Abstract
Purpose
Fibrosis or scarring is a pathological outcome of wound healing and is characterized by terminally differentiated myofibroblasts. Heavy chain-hyaluronic acid/pentraxin 3 (HC-HA/PTX3) is a unique matrix component purified from amniotic membrane that exerts an anti-inflammatory effect. Herein, we investigate whether HC-HA/PTX3 can also exert an antiscarring effect.
Methods
Human corneal fibroblasts and myofibroblasts were seeded on plastic, immobilized HA or HC-HA/PTX3 or on plastic with or without soluble HA and HC-HA/PTX3 in DMEM+10% FBS, with or without AMD3100 or SB431542 in DMEM+ITS with or without transforming growth factor–β1 (TGF-β1). Transcript expression of keratocyte and signaling markers was determined by RT-qPCR. Immunostaining was performed to monitor cytolocalization of signaling markers and α-SMA. Western blotting was used to measure relative protein level.
Results
Human corneal fibroblasts and myofibroblasts cultured in or on HC-HA/PTX3, but not HA, were refrained from cytoplasmic expression of αSMA and nuclear translocation of pSMAD2/3 when challenged with exogenous TGF-β1. Such an antiscarring action by suppressing canonical TGF-β1 signaling was surprisingly accompanied by phenotypic reversal to keratocan-expressing keratocytes through activation of BMP signaling. Further investigation disclosed that such phenotypic reversal was initiated by cell aggregation mediated by SDF1-CXCR4 signaling highlighted by nuclear translocation of CXCR4 and upregulation of CXCR4 transcript and protein followed by activation of canonical BMP signaling.
Conclusions
These findings collectively provide mechanistic understanding explaining how amniotic membrane transplantation exerts an antiscarring action. In addition, HC-HA/PTX3 and derivatives may be developed into a new biologic to treat corneal blindness caused by stromal scar or opacity in the future.
Keywords: HC-HA/PTX3, corneal fibroblasts, keratocytes, CXCR4, BMP
Fibrosis is considered as aberrant wounding healing resulting in progression rather than resolution of scarring. Fibrosis involves unabated fibroblast activity leading to excessive accumulation of extracellular matrix causing disruption of normal tissue architecture and function. In the cornea, this pathological process may occur in wound healing where relatively quiescent stromal keratocan-expressing keratocytes are activated and differentiated into fibroblasts and myofibroblasts expressing α–smooth muscle actin (α-SMA) as a contractile cytoskeletal element organized in stress fibers.1
Clinically, transplantation of cryopreserved amniotic membrane (AM) is effective in reducing scar during ocular surface reconstruction in ophthalmology (for reviews, see reference 2). We have previously shown downregulation of TGF-β ligands and TGF-β receptors and α-SMA transcripts by human corneal and limbal fibroblasts3 and human conjunctival and pterygium fibroblasts4 when cultured on the AM stromal surface. In addition, human5 and murine6 keratocytes can maintain their normal phenotypes when cultured on AM stromal surface without activation of and expression of α-SMA even when TGF-β is added in a serum-containing medium.6 Furthermore, water-soluble AM stromal extract can reverse myofibroblasts derived from the AM stroma to fibroblasts with downregulation of α-SMA.7 These data suggest that the AM stromal matrix can prevent differentiation of corneal keratocytes and fibroblasts into myofibroblasts although the exact molecular mechanism remains unclear.
Our laboratory has successfully purified HC-HA/PTX3 from water-soluble AM extract as a unique matrix consisting of high molecular weight hyaluronic acid (HA) covalently linked with heavy chain 1 (HC1) from inter-α-trypsin inhibitor (“-” denotes covalent linkage) and further complexed with pentraxin 3 (PTX3) (“/” denotes noncovalent linkage).8,9 HC-HA/PTX3 has been shown to exert an anti-inflammatory action that extends from innate immune responses by facilitating apoptosis of stimulated neutrophils and polarizing M2 macrophages to adaptive immune responses by suppressing activation of Th1 and Th17 lymphocytes to downregulate alloreactive immune responses.10,11 Herein, we demonstrate that HC-HA/PTX3, but not HA, can revert human corneal fibroblasts and myofibroblasts to keratocytes by inhibiting canonical TGF-β signaling and by activating BMP signaling following an early event of cell aggregation mediated by SDF1-CXCR4 signaling.
Materials and Methods
Materials
All materials used for assays, cell culture, expansion, and experiments were listed in Supplementary Table S1. All primers used for quantitative real time polymerase chain reaction (qRT-PCR) were listed in Supplementary Table S2, whereas all antibodies used for immunofluorescence staining or Western blotting were listed in Supplementary Table S3, and for the validation data for these antibodies see also the Supplementary Figures.
Purification and Immobilization of HC-HA/PTX3
HC-HA/PTX3 was purified from cryopreserved amniotic membrane after retrieval from human placenta provided by TissueTech, Inc. (Miami, FL, USA) as reported8,9 with modification. In brief, AM was cryopulverized by FreezeMill (FreezerMill 6870; SPEX SamplePrep, Metuchen, NJ, USA), extracted by phosphate-buffered saline solution (PBS; pH 7.4) at 4°C for 1 hour and then centrifuged at 48,000g at 4°C for 30 minutes. The supernatant designated as water-soluble AM extract was fractionated by ultracentrifugation in a CsCl gradient at an initial density of 1.35 g/mL in 4 mol/L GnHCl at 125,000 rpm at 15°C for 48 hours (Optima L-80 X, SW41 rotor; Beckman Coulter, Indianapolis, IN, USA). A total of 12 fractions (1 mL/fraction) were collected and subjected to the measurement of HA and protein contents by the enzyme-linked immunosorbent HA Quantitative Test Kit and the BCA Protein Assay Kit, respectively. The fractions of 2–12, which contained most of HC-HA/PTX3, were pooled and further subjected to three consecutive runs of ultracentrifugation at 125,000g in CsCl/4 mol/L guanidine HCl at a density of 1.40 g/mL for the second, third, and fourth runs, each run at 15°C for 48 hours. The fractions 3–9 after the fourth run were pooled and dialyzed against distilled water at 4°C for 48 hours, lyophilized, stored at −80°C and designated as HC-HA/PTX3. Before use, HC-HA/PTX3 was qualified by verifying its biochemical composition containing high molecular weight HA based on agarose gel electrophoresis and HC-HA/PTX3 based on Western blotting to HC1 and PTX3 with or without hyaluronidase (1 U/µg HA) digestion and with or without reduction by 100 mmol/L dithiothreitol in the presence of proteinase inhibitors (10 mmol/L ethylenediamine tetra-acetic acid [EDTA], 10 mmol/L aminocaproic acid, 10 mmol/L N-ethylmaleimide, and 1 mmol/L phenylmethanesulfonyl fluoride (PMSF)) as reported.8,9 Because the negligible amount of protein therein, the amount of HC-HA/PTX3 used in the experiment was expressed based on the HA amount.
HA or HC-HA/PTX3 was immobilized on Covalink-NH 96 wells (Nalge Nunc International, Rochester, NY, USA) as reported11 by first sterilizing the Covalink-NH 96 wells in 70% alcohol for 30 minutes, and then the wells were washed with distilled water two times. HA (2 µg/well) or HC-HA/PTX3 (2 µg/well) with the cross-linking reagents of Sulfo-NHS at 9.2 mg/mL (Pierce) and 1-ethyl-3(3-dimethylaminopropyl) carbodiimide (Pierce) at 6.15 mg/mL were added to each well (100 µL) and incubated at 4˚C overnight. After that, the un-cross-linked HC-HA/PTX3 and cross-linking reagents were removed, and the wells were washed twice with 2 mol/L NaCl/ 50 mmol/L MgSO4 /PBS, followed by two washes of PBS.
Isolation, Culture, and Treatment of Human Corneal Fibroblasts and Myofibroblasts
Human corneas from donors aged 18 to 76 years and maintained at 4°C in Optisol (Chiron Vision, Irvine, CA, USA) for less than seven days after death were obtained from the Florida Lions Eye Bank (Miami, FL, USA) and handled according to the Declaration of Helsinki.
Human corneal fibroblasts (HCF) were isolated and cultured as reported.12 Briefly, the endothelium was peeled off from cornea by forceps and the epithelium removed by 10 mg/mL dispase overnight. The remaining corneal stroma was cut into cubes of approximately 1 mm3, incubated in 2 mg/mL collagenase for 16 hours at 37°C, and then 1 × 104 cm2 cells were placed on plastic in a culture medium consisting of Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (DMEM+10%FBS) containing 50 mg/mL gentamicin and 1.25 mg/mL amphotericin B. The culture medium was changed twice a week. HCF cultured on plastic in DMEM+10% FBS until 70% confluence at passage 3 were switched to a serum free medium (DMEM+ITS), which contained DMEM plus 5 µg/mL Insulin, 5 µg/mL Transferrin, 5 ng/mL sodium selenite for one day before being added with 10 ng/mL TGF-β1 in DMEM+ITS medium for three days to induce myofibroblasts. Passage 3 HCF or myofibroblasts detached by 0.25% trypsin were pretreated for 30 minutes before being seeded at 5000 cells/96-well and continuously cultured in DMEM+10% FBS with or without 0.1% DMSO with or without 20 µg/mL AMD3100 or 10 µmol/L SB431542 and on plastic with or without immobilized HA or HC-HA/PTX3 and soluble HA or HC-HA/PTX3, each at 20 µg/mL for seven days before being harvested for analysis.
RNA Extraction, Reverse Transcription and qRT-PCR
Total RNAs were extracted using RNeasy Mini Kit and reverse-transcribed using High Capacity Reverse Transcription Kit (Applied Biosystems). The cDNA was amplified by real-time RT-PCR using specific primers obtained from Applied Biosystems (Supplementary Table S2) and DNA polymerase in Quant Studio 5 Real-time PCR System (Applied Biosystems). Real-time RT-PCR profile consisted of 10 minutes of initial activation at 95°C, followed by 40 cycles of 15 seconds denaturation at 95°C, and 1 minute annealing and extension at 60°C. The genuine identity of each PCR product was confirmed by the size determination using 2% agarose gels followed by ethidium bromide staining together with PCR marker according to EC3 Imaging System (BioImaging System, Upland, CA, USA).
Immunofluorescence Staining
HCF or myofibroblasts were harvested with 0.05% trypsin and 1 mmol/L EDTA at 37°C for 10 minutes and prepared for cytospin using Cytofuge (StatSpin Inc., Norwood, MA, USA) at 1000 rpm for eight minutes. Cells were fixed with 4% formaldehyde, pH 7.0, for 15 minutes at room temperature, permeabilized with 0.2% Triton X-100 in PBS for 15 minutes, and blocked with 2% bovine serum albumin for 1 hour before being incubated with primary antibodies for 16 hours at 4°C. After three washes with PBS, the corresponding Alexa Fluor-conjugated secondary immunoglobulin G (IgG; all 1:100 dilution) were incubated for 60 minutes and three washings with PBS. After three washes with PBS, the second antibodies were incubated for 60 minutes and followed with the corresponding Alex Fluor-conjugated secondary IgG. The nucleus was counterstained with Hoechst 33342 before being analyzed with Zeiss LSM 700 confocal microscope (Carl Zeiss, Thornwood, NY, USA). Corresponding mouse and rabbit sera were used as negative controls for primary monoclonal and polyclonal antibodies, respectively.
Western Blotting
Cell lysates and nuclear extracts were prepared in RIPA buffer, the protein concentrations determined by The BCA Protein Assay Kit (ThermoFisher Scientific, Waltham, MA, USA), and 10 µg of protein for each sample resolved on 4% to 15% (w/v) gradient acrylamide gels under denaturing and reducing conditions for Western blotting. The protein extracts were transferred to the nitrocellulose membrane, which was then blocked with 5% (w/v) fat-free milk in Tris-Buffered Saline, 0.1% Tween 20 Detergent (TBST) (50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 0.05% [v/v] Tween-20), followed by sequential incubation with the specific primary antibody against keratocan and its respective horseradish peroxidase–conjugated secondary antibody using β-actin as the loading control. Immunoreactive proteins were detected with Western Lighting Chemiluminescence (PerkinElmer, Waltham, MA, USA). For nuclear proteins, NE-PER Nuclear and Cytoplasmic Extraction Reagents were used to separate membranous, cytoplasmic and nuclear proteins before we do Western blotting.
Statistical Analysis
All summary data were reported as mean ± SD. calculated for each group and compared using analysis of variance and the Student unpaired t-test by Microsoft Excel (Microsoft, Redmon, WA, USA). Test results were reported as two-tailed P values, where P < 0.05 was considered statistically significant.
Results
Human Corneal Myofibroblasts Form Aggregates and Revert to Keratocytes by HC-HA/PTX3
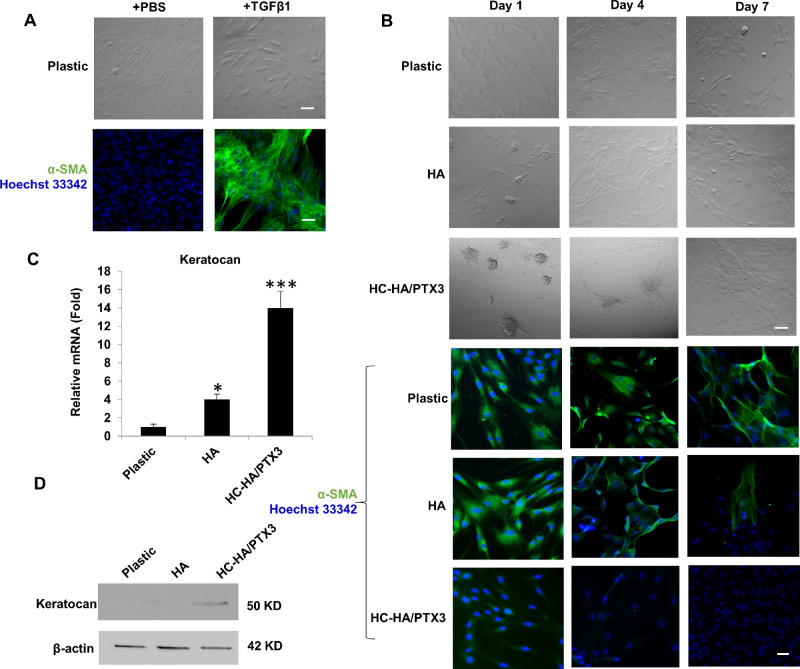
To examine whether HC-HA/PTX3 purified from water-soluble AM extract might be responsible for preventing human and mouse keratocytes from differentiating to myofibroblasts8,9 and for reverting AM stromal myofibroblasts to fibroblasts,7 we induced HCF into myofibroblasts by adding 10 ng/mL TGF-β1 in DMEM+ITS for three days and verified their phenotype by positive cytoplasmic immunofluorescence staining of α-SMA (Fig. 1A). We then seeded human corneal myofibroblasts on plastic in DMEM+10% FBS with or without immobilized HA or HC-HA/PTX3 for seven days. The results showed that human corneal myofibroblasts uniquely formed aggregates on HC-HA/PTX3 but not plastic or HA at day 1, and such aggregates became to spread to a monolayers of spindle cells (Fig. 1B). Immunofluorescence staining confirmed the expression of α-SMA by spindle cells seeded on plastic throughout seven days and by those on immobilized HA until day 4 with a slight reduced expression at day 7 (Fig. 1B). In contrast, expression of α-SMA was absent in aggregated cells on HC-HA/PTX3 at day 1 and continued to be negative until day 7, even when spindle cells spread out (Fig. 1B). Such reversal of myofibroblasts was accompanied by emergence of 14-fold increase of keratocan mRNA expression at day 1 (Fig. 1C) and positive expression of keratocan protein at day 2 (Fig. 1D). These results collectively suggested that human corneal myofibroblasts were reverted to keratocan-expressing keratocytes by immobilized HC-HA/PTX3.
Figure 1.
Human corneal myofibroblasts on HC-HA/PTX3 form aggregation and revert to keratocytes. Passage 3 HCF were induced into myofibroblasts by addition of TGFβ1 as verified by immunofluorescence staining to α-SMA (A) and passaged on plastic with or without immobilized HA or HC-HA/PTX3 for 7 days for phase contrast micrography and immunostaining to α-SMA (B, nuclear staining by Hoechst 33342, Bar = 100 µm). Expression of keratocan mRNA and protein was determined at Day 1 by quantitative RT-PCR using the expression level on plastic set as 1 (C, *P < 0.05, ***P < 0.001, n = 3) and by Western blot using β-actin as the loading control (D), respectively.
HCF Form Aggregates, Revert to Keratocytes, and Resist to TGF-β1 Challenge on HC-HA/PTX3
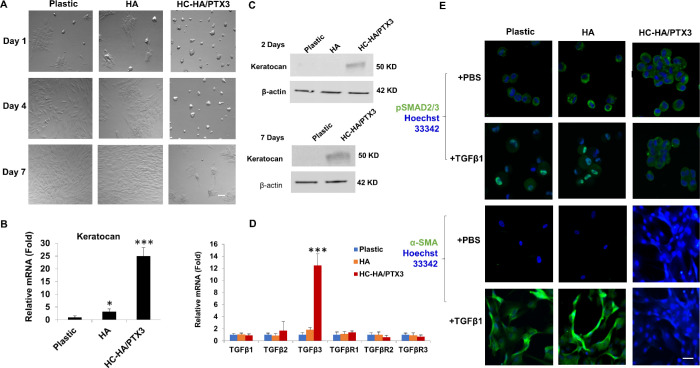
To determine whether HCF might also respond to HC-HA/PTX3 in the same manner, we seeded passage 3 HCF in DMEM+10% FBS on plastic with or without immobilized HA or HC-HA/PTX3 for seven days. Phase contrast micrographs showed that HCF also formed cell aggregates on HC-HA/PTX3 on day 1 that last until day 7 whereas HCF only formed few aggregates on plastic or HA at Day 1 (Fig. 2A). Expression of keratocan transcript was elevated 24-fold (Fig. 2B), together with positive expression of keratocan protein by HCF on HC-HA/PTX3 at day 1 and extended to day 7, when cells began to migrate from the aggregate (Fig. 2C). When compared to plastic or HA, the transcript expression of TGF-β1, TGF-β2, TGF-βRI, TGF-βRII, and TGF-βRIII 3 did not show any significant changes while that of TGF-β3 was significantly upregulated by HCF on HC-HA/PTX (Fig. 2D, P < 0.001 and P < 0.001 c.f., plastic and HA, respectively). To demonstrate whether such a phenotypic reversal was resulted from the resistance to TGF-β activation, HCF were challenged by exogenous addition of 10 ng/mL of TGF-β1 in DMEM+ITS medium at day 1 for 3 days. Immunofluorescence staining of resultant cells showed nuclear staining of pSMAD2/3 and cytoplasmic staining of α-SMA in HCF cultured on plastic or immobilized HA but cytoplasmic staining of nuclear pSMAD2/3 and the lack of cytoplasmic staining of α-SMA in HCF cultured on immobilized HC-HA/PTX3 after TGF-β1 challenge (Fig. 2E). These findings collectively suggested that HCF also formed cell aggregation and reverted to keratocan-expressing keratocytes on immobilized HC-HA/PTX3 and that such a change was accompanied by withstanding the challenge of exogenous TGF-β1, which did not yield canonical TGF-β signaling mediated by Smad2/3.
Figure 2.
HCF on HC-HA/PTX3 Form Aggregates, Revert to Keratocytes, and Resist to TGFβ1. Passage 3 HCF were seeded on plastic with or without immobilized HA or HC-HA/PTX3 for 7 days (A, Bar = 100 µm). Transcript expression of keratocan (B) and TGFβs and TGFβRs (D) was determined at day 1 by quantitative RT-PCR using the expression level on plastic set as 1 (*P < 0.05, ***P < 0.001, n = 3). Expression of keratocan protein was determined at Day 2 and Day 7 by Western blot using β-actin as the loading control (C). HCF seeded on plastic with or without immobilized HA or HC-HA/PTX3 in DMEM+10% FBS were switched to DMEM+ITS for 24 hours before being added with 10 ng/mL TGFβ1 for 24 hours before immunostaining of pSmad2/3 and for 72 hours before immunostaining to α-SMA (E, nuclear staining by Hoechst 33342, Bar = 100 µm).
Cell Aggregation and Reversal to Keratocytes Promoted by HC-HA/PTX3 Is Mediated by SDF1-CXCR4 Signaling and BMP Signaling but not by Integrin β1 Signaling
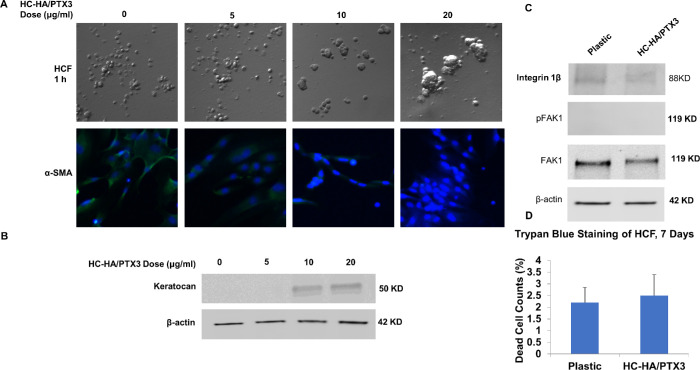
Cell aggregation of human corneal myofibroblasts and HCF was an early event uniquely promoted by immobilized HC-HA/PTX3 (Figs. 1 and 2). Such aggregation mediated by HC-HA/PTX3 differs from that caused by low attachment condition because that by HC-HA/PTX3 was dose-dependent. By adding an increasing concentration of soluble HC-HA/PTX3, we noted that cell aggregation, increase of keratocan expression, and reduction of α-SMA were all dose-dependently affected by soluble HC-HA/PTX3 (Figs. 3A, 3B). We have excluded the likelihood that cell aggregation by HC-HA/PTX3 was mediated by integrin β1 and FAK (phosphorylation at Tyr-397) (Figs. 3C and 3D), of which the latter is an integrin-mediated cell adhesion-dependent phosphorylation event.13 Furthermore, we have also excluded the possibility that the observed reversal to keratocytes was due to the survival of nonmyofibroblastic cells because we did not observe any cell death measured by trypan blue (Fig. 3C).
Figure 3.
Promotion of cell aggregation, keratocan expression and inhibition of α-SMA in HCF are dose-dependent and not mediated by integrin β1 signaling or cell death. Passage 3 HCF were seeded on plastic with or without immobilized HC-HA/PTX3 or soluble HC-HA/PTX3 for 1 day at the concentration of 0, 5, 10, or 20 µg/mL (A, Bar = 100 µm). HCF seeded on immobilized HC-HA/PTX3 in DMEM+10% FBS were switched to DMEM+ITS for 24 hours before being added with 10 ng/mL TGFβ1 for 72 hours before immunostaining to α-SMA (A, nuclear staining by Hoechst 33342, Bar = 100 µm). Expression of keratocan was determined in HCF on immobilized HC-HA/PTX3 (20 µg/ml) at Day 2 (B) while that of integrin β1 (activated) and pFAK (Tyr-397) at 15 minutes (C) by Western blot using β-actin and total FAK as the loading controls. Trypan blue staining was performed on HCF after cultured on immobilized HC-HA/PTX3 for 7 days and counted as stained cells in total cells (%) (D, n = 3, P > 0.05).
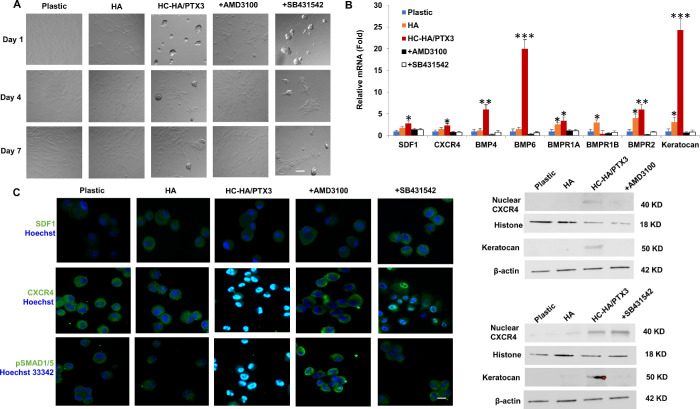
We previously reported that immobilized HC-HA/PTX3, but not 3D Matrigel, uniquely upregulates the BMP signaling in limbal niche cells and that this action is responsible for the maintenance of limbal epithelial stem cell quiescence maintained in HC-HA/PTX3.14 In addition, the aforementioned outcome requires a close contact between limbal niche cells and epithelial stem cells through SDF-1/CXCR4 signaling15 to prevent limbal epithelial stem cells from adopting corneal fate decision.16,17 Thus we decided to perturb the above two signaling by adding AMD3100 known to block CXCR418 or SB431542, a small molecule BMP inhibitor,19 30 minutes before and continuously after seeding passage 3 HCF in DMEM+10% FBS on plastic with or without immobilized HA or HC-HA/PTX3 for seven days. The results showed that addition of AMD3100, but not SB431542, abolished cell aggregation that occurred uniquely on immobilized HC-HA/PTX3 but not plastic or immobilized HA (Fig. 4A), indicating that SDF-1/CXCR4 signaling was, but BMP signaling was not, involved in cell aggregation triggered by HC-HA/PTX3. Compared to plastic and HA, the upregulation of keratocan transcript by 24-fold (Fig. 4B) and keratocan protein (Fig. 4D) by immobilized HC-HA/PTX3 was abolished by both AMD3100 and SB431542, suggesting that the aforementioned phenotypic reversal to keratocytes by HC-HA/PTX3 was controlled by both SDF-1/CXCR4 and BMP signaling. Expression of SDF-1 transcript was upregulated 3-fold whereas that of CXCR4 transcript was upregulated 2-fold by HC-HA/PTX3, and such upregulation was inhibited by AMD3100, but not SB431542 (Fig. 4B), suggesting that cell aggregation was accompanied by upregulation of SDF-1 and CXCR4 transcript, and such an event was not affected by BMP signaling. Immunofluorescence staining to CXCR4 was membranous and cytoplasmic in HCF seeded on plastic or immobilized HA but nuclear in those seeded on immobilized HC-HA/PTX3 (Fig. 4C). Such nuclear CXCR4 staining and presence of CXCR4 protein in the nuclear extract were abolished by AMD3100 but not SB431542 (Figs. 4C and 4D). As expected, HC-HA/PTX3 uniquely upregulated 6-fold and 20-fold expression of BMP4 and BMP6 transcripts and 3-fold and 5-fold expression of BMPR1A and BMPR2 transcripts (Fig. 4B), and such transcript upregulation was accompanied by nuclear staining of pSMAD1/5 (Fig. 4C), indicating the activation of canonical BMP signaling by HC-HA/PTX3. Although also as expected, such BMP signaling was abolished by SB431542 (Fig. 4B), it was also abolished by AMD3100. Therefore we concluded that cell aggregation was mediated by SDF-1/CXCR4 signaling but not BMP signaling and led to the phenotypic reversal to keratocytes, of which the latter was mediated by both SDF-1/CXCR4 and BMP signaling.
Figure 4.
Cell Aggregation and Reversal to Keratocytes Promoted by HC-HA/PTX3 Is Mediated by SDF1-CXCR4 Signaling and BMP Signaling. Passage 3 HCF were pre-treated for 30 minutes before seeding and continuously culturing on plastic with or without immobilized HA or HC-HA/PTX3 for 7 days with or without AMD3100 or SB431542 (A, bar =100 µm). Cells were harvested at Day 1 for quantitative RT-PCR using the expression level on plastic set as 1 (B, *P < 0.05, **P < 0.01, ***P < 0.001, n = 3) and for immunofluorescence staining (C, nuclear staining by Hoechst 33342, Bar = 100 µm) and at Day 2 for Western blot using β-actin or histone as the loading control for total or nuclear extract, respectively (D).
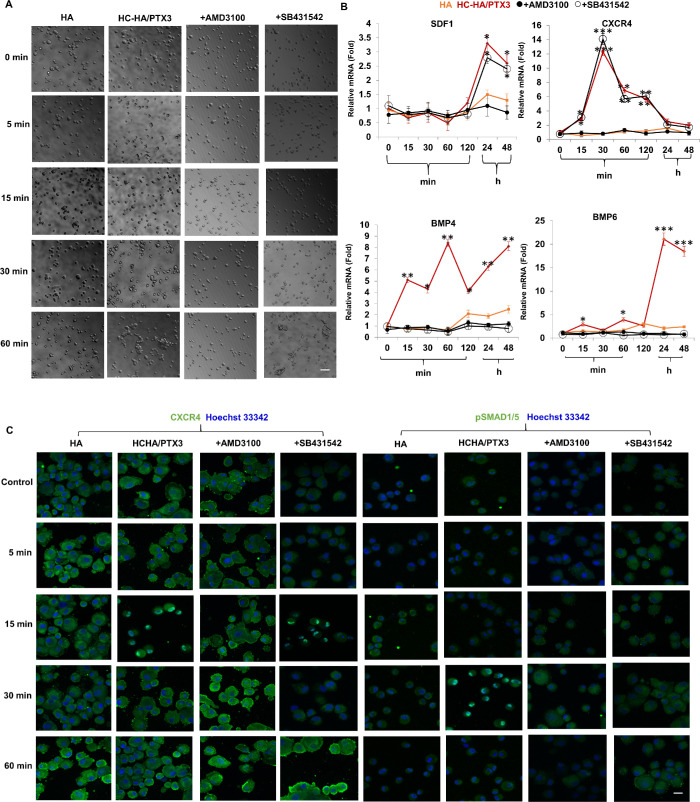
SDF-1/CXCR4 Signaling Precedes BMP Signaling
Although both SDF-1/CXCR4 and BMP signaling were involved in the aforementioned phenotypic reversal to keratocytes, the former, but not the latter, was involved in cell aggregation uniquely triggered by immobilized HC-HA/PTX3 (Fig. 4). Because cell aggregation perturbed by AMD3100 also resulted in suppression of BMP signaling, we thus speculated that SDF-1/CXCR4 signaling that was critical to cell aggregation also led to the activation of BMP signaling, which was pivotal for phenotypic reversal. To further substantiate this notion, we repeated the above experiment using passage 3 HCF cultured in plastic with or without addition of soluble HA or HC-HA/PTX3 in DMEM+10% FBS with or without AMD3100 or SB431542 and performed analysis at different time points in a period of 60 minutes. Phase contrast microscopy showed that cell aggregation was also promoted by soluble HC-HA/PTX3, but not HA, on plastic and such aggregation was abolished by AMD3100, but not SB431542, at the end of 60 minutes (Fig. 5A). Immunofluorescence staining showed nuclear staining of CXCR4 at 15 minutes, but nuclear staining of pSMAD1/5 at 30 minutes in HCF seeded on plastic with soluble HC-HA/PTX3 but not soluble HA (Fig. 5B). AMD3100 abolished nuclear staining of both CXCR4 and pSMAD1/5 whereas SB431542 abolished only nuclear pSMAD1/5 staining but not nuclear CXCR4 staining (Fig. 5C). When the transcript expression levels were analyzed by qRT-PCR, the expression of both CXCR4 and BMP4 transcripts were upregulated by HC-HA/PTX3 as early as 15 minutes and CXCR4 upregulation reached a peak level by 30 minutes whereas BMP4 upregulation gradually increased by 24 hours and thereafter (Fig. 5B). Expression of SDF-1 and BMP6 transcript was upregulated by HC-HA/PTX3 only after 24 hours (Fig. 5B). Such upregulation of CXCR4, SDF-1, BMP4 and BMP6 transcripts by HC-HA/PTX3 was abolished by AMD3100 whereas upregulation of BMP4 and BMP 6 transcripts, but not that of CXCR4 and SDF-1 transcripts, was downregulated by SB431542 (Fig. 5B). Collectively, these results indicated that cell aggregation was mediated by SDF-1/CXCR4 signaling but not BMP signaling and that SDF-1/CXCR4 signaling preceded BMP signaling causing the phenotypic reversal.
Figure 5.
SDF-1/CXCR4 signaling precedes BMP signaling. Passage 3 HCF were pretreated before seeding and continuously cultured on plastic with addition of soluble HA or HC-HA/PTX3 with or without inhibitors AMD3100 or SB431542 for different times in a period of 60 min before being harvested for phase contrast micrography (A, Bar = 100 µm) and immunostaining to CXCR4 and pSMAD1/5 with nuclear staining by Hoechst 33342 (C, Bar = 100 µm) and for a total of 48 hours before quantitative RT-PCR using the expression level at time 0 as 1 (B, *P < 0.05, **P < 0.01, ***P < 0.001, n = 3).
Discussion
Transplantation of cryopreserved AM during ocular surface reconstruction has been shown to reduce scarring.2,20–22 The mode of action for such antiscarring action is thought to be mediated indirectly through the AM's anti-inflammatory action. From water-soluble AM extract, our laboratory has successfully purified and characterized HC-HA/PTX3 as a unique matrix8,9 to exert an anti-inflammatory action extends from innate to adaptive immune responses.10,11 Herein, we demonstrate that HC-HA/PTX3 also exerts a direct antiscarring action as evidenced by the lack of cytoplasmic staining of α-SMA and activation of pSMAD2/3-mediated canonical TGF-β signaling under the challenge of exogenous TGF-β1 (Fig. 2). Interestingly, on immobilized HC-HA/PTX3, addition of TGFβ1 actually upregulated transcript expression of TGFβ3, which is an isoform known to be more abundantly upregulated during fetal development and exerts an antiscarring action.23,24 This finding explains the reported antiscarring action in human corneal and conjunctival fibroblasts3,4 and AM stromal fibroblasts7 when cultured on human AM stroma. It also explains why human and murine keratocyte phenotype can be maintained if cultured on AM stromal surface even challenged with exogenous TGF-β1 in a serum free medium.6,25
To our surprise, the aforementioned antiscarring action by HC-HA/PTX3 was accompanied by phenotypic reversal of both human corneal fibroblasts and myofibroblasts to keratocan-expressing keratocytes (Figs. 1 and 2). Our study further disclosed that such phenotypic reversal was mediated by BMP signaling as evidenced by the upregulation of transcript expression of BMP4, BMP6, BMPR1A, BMPR1B, and BMPR2 and nuclear staining pf pSMAD1/5 in HCF 24 h on plastic with immobilized HC-HA/PTX3 but not plastic or plastic with immobilized HA (Fig. 4). Addition of SB431542, a small molecular BMP inhibitor,19 abolished the aforementioned transcript expression and nuclear staining of pSMAD1/5, resulting in the loss of expression of keratocan transcript and protein (Fig. 4). BMPs are a subgroup of the TGF-β superfamily26 and bind with BMPRII to phosphorylate and activate BMPRI to recruit and activate SMAD1/5/8 for nuclear translocation in order to activate canonical BMP signaling.27 The finding that HC-HA/PTX3 upregulates BMP signaling might be a broader phenomenon because it has also been reported in human limbal niche cells, which upon close contact with limbal epithelial stem cells also help prevent the latter from adopting the corneal fate decision14–16 and maintain BMP signaling in the latter for SC renewal28 in 3D Matrigel but SC quiescence in HC-HA/PTX3.14
BMP signaling is also involved in the early stage of somatic cell reprogramming, which is also highlighted by cell aggregation.14,28 Herein, we noted that phenotypic reversal to keratocytes by immobilized HC-HA/PTX3 was initiated by an early event of cell aggregation (Figs. 1 and 2) and that such cell aggregation could be replicated dose-dependently by soluble HC-HA/PTX3 but not soluble HA in 60 minutes (Fig. 5). Cell aggregation caused by HC-HA/PTX3 immobilized on plastic has been observed in other human primary cultured cells such as limbal niche cells,29 bone marrow mesenchymal stem cells, and trabecular meshwork cells (Tighe et al., unpublished observations, 2020; Zhang et al., unpublished observations, 2020). Our data suggest that cell aggregation by soluble or immobilized HC-HA/PTX3 is not mediated by integrin β1. This notion was consistent with the finding that the cell adhesion of RAW264.7 cells on immobilized HC-HA/PTX3 was not affected by adding neutralized anti-integrin β1 antibody before seeding (unpublished data). Because we did not observe any cell death measured by trypan blue (Fig. 3), we also exclude the likelihood that the reversal to keratocytes was due to the survival of non-myofibroblastic cells. Because cell aggregation, reduction of α-SMA and upregulation of keratocan were dose-dependent on addition of soluble HC-HA/PTX3 (Fig. 3), we conclude that cell aggregation is specific to HC-HA/PTX3. In fact, this notion is further supported by upregulation of transcript expression of BMP4 as early as 15 minutes followed by nuclear staining of pSmad1/5 at 30 minutes and BMP6 at 24 hours on addition of soluble HC-HA/PTX3 (Fig. 5). Furthermore, cell aggregation was mediated by SDF-1/CXCR4 signaling before activation of BMP signaling. Addition of AMD3100, known to block CXCR4,18 aborted cell aggregation, upregulation of BMP transcripts, nuclear staining of pSMAD1/5, and phenotypic reversal to keratocytes in HCF seeded on plastic with immobilized HC-HA/PTX3 (Figs. 4 and 5). In contrast, disruption of BMP signaling by SB431542 did not affect cell aggregation and upregulation of CXCR4 and SDF-1 transcripts (Figs. 4 and 5).
The notion that cell aggregation mediated by SDF-1/CXCR4 preceded BMP signaling was further supported by the finding that nuclear staining of CXCR4 occurred at 15 minutes whereas nuclear staining of pSMAD1/5 was noted at 30 minutes before cell aggregation at 60 minutes facilitated by HC-HA/PTX3 (Figs. 3 and 5). The nuclear translocation of CXCR4 at 15 minutes was accompanied by remarkable early upregulation of CXCR4 transcript at 15 minutes peaking at 30 minutes. Interestingly, such nuclear translocation and transcript upregulation of CXCR4 were abolished by the addition of AMD3100 but not SB431542, of which the latter only suppressed upregulation of BMP4 and BMP6 (Fig. 5). We thus speculate that cell aggregation mediated by SDF-1/CXCR4 signaling highlighted by nuclear translocation of CXCR4 should be a unique cellular hallmark particularly in view of the finding that nuclear CXCR4 before this time has been regarded as a strong indicator for high malignancy in several cancer cells.30–34 We have also observed that SDF1/CXCR4 signaling is also involved om cell aggregation of human limbal niche cells induced by HC-HA/PTX3 (manuscript submitted). Future studies are needed to determine the mechanism leading to nuclear translocation of CXCR4 by HC-HA/PTX3.
In summary, HC-HA/PTX3 is a unique matrix in AM that may exert not only anti-inflammatory actions by modulating both innate and adaptive immune responses8,11 but also a direct antiscarring action. Consequently, we also envisage that HC-HA/PTX3 and derivatives may be developed into a new biologic to treat corneal blindness caused by stromal scar or opacity in the future. These actions together with its ability of supporting limbal niche cells for maintaining SC renewal and quiescence14 further support why transplantation of AM may promote regenerative healing35 and augments the success of in vivo36–38 and ex vivo39–41 expansion of limbal SCs to treat corneal blindness caused by limbal SC deficiency.
Supplementary Material
Acknowledgments
Supported by Grant RO1 EY06819 (to SCGT). The authors alone are responsible for the content and writing of the paper.
Disclosure: Y.-T. Zhu, None; F. Li, None; Y. Zhang, None; S.-Y. Chen, None; S. Tighe, None; S.-Y. Lin, None; S.C.G. Tseng, None
References
- 1. Netto MV, Wilson SE. Flap lift for LASIK retreatment in eyes with myopia. Ophthalmology. 2004; 111: 1362–1367. [DOI] [PubMed] [Google Scholar]
- 2. Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004; 49: 51–77. [DOI] [PubMed] [Google Scholar]
- 3. Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999; 179: 325–335. [DOI] [PubMed] [Google Scholar]
- 4. Lee SB, Li DQ, Tan DT, Meller DC, Tseng SC. Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Current Eye Research. 2000; 20: 325–334. [PubMed] [Google Scholar]
- 5. Espana EM, He H, Kawakita T, et al.. Human keratocytes cultured on amniotic membrane stroma preserve morphology and express keratocan. Invest Ophthalmol Vis Sci. 2003; 44: 5136–5141. [DOI] [PubMed] [Google Scholar]
- 6. Kawakita T, Espana EM, He H, et al.. Keratocan expression of murine keratocytes is maintained on amniotic membrane by downregulating TGF-beta signaling. J Biol Chem. 2005; 280: 27085–27092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li W, He H, Chen YT, Hayashida Y, Tseng SC. Reversal of myofibroblasts by amniotic membrane stromal extract. J Cell Physiol. 2008; 215: 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He H, Li W, Tseng DY, et al.. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-alpha-inhibitor (HC*HA) purified from extracts of human amniotic membrane. J Biol Chem. 2009; 284: 20136–20146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang S, Zhu YT, Chen SY, He H, Tseng SC. Constitutive expression of pentraxin 3 (PTX3) protein by human amniotic membrane cells leads to formation of the heavy chain (HC)-hyaluronan (HA)-PTX3 complex. J Biol Chem. 2014; 289: 13531–13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He H, Zhang S, Tighe S, Son J, Tseng SC. Immobilized Heavy Chain-Hyaluronic Acid Polarizes Lipopolysaccharide-activated Macrophages toward M2 Phenotype. J Biol Chem. 2013; 288: 25792–25803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sheha HTSG. Self-retained amniotic membrane for post-PRK keratitis. ASCRS ASOA Symposium and Congress; 2014. [Google Scholar]
- 12. Li D-Q, Tseng SCG. Differential regulation of cytokine and receptor transcript expression in human corneal and limbal fibroblasts by EGF, TGF-a, PDGF-BB and IL-1b. Invest Ophthalmol Vis Sci. 1996; 37: 2068–2080. [PubMed] [Google Scholar]
- 13. Wu C. Focal adhesion: a focal point in current cell biology and molecular medicine. Cell Adhesion & Migration. 2007; 1: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen SY, Han B, Zhu YT, et al.. HC-HA/PTX3 purified from amniotic membrane promotes BMP signaling in limbal niche cells to maintain quiescence of limbal epithelial progenitor/stem cells. Stem Cells. 2015; 33: 3341–3355. [DOI] [PubMed] [Google Scholar]
- 15. Xie HT, Chen SY, Li GG, Tseng SC. Limbal epithelial stem/progenitor cells attract stromal niche cells by SDF-1/CXCR4 signaling to prevent differentiation. Stem Cells. 2011; 29: 1874–1885. [DOI] [PubMed] [Google Scholar]
- 16. Li GG, Chen SY, Xie HT, Zhu YT, Tseng SC. Angiogenesis potential of human limbal stromal niche cells. Invest Ophthalmol Vis Sci. 2012; 53: 3357–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li GG, Zhu YT, Xie HT, Chen SY, Tseng SC. Mesenchymal stem cells derived from human limbal niche cells. Invest Ophthalmol Vis Sci. 2012; 53: 5686–5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De CE. Inhibition of HIV infection by bicyclams, highly potent and specific CXCR4 antagonists. Mol Pharmacol. 2000; 57: 833–839. [PubMed] [Google Scholar]
- 19. Neely MD, Litt MJ, Tidball AM, et al.. DMH1, a highly selective small molecule BMP inhibitor promotes neurogenesis of hiPSCs: comparison of PAX6 and SOX1 expression during neural induction. ACS Chem Neurosci. 2012; 3: 482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sippel KC, Ma JJ, Foster CS. Amniotic membrane surgery. Curr Opin Ophthalmol. 2001; 12: 269–281. [DOI] [PubMed] [Google Scholar]
- 21. Tseng SC, Meller D, Anderson DF, et al.. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane for treating corneal diseases with total limbal stem cell deficiency. Adv Exp Med Biol. 2002; 506: 1323–1334. [DOI] [PubMed] [Google Scholar]
- 22. Bouchard CS, John T. Amniotic membrane transplantation in the management of severe ocular surface disease: indications and outcomes. Ocul Surf. 2004; 2: 201–211. [DOI] [PubMed] [Google Scholar]
- 23. Li D-Q, Lee A, Tseng SCG. Differential expression and regulation of TGF-b1, TGF-2, TGF-3, TGF-bRI, TGF-bRII and TGF-bRIII in human corneal, limbal and conjunctival fibroblasts. Curr Eye Res. 1999; 19: 154–161. [DOI] [PubMed] [Google Scholar]
- 24. Hao J, Varshney RR, Wang DA. TGF-beta3: A promising growth factor in engineered organogenesis. Expert Opinion on Biological Therapy. 2008; 8: 1485–1493. [DOI] [PubMed] [Google Scholar]
- 25. Espana EM, Grueterich M, Touhami A, Tseng SCG. Corneal stromal changes following reconstruction by ex vivo expanded limbal epithelial cells in rabbits with total limbal stem cell deficiency. Br J Ophthalmol. 2003; 87: 1509–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lavery LA, Peters EJ, Armstrong DG, Wendel CS, Murdoch DP, Lipsky BA. Risk factors for developing osteomyelitis in patients with diabetic foot wounds. Diabetes Res Clin Pract. 2009; 83: 347–352. [DOI] [PubMed] [Google Scholar]
- 27. Wang W, Rigueur D, Lyons KM. TGFβ signaling in cartilage development and maintenance. Birth Defects Research C Embryo Today. 2014; 102: 37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Han B, Chen SY, Zhu YT, Tseng SC. Integration of BMP/Wnt signaling to control clonal growth of limbal epithelial progenitor cells by niche cells. Stem Cell Res. 2014; 12: 562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen S-Y, Cheng AMS, Zhang Y, et al.. Pax 6 Controls Neural Crest Potential of Limbal Niche Cells to Support Self-Renewal of Limbal Epithelial Stem Cells. Scientific Reports. 2019; 9: 9763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Na IK, Scheibenbogen C, Adam C, et al.. Nuclear expression of CXCR4 in tumor cells of non-small cell lung cancer is correlated with lymph node metastasis. Hum Pathol. 2008; 39: 1751–1755. [DOI] [PubMed] [Google Scholar]
- 31. Wang L, Wang Z, Yang B, Yang Q, Wang L, Sun Y. CXCR4 nuclear localization follows binding of its ligand SDF-1 and occurs in metastatic but not primary renal cell carcinoma. Oncol Rep. 2009; 22: 1333–1339. [DOI] [PubMed] [Google Scholar]
- 32. Masuda T, Nakashima Y, Ando K, et al.. Nuclear expression of chemokine receptor CXCR4 indicates poorer prognosis in gastric cancer. Anticancer Res. 2014; 34: 6397–6403. [PubMed] [Google Scholar]
- 33. Spano JP, Andre F, Morat L, et al.. Chemokine receptor CXCR4 and early-stage non-small cell lung cancer: pattern of expression and correlation with outcome. Ann Oncol. 2004; 15: 613–617. [DOI] [PubMed] [Google Scholar]
- 34. Yoshitake N, Fukui H, Yamagishi H, et al.. Expression of SDF-1 alpha and nuclear CXCR4 predicts lymph node metastasis in colorectal cancer. Br J Cancer. 2008; 98: 1682–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tseng S. HC-HA/PTX3 purified from amniotic membrane as novel regenerative matrix: insight into relationship between inflammation and regeneration. Invest Ophthalmol Vis Sci. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anderson DF, Ellies P, Pires RT, Tseng SC. Amniotic membrane transplantation for partial limbal stem cell deficiency. Br J Ophthalmol. 2001; 85: 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gomes JA, dos Santos MS, Cunha MC, Mascaro VL, Barros JN, de Sousa LB. Amniotic membrane transplantation for partial and total limbal stem cell deficiency secondary to chemical burn. Ophthalmology. 2003; 110: 466–473. [DOI] [PubMed] [Google Scholar]
- 38. Meallet MA, Espana EM, Grueterich M, Ti SE, Goto E, Tseng SC. Amniotic membrane transplantation with conjunctival limbal autograft for total limbal stem cell deficiency. Ophthalmology. 2003; 110: 1585–1592. [DOI] [PubMed] [Google Scholar]
- 39. Tsai RJ, Li L, Chen J. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells(1). Am J Ophthalmol. 2000; 130: 543. [DOI] [PubMed] [Google Scholar]
- 40. Sangwan VS, Sridhar MS, Vemuganti GK. Treatment of complex choristoma by excision and amniotic membrane transplantation. Arch Ophthalmol. 2003; 121: 278–280. [DOI] [PubMed] [Google Scholar]
- 41. Kawashima M, Kawakita T, Satake Y, Higa K, Shimazaki J. Phenotypic study after cultivated limbal epithelial transplantation for limbal stem cell deficiency. Arch Ophthalmol. 2007; 125: 1337–1344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.