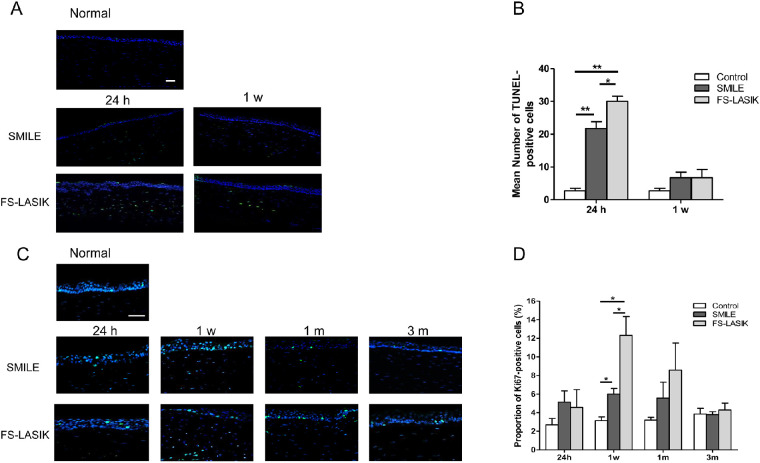
Due to differences observed in corneal edema between the two surgical groups, we investigated whether this could be attributed to differences in apoptosis and proliferation. Both procedures induced increased apoptosis at 24 hours postoperatively, with significantly more apoptotic cells detected in post-LASIK corneas (P = 0.019, Figs. 4A, 4B). Although the number of apoptotic cells was comparable to the control group 1 week after the surgery, TUNEL-positive cells were still detected in the stroma of corneas for both surgical groups but not the control group.
Figure 4.
Apoptosis and cell proliferation at different time points in the corneas underwent SMILE and FS-LASIK. (A) Representative images of TUNEL assay to assess the level of apoptosis in the cornea for all groups postoperatively (blue: DAPI; green: TUNEL-positive cells). Scale bar: 100 μm. (B) Bar chart showing the mean number of TUNEL-positive cells in the cornea at 24 hours and 1 week after both surgeries. (C) Expression for Ki67 immunofluorescence staining to detect the level of proliferation in the cornea of all groups postoperatively (blue: DAPI; green: Ki67-positive cells). Scale bar: 100 μm. (D) Bar chart showing the percentage of Ki67-positive cells in the cornea at all follow-up time points postoperatively. *P < 0.05. **P < 0.01.
A peak in cell proliferation for both surgical groups was observed 1 week postoperatively, and the FS-LASIK group had significantly more Ki67-positive cells in comparison to the SMILE group (P = 0.014, Figs. 4C, 4D). Ki67-positive cells were observed in corneas up to 1 month postoperatively, with no significant difference between the two surgical groups (P = 0.503). At 3 months, the expression of Ki67 was similar among all three groups.
Corneal Wound-Healing Response
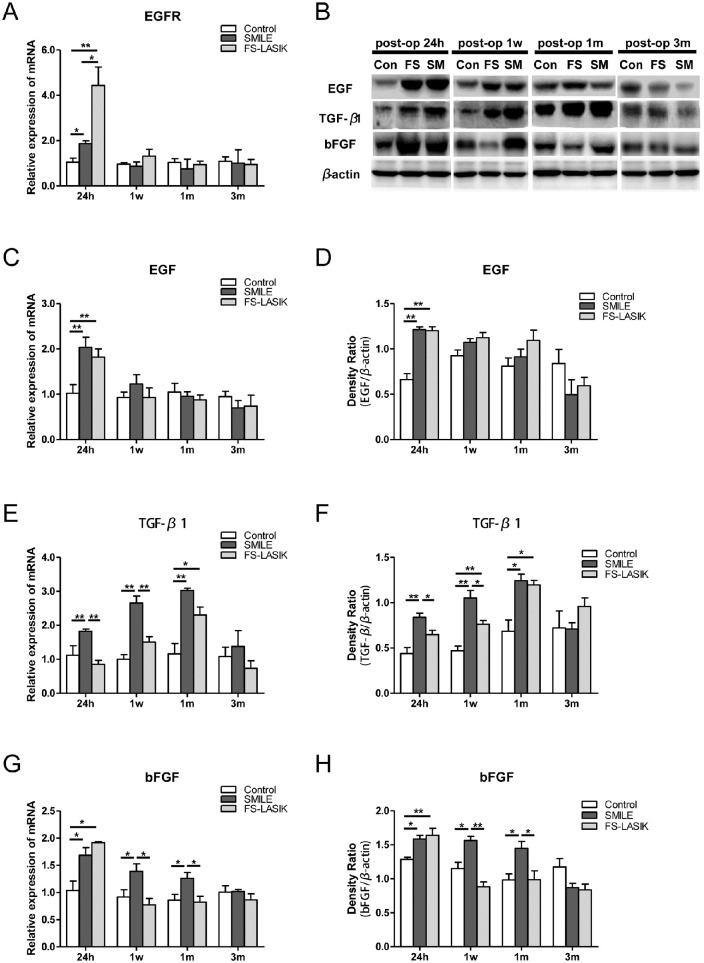
To further understand the differences between SMILE and FS-LASIK at the molecular level, the expression of GFs (EGF, TGF-β1, BFGF) and the EGF receptor (EGFR) were measured in corneal tissue. At postoperative 24 hours, mRNA levels for EGFR in both surgical groups increased compared to the control, with a twofold increase induced by FS-LASIK compared to SMILE (P = 0.035, Fig. 5A). Similarly, the mRNA and protein level of EGF in both treatment groups significantly increased at 24 hours postoperatively (P < 0.05, Figs. 5B–5D). Elevated EGFR and EGF levels returned to baseline 1 week after surgery for both groups.
Figure 5.
Relative expressions of wound-healing associated growth factors in corneas of all groups evaluated by RT-qPCR and Western blot. (A) Bar chart showing EGFR mRNA expression in the corneas at different time points postoperatively. *P < 0.05. **P < 0.01. (B) Representative immunoblots of tissue lysates showing detection of EGF, TGF-β1, and BFGF in the postoperative corneas, respectively. β-Actin was used to normalize protein levels. (C–H) Bar charts showing the mRNA and relative protein expression of EGF (C, D), TGF-β1 (E, F), and BFGF (G, H) in the postsurgical and control corneas at 24 hours, 1 week, 1 month, and 3 months after the procedures. *P < 0.05. **P < 0.01.
There was a gradual, yet significant, increase in mRNA and protein (25 kDa) levels of TGF-β1 in the SMILE group up to 1 month postoperatively. Notably, the SMILE group had higher TGF-β1 expression, compared with the FS-LASIK group, at 24 hours and 1 week postoperatively (P < 0.05, Figs. 5E, 5F). TGF-β1 levels peaked at 1 month postoperatively for both procedures, followed by a decrease to control group levels after 3 months. Both mRNA and protein expression of BFGF increased significantly at 24 hours postoperatively (Figs. 5G, 5H) in both treatment groups. BFGF levels remained elevated in the SMILE group up to 1 month postoperatively, whereas a decrease to control group levels was observed in the FS-LASIK group at 1 week postoperatively. By 3 months after the surgery, BFGF levels were similar for all three groups.
Corneal Inflammatory Reaction
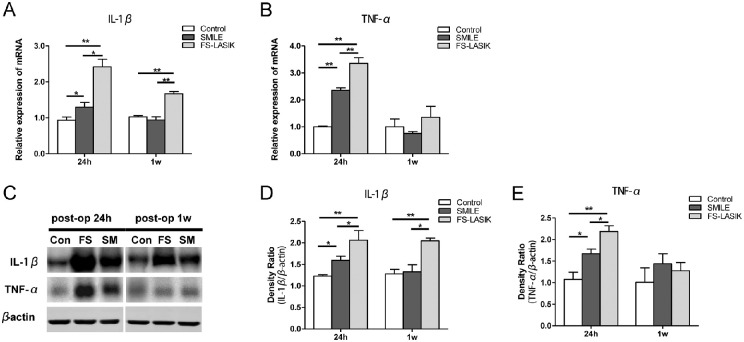
Major changes were observed in corneal edema, apoptosis, and proliferation in rabbit corneas after SMILE and FS-LASIK, mainly within the first week postoperatively. We next evaluated changes in the expression of inflammatory cytokines. Compared to the control group, the SMILE group induced increased IL-1β levels at postoperative 24 hours. FS-LASIK induced significantly higher levels of IL-1β than SMILE at 24 hours and 1 week postoperatively (P < 0.05, Figs. 6A, 6C and 6D). TNF-α was significantly increased in both groups 24 hours after surgery, with the highest level detected in the FS-LASIK group (P < 0.05, Figs. 6B, 6E).
Figure 6.
Relative expressions of proinflammatory cytokines in corneas of all groups evaluated by RT-qPCR and Western blot. (A, B) Bar charts showing the mRNA expression of IL-1β and TNF-α in postsurgical and control corneas at 24 hours and 1 week postoperatively. *P < 0.05. **P < 0.01. (C) Representative immunoblots of tissue lysates showing detection of IL-1β and TNF-α in the postoperative and control corneas, respectively. β-Actin was used to normalize protein levels. (D, E) Bar charts showing the relative protein expression of IL-1β and TNF-α in the corneas within 1 week postoperatively. *P < 0.05. **P < 0.01.
Inflammatory Responses of the Anterior Chamber
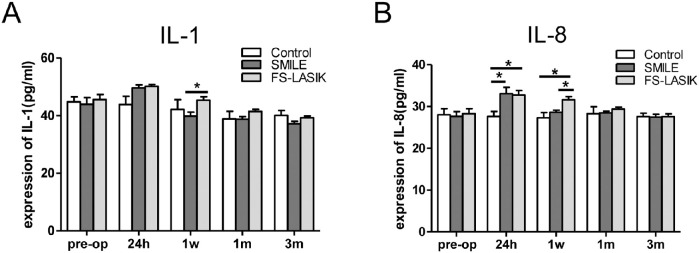
Beyond local reactions within the cornea, we investigated the inflammatory response in AqH, focusing on IL-1 and IL-8 expression. IL-1 levels in the AqH of FS-LASIK group were significantly higher than those in the SMILE group at 1 week postoperatively (P = 0.038, Fig. 7A). IL-8 levels for both surgical groups were significantly higher than those of the control group at 24 hours (P < 0.05), and IL-8 level for the FS-LASIK group remained elevated up to 1 week postoperatively (FS-LASIK versus SMILE: P = 0.02, Fig. 7B). IL-1 and IL-8 levels were similar among all three groups 1 month postoperatively.
Figure 7.
The level of proinflammatory cytokine in aqueous humor of rabbits of all groups measured by ELISA. Bar chart showing the changes of IL-1 (A) and IL-8 (B) expression in aqueous humor before and at 24 hours, 1 week, 1 month, and 3 months after both procedures. *P < 0.05.
Discussion
In this study, we provide evidence that SMILE is associated with reduced edema of the corneal epithelium and less apoptosis and proliferation in rabbit corneas 1 week postoperatively in comparison to FS-LASIK. Moreover, SMILE resulted in less acute inflammation than FS-LASIK in the cornea and anterior chamber of rabbits. SMILE induced a significant increase in TGF-β1 and BFGF expressions from postoperative 24 hours until 1 month, whereas FS-LASIK induced only high TGF-β1 level at 1 month postoperatively, which might be the possible reason of delayed recovery after SMILE.
Our study first used AS-OCT to compare CET at all follow-up time points in rabbits. Since none of the procedures make ablations on epithelium, the differences in changes of CET could be mainly attributed to the differences in surgical approach between the two procedures. CET significantly increased at 24 hours and 1 week after FS-LASIK compared with after SMILE. This transient increase may due to postoperative edema resulting from the different amounts of energy delivered to the corneal stroma for high refractive correction. Femtosecond laser use for corneal lenticule creation during SMILE is constant and independent of refractive correction degree, whereas laser use during FS-LASIK depends on the refractive correction.21,22 The greater the refractive correction, the more energy delivered to the corneal stroma during FS-LASIK, causing stroma injury and corneal edema. Increased edema severity of the FS-LASIK flap compared with the SMILE cap within 1 week postoperatively in either rabbits or patients has been reported.21,23–25 However, postoperative edema might be enlarged in the rabbit model owing to species-related biologic differences.
To study the cellular changes in the cornea, we detected apoptosis using the TUNEL assay and proliferation using Ki-67 immunofluorescent staining in postoperative rabbit corneas. Improper corneal stromal wound healing was related to stromal haze formation with reduced corneal transparency postoperatively.16 Similar to Dong et al.,26 our data showed that SMILE induced less keratocyte apoptosis than FS-LASIK 24 hours after surgery. Our results were also consistent with the report by Sun et al.,27 which revealed that reduced apoptosis disappeared within 1 week. Supporting previous reports,26,27 corneal cell proliferation was significantly higher after FS-LASIK than after SMILE at 1 week postoperatively and returned to normal levels by 3 months.
Corneal wound healing occurs through a complex cascade involving multiple cell types and GFs.28 EGF, through binding to the EGFR, stimulates proliferation of corneal epithelial cells and accelerates epithelial wound healing.29,30 In our study, significantly higher EGFR expression in the FS-LASIK group suggests increased EGF binding and accelerated epithelial wound healing; however, this may also due to the larger flap incision.
TGF-β1 promotes corneal stromal cell proliferation and migration, a process necessary to repopulate wounded tissue.16 However, TGF-β1 also generates adherent myofibroblasts, which may cause tissue fibrosis and affect the transparency of cornea.31 Our study showed elevated TGF-β1 levels up to 1 month after both procedures, but SMILE induced significantly higher levels than FS-LASIK at 24 hours and 1 week postoperatively. We speculate that although the femtosecond laser goes through the ocular surface into the corneal stroma to create the lenticule or flap, which is a precise laser ablation, extra energy could escape during the ablation process and mildly injure the epithelium and activate cytokine and growth factor release.32 SMILE requires two femtosecond laser scans, whereas FS-LASIK requires only one. Therefore, it is reasonable to assume that the epithelium was more affected by the laser in SMILE than FS-LASIK, causing increased TGF-β1 production. Moreover, the rabbit cornea differs from the human cornea in that it lacks a Bowman layer, resulting in a weaker barrier between the epithelium and stroma.
Although elevated TGF-β1 levels suggests a strong wound-healing response and potential fibrosis, corneal wound healing is an exceedingly complex process involving multiple cytokine- and GF-mediated interactions.28 Interestingly, we also found increased BFGF levels in rabbit cornea up to 1 month in the SMILE group and up to 24 hours in the FS-LASIK group. TGF-β1 and BFGF act in a coordinated manner to determine stromal wound-healing patterns, a process directly associated with corneal stromal opacity and visual outcomes.33 It has been shown that BFGF accelerates wound closure by increasing cell proliferation and inhibiting myofibroblast differentiation.34 Gallego-Muñoz et al.35 reported that upon adding TGF-β1 and BFGF to the culture medium of human corneal fibroblasts, the proliferation process is strengthened and myofibroblast differentiation and migration are remarkably reduced, in comparison to TGF-β1 treatment alone. Given the expression levels observed for TGF-β1 and BFGF within the first month, our study suggests that corneal wound healing is activated earlier in SMILE, in comparison to FS-LASIK, and the difference disappears at 3 months postoperatively. This is consistent with a previous biomechanical study that reported earlier tissue healing after SMILE, in comparison to FS-LASIK.13 Also, the trend in GF expression is in agreement with clinical findings1 and might contribute to post-SMILE delayed visual acuity recovery. In addition, our study suggested that wound healing continues over the first month postoperatively, demonstrating the importance of postoperative care.
Inflammatory cytokines may also be involved in corneal wound healing, as elevated IL-1β has been reported in corneal inflammation.36,37 TNF-α is known to be involved in corneal inflammation, activating neutrophils and inducing chemokine secretion.38 We found increased levels of IL-1β and TNF-α in the cornea 24 hours after FS-LASIK, in comparison to SMILE, with IL-1β levels remaining elevated in post-LASIK corneas at 1 week postoperatively, suggesting that SMILE induces less inflammation in the whole cornea tissue than FS-LASIK within the first week after surgery. This was consistent with the previous rabbit experiments26,39 and a human ex vivo experiment40 showing the inflammation in the corneal stroma by immunofluorescent staining of CD11b on the corneal section.
As noninfectious inflammation after photoablative procedures has been reported to limit visual outcomes,41 changes in the anterior chamber may also be of importance. IL-8, a neutrophil chemotactic factor, plays a key role in the defense mechanism through its effects on neutrophil activity, but prolonged presence of IL-8 in circulation may cause tissue injury.42,43 Our data revealed a significant increase in IL-8 levels in AqH samples 24 hours after both procedures, which remained elevated up to 1 week in the FS-LASIK group. This may due to increased immune cells in the cornea and conjunctiva44 or changes in intraocular pressure after surgery.45 The presence of increased levels of inflammatory cytokines up to 1 week in post-LASIK corneas and AqH supports the notion that the inflammatory response was more severe after FS-LASIK compared to SMILE.
The current study does present some limitation. First, since species-related biologic difference exists, the use of a rabbit model is the main limitation of our study. A rabbit model is more prone to inflammatory reactions compared with humans. However, the main aim of the study was to compare time-sensitive molecular changes between the two procedures. Notably, limitations associated with experiments using human samples, including lack of dynamic changes after refractive surgery in an ex vivo study and difficulty with follow-up cornea sample harvest for in vivo applications, make such studies difficult to perform. The rabbit model allowed us to investigate critical factors at specific time points after surgery and is a common approach used to study corneal wound healing after refractive surgery.3,21,24 Second, there are multiple cytokines and GFs involved in the corneal wound-healing response, such as platelet-derived growth factors and keratinocyte growth factor. Our study focused on three such factors to evaluate would healing. Finally, examination of postoperative retina tissue is imperative to study the effects of refractive surgery on the posterior chamber.
In conclusion, our results suggest that in comparison to FS-LASIK, SMILE resulted in a significant reduction in acute inflammatory responses in both cornea and the aqueous humor, as well as less keratocyte apoptosis and proliferation. Importantly, we present the first study, to our knowledge, characterizing different GF expression profiles during corneal wound healing after SMILE and FS-LASIK, which might contribute to the possible cause of delayed recovery at 1 month after SMILE. The study implies the need for refractive surgeons to continuously monitor and provide adequate postoperative care for the patients during the first month after surgery.
Supplementary Material
Acknowledgments
The authors thank Yuchuan Wang, Tianjin Eye Hospital and Eye Institute, for his technical support and the Tianjin Eye Hospital and Eye Institute as well as Tianjin Key Lab of Ophthalmology and Visual Science.
Supported by the National Natural Science Foundation of China (No. 81670884 and No. 81873684). The funding organization had no role in the design or conduct of this research.
Disclosure: L. Liu, None; W. Cheng, None; D. Wu, None; L. Chen, None; S. Yu, None; T. Zuo, None; L. Zhang, None; K. Yang, None; H. Li, None; H. Zhang, None; P. Wei, None; A.L.K. Ng, None; G.P.-M. Cheng, None; V.C.-P. Woo, None; J. Yin, None; K. Chiu, None; Y. Wang, None
References
- 1. Lau YT, Shih KC, Tse RH, Chan TC, Jhanji V. Comparison of visual, refractive and ocular surface outcomes between small incision lenticule extraction and laser-assisted in situ keratomileusis for myopia and myopic astigmatism. Ophthalmol Ther. 2019; 8: 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim TI, Alio Del Barrio JL, Wilkins M, Cochener B, Ang M. Refractive surgery. Lancet. 2019; 393: 2085–2098. [DOI] [PubMed] [Google Scholar]
- 3. Wei S, Wang Y, Wu D, Zu P, Zhang H, Su X. Ultrastructural changes and corneal wound healing after SMILE and PRK procedures. Curr Eye Res. 2016; 41: 1316–1325. [DOI] [PubMed] [Google Scholar]
- 4. Reinstein DZ, Archer TJ, Gobbe M, Bartoli E. Corneal sensitivity after small-incision lenticule extraction and laser in situ keratomileusis. J Cataract Refract Surg. 2015; 41: 1580–1587. [DOI] [PubMed] [Google Scholar]
- 5. Wu D, Wang Y, Zhang L, Wei S, Tang X. Corneal biomechanical effects: small-incision lenticule extraction versus femtosecond laser-assisted laser in situ keratomileusis. J Cataract Refract Surg. 2014; 40: 954–962. [DOI] [PubMed] [Google Scholar]
- 6. Damgaard IB, Reffat M, Hjortdal J. Review of corneal biomechanical properties following LASIK and SMILE for myopia and myopic astigmatism. Open Ophthalmol J. 2018; 12: 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li M, Li M, Chen Y, Miao H, Yang D, Ni K, Zhou X. Five-year results of small incision lenticule extraction (SMILE) and femtosecond laser LASIK (FS-LASIK) for myopia. Acta Ophthalmologica. 2019; 97: e373–e380. [DOI] [PubMed] [Google Scholar]
- 8. Blum M, Taubig K, Gruhn C, Sekundo W, Kunert KS. Five-year results of small incision lenticule extraction (ReLEx SMILE). Br J Ophthalmol. 2016; 100: 1192–1195. [DOI] [PubMed] [Google Scholar]
- 9. Liu T, Lu G, Chen K, Kan Q, Bai J. Visual and optical quality outcomes of SMILE and FS-LASIK for myopia in the very early phase after surgery. BMC Ophthalmol. 2019; 19: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ang M, Ho H, Fenwick E, et al.. Vision-related quality of life and visual outcomes after small-incision lenticule extraction and laser in situ keratomileusis. J Cataract Refract Surg. 2015; 41: 2136–2144. [DOI] [PubMed] [Google Scholar]
- 11. Agca A, Ozgurhan EB, Yildirim Y, Cankaya KI, Guleryuz NB, Alkin Z. Corneal backscatter analysis by in vivo confocal microscopy: fellow eye comparison of small incision lenticule extraction and femtosecond laser-assisted LASIK. J Ophthalmol. 2014; 2014: 265012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Ma J, Zhang L, Zou H, Li J, Zhang Y, Jhanji V. Postoperative corneal complications in small incision lenticule extraction: long-term study. J Refract Surg. 2019; 35: 146–152. [DOI] [PubMed] [Google Scholar]
- 13. Shetty R, Francis M, Shroff R, et al.. Corneal biomechanical changes and tissue remodeling after SMILE and LASIK. Invest Ophthalmol Vis Sci. 2017; 58: 5703–5712. [DOI] [PubMed] [Google Scholar]
- 14. Ji YW, Kim M, Kang DSY, et al.. Lower laser energy levels lead to better visual recovery after small-incision lenticule extraction: prospective randomized clinical trial. Am J Ophthalmol. 2017; 179: 159–170. [DOI] [PubMed] [Google Scholar]
- 15. Baldwin HC, Marshall J. Growth factors in corneal wound healing following refractive surgery: a review. Acta Ophthalmol Scand. 2002; 80: 238–247. [DOI] [PubMed] [Google Scholar]
- 16. Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res. 2015; 49: 17–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leonardi A, Tavolato M, Curnow SJ, Fregona IA, Violato D, Alio JL. Cytokine and chemokine levels in tears and in corneal fibroblast cultures before and after excimer laser treatment. J Cataract Refract Surg. 2009; 35: 240–247. [DOI] [PubMed] [Google Scholar]
- 18. Zhang C, Ding H, He M, et al.. Comparison of early changes in ocular surface and inflammatory mediators between femtosecond lenticule extraction and small-incision lenticule extraction. PLoS One. 2016; 11: e0149503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao S, Li S, Liu L, Wang Y, Ding H, Li L, Zhong X. Early changes in ocular surface and tear inflammatory mediators after small-incision lenticule extraction and femtosecond laser-assisted laser in situ keratomileusis. PLoS One. 2014; 9: e107370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mastropasqua L, Calienno R, Curcio C, Mastropasqua R, Nubile M, Salgari N, Lanzini M. In vivo and ex vivo evaluation of inflammation and apoptosis induced after SMILE procedures for different refractive error range. Curr Eye Res. 2017; 42: 701–707. [DOI] [PubMed] [Google Scholar]
- 21. Riau AK, Angunawela RI, Chaurasia SS, Lee WS, Tan DT, Mehta JS. Early corneal wound healing and inflammatory responses after refractive lenticule extraction (ReLEx). Invest Ophthalmol Vis Sci. 2011; 52: 6213–6221. [DOI] [PubMed] [Google Scholar]
- 22. Hou J, Wang Y, Lei Y, Zheng X. Comparison of effective optical zone after small-incision lenticule extraction and femtosecond laser-assisted laser in situ keratomileusis for myopia. J Cataract Refract Surg. 2018; 44: 1179–1185. [DOI] [PubMed] [Google Scholar]
- 23. Netto MV, Mohan RR, Medeiros FW, et al.. Femtosecond laser and microkeratome corneal flaps: comparison of stromal wound healing and inflammation. J Refract Surg. 2007; 23: 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tay E, Li X, Chan C, Tan DT, Mehta JS. Refractive lenticule extraction flap and stromal bed morphology assessment with anterior segment optical coherence tomography. J Cataract Refract Surg. 2012; 38: 1544–1551. [DOI] [PubMed] [Google Scholar]
- 25. Heisterkamp A, Mamom T, Kermani O, et al.. Intrastromal refractive surgery with ultrashort laser pulses: in vivo study on the rabbit eye. Graefes Arch Clin Exp Ophthalmol. 2003; 241: 511–517. [DOI] [PubMed] [Google Scholar]
- 26. Dong Z, Zhou X, Wu J, et al.. Small incision lenticule extraction (SMILE) and femtosecond laser LASIK: comparison of corneal wound healing and inflammation. Br J Ophthalmol. 2014; 98: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun Y, Zhang T, Liu M, Zhou Y, Weng S, Yang X, Liu Q. Early corneal wound healing response after small incision lenticule extraction. Cornea. 2019; 38: 1582–1588. [DOI] [PubMed] [Google Scholar]
- 28. Yin H, Lu Q, Wang X, et al.. Tissue-derived microparticles reduce inflammation and fibrosis in cornea wounds. Acta Biomater. 2019; 85: 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tuli SS, Liu R, Chen C, Blalock TD, Goldstein M, Schultz GS. Immunohistochemical localization of EGF, TGF-alpha, TGF-beta, and their receptors in rat corneas during healing of excimer laser ablation. Curr Eye Res. 2006; 31: 709–719. [DOI] [PubMed] [Google Scholar]
- 30. Zieske JD, Takahashi H, Hutcheon AE, Dalbone AC. Activation of epidermal growth factor receptor during corneal epithelial migration. Invest Ophthalmol Vis Sci. 2000; 41: 1346–1355. [PubMed] [Google Scholar]
- 31. Wang L, Ko CY, Meyers EE, Pedroja BS, Pelaez N, Bernstein AM. Concentration-dependent effects of transforming growth factor beta1 on corneal wound healing. Mol Vis. 2011; 17: 2835–2846. [PMC free article] [PubMed] [Google Scholar]
- 32. Homer N, Jurkunas UV. The use of femtosecond laser in refractive and cataract surgery. Int Ophthalmol Clin. 2017; 57: 1–10. [DOI] [PubMed] [Google Scholar]
- 33. Gallego-Muñoz P, Ibares-Frias L, Valsero-Blanco MC, Cantalapiedra-Rodriguez R, Merayo-Lloves J, Martinez-Garcia MC. Effects of TGFbeta1, PDGF-BB, and bFGF, on human corneal fibroblasts proliferation and differentiation during stromal repair. Cytokine. 2017; 96: 94–101. [DOI] [PubMed] [Google Scholar]
- 34. Song QH, Klepeis VE, Nugent MA, Trinkaus-Randall V. TGF-beta1 regulates TGF-beta1 and FGF-2 mRNA expression during fibroblast wound healing. Mol Pathol. 2002; 55: 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gallego-Muñoz P, Ibares-Frias L, Garrote JA, Valsero-Blanco MC, Cantalapiedra-Rodriguez R, Merayo-Lloves J, Carmen Martinez-Garcia M. Human corneal fibroblast migration and extracellular matrix synthesis during stromal repair: role played by platelet-derived growth factor-BB, basic fibroblast growth factor, and transforming growth factor-beta1. J Tissue Eng Regen Med. 2018; 12: e737–e746. [DOI] [PubMed] [Google Scholar]
- 36. Matsumoto K, Ikema K, Tanihara H. Role of cytokines and chemokines in pseudomonal keratitis. Cornea. 2005; 24: S43–S49. [DOI] [PubMed] [Google Scholar]
- 37. Zhang Y, Liang Q, Liu Y, Pan Z, Baudouin C, Labbe A, Lu Q. Expression of cytokines in aqueous humor from fungal keratitis patients. BMC Ophthalmol. 2018; 18: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008; 132: 344–362. [DOI] [PubMed] [Google Scholar]
- 39. Liu YC, Teo EP, Lwin NC, Yam GH, Mehta JS. Early corneal wound healing and inflammatory responses after SMILE: comparison of the effects of different refractive corrections and surgical experiences. J Refract Surg. 2016; 32: 346–353. [DOI] [PubMed] [Google Scholar]
- 40. Luft N, Schumann RG, Dirisamer M, et al.. Wound healing, inflammation, and corneal ultrastructure after SMILE and femtosecond laser-assisted LASIK: a human ex vivo study. J Refract Surg. 2018; 34: 393–399. [DOI] [PubMed] [Google Scholar]
- 41. Alio JL, Javaloy J. Corneal inflammation following corneal photoablative refractive surgery with excimer laser. Surv Ophthalmol. 2013; 58: 11–25. [DOI] [PubMed] [Google Scholar]
- 42. Ghasemi H, Ghazanfari T, Yaraee R, Faghihzadeh S, Hassan ZM. Roles of IL-8 in ocular inflammations: a review. Ocul Immunol Inflamm. 2011; 19: 401–412. [DOI] [PubMed] [Google Scholar]
- 43. Zenkel M, Lewczuk P, Junemann A, Kruse FE, Naumann GO, Schlotzer-Schrehardt U. Proinflammatory cytokines are involved in the initiation of the abnormal matrix process in pseudoexfoliation syndrome/glaucoma. Am J Pathol. 2010; 176: 2868–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aketa N, Yamaguchi T, Asato T, et al.. Elevated aqueous cytokine levels in eyes with ocular surface diseases. Am J Ophthalmol. 2017; 184: 42–51. [DOI] [PubMed] [Google Scholar]
- 45. Cheng W, Liu L, Yu S, et al.. Real-time intraocular pressure measurements in the vitreous chamber of rabbit eyes during small incision lenticule extraction (SMILE). Curr Eye Res. 2018; 43: 1260–1266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.