Abstract
Advanced central nervous system (CNS) therapies exhibited high efficacy but complete treatment of CNS diseases remains challenging owing to limited delivery of therapeutic agents to the brain. Multifunctional magnetic nanoparticles are investigated not only for site-specific drug delivery but also for theranostic applications aiming for an effective CNS therapy. Recently, surface engineering of magnetic nanoparticles was recognized as a crucial area of research to achieve precise therapy and imaging at molecular and cellular levels. This review reports state-of-the-art advancement in the development of surface-engineered magnetic nanoparticles targeting CNS diagnostics and therapies. The challenges and future prospects of magnetic theranostics are also discussed by considering the translation from bench to bedside. Successful translation of magnetic theranostics to the clinical setting will enable precise and efficient diagnostics and therapy to manage CNS diseases.
Keywords: Surface engineering, multifunctional magnetic nanoparticles, CNS diseases, image-guided therapy, theranostics
Central nervous system diseases and therapeutic challenges
Central nervous system (CNS) diseases are functional disorders and infections in the brain and spinal cord that affect millions of people globally and cause economic burden [1]. Neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease and other neurological disorders such as epilepsy lead to significant declines in quality of life for patients. Although several drugs and therapeutic agents have been investigated to treat or manage CNS diseases, therapeutic effects are limited owing to inadequate delivery of these agents to the brain. The main obstacle is the existence of the blood–brain barrier (BBB), a diffusion barrier between the circulating blood and the brain [2]. The BBB is composed of endothelial cells linked by tight junctions, astrocyte end-feet and pericytes. The tight junctions between the endothelial cells selectively limit systemically circulating molecules, including therapeutic agents, from entering the brain [3]. Therefore, a breakthrough in delivery strategy to the brain as well as development of novel therapeutic cargos with higher efficacy and minimum side-effects are urgently required for successful treatment of CNS diseases.
To achieve these objectives, nanomedicine, a therapeutic and diagnostic approach using therapeutic agents composed of nanoparticles, has emerged with great potential to improve therapeutic efficacy and disease monitoring in the brain. Among various nanoparticles, magnetic nanoparticles have emerged as multifunctional nanoparticles with a wide range of biomedical applications owing to easy surface functionalization and tunable unique properties under external stimuli such as magnetic fields and electric fields, among others. One of the most attractive applications of magnetic nanoparticles is molecular imaging. Imaging agents have been used since the discovery of X-rays by Wilhelm Roentgen, who used the technique to image his wife’s hand and distinguish between bones of different densities [4]. Over time, various contrast agents were used for X-rays, such as colloidal silver, thorium nitrate and iodinated products, each of which had its own advantages and disadvantages [5]. Medical imaging can be classified into anatomic and functional imaging, each providing information that is crucial for diagnosis and treatment. To enhance the quality of the image created, exogenous imaging agents are used; nanoparticles belong to a unique class owing to their distinct properties. Iron oxide nanoparticles (IONPs) reduce the MRI signal intensities on T2 and T2* weighted images, resulting in magnetic field inhomogeneity [6]. Various preparation techniques, such as aqueous co-precipitation and thermal decomposition, among others, are used to produce IONPs [7]. The strategy employed by various research groups to avoid aggregation and increase colloidal stability is to coat and package IONPs with polymers, dendrimers, emulsions and micelles. The surface charges of these particles and the administration route have significant roles in the biodistribution of IONPs [8]. Depending on whether they are coated with dextran, carboxydextran or polyglucose sorbitol carboxymethylether, IONPs are used for mononuclear phagocyte imaging, lymph node imaging or treatment in iron deficiency anemia. Magnetic particle imaging (MPI), which directly images magnetic nanoparticles, is another emerging imaging modality for molecular imaging application [9]. Moreover, magnetic nanoparticles show excellent properties as nanocarriers for drug and gene delivery [10]. Magnetic guidance with a combination of other passive and active targeting approaches enables efficient delivery of therapeutic agents to disease sites [11]. Hyperthermia, which utilizes heat generation of magnetic nanoparticles for thermal treatment of cancer, is another promising application of magnetic nanoparticles [12]. In recent times, nanoparticles with multifunctional properties have been developed for theranostics, combining monitoring and tracking of disease progression with therapeutic capabilities into a single nanoparticle system [10,13,14]. In this review, we introduce synthesis and surface functionalization of magnetic nanoparticles and their applications; and discuss the recent development of magnetic nanoparticles for CNS disease diagnostics and therapeutics.
Investigation of multimodal magnetic nanoparticles
In principle, there are two strategies for designing and developing functionalized magnetic nanoparticles. First, synthesis of core inorganic magnetic nanocrystals and, second, converting hydrophobic core nanocrystals to be hydrophilic and biocompatible with suitable biomaterials, such as antibodies, oligonucleotides, polymers and lipids. The synthesis of core nanocrystals is crucial for biomedical applications to improve their magnetic properties, sizes, shapes and biocompatibility. Among various core nanocrystals, IONPs and cobalt ferrite (CF) [15] are most widely used for biomedical applications owing to their high magnetization and biocompatibility [16]. Magnetic nanoparticles are synthesized by various wet chemical methods including co-precipitation [17] and thermal decomposition [18]. Co-precipitation is extensively used to synthesize hydrophilic IONPs. IONPs are synthesized by simultaneous precipitation of Fe2+ and Fe3+ aqueous salt solutions under alkaline conditions. The size and shape of the nanoparticles can be controlled by changing the type of salts, the pH of media and the capping agents [19]. Highly monodispersed IONPs can be synthesized by thermal decomposition of iron precursors such as iron oleate and iron pentacarbonyl [20,21]. Compared with the broader size distribution of IONPs synthesized by co-precipitation, thermal decomposition can result in highly crystalized and monodispersed IONPs with hydrophobic surfaces as a result of the surfactant capping. Therefore, phase transfer is required for the IONPs synthesized by thermal decomposition to be used for biomedical applications.
The surface functionalization of IONPs is an essential step to achieve specific targeting for the detection or treatment of CNS diseases using IONPs. Many therapeutic agents and nanoparticles show low penetration to the brain owing to the existence of the BBB. To improve brain-targeting capacity, IONPs are functionalized with a ligand that promotes transcytosis of IONPs such as transferrin, lactoferrin and cell-penetrating peptides (CPPs). Transferrin receptors are expressed on the surface of brain microvascular endothelial cells. Transferrin and lactoferrin bind to the transferrin receptors and cross the BBB through receptor-mediated transcytosis [22]. Similarly, CPPs are a group of peptides that have the capacity to cross cell membrane bilayers mainly by endocytosis [23]. Some CPPs such as transactivator of transcription (TAT), derived from HIV-1, and low molecular weight protamine (LMWP) facilitate brain delivery of nanoparticles [24,25]. Besides, magnetic nanomaterials have been shown to enhance BBB transmigration by magnetic targeting [26–28]. Magnetic targeting is a delivery approach that uses the magnetic properties of nanoparticles and an external magnetic field gradient to control the motion of the nanoparticles and deliver therapeutic agents to the desired site [29,30]. A magnetic force exerted on magnetic nanoparticles is governed by the total magnetic moment of the nanoparticles, the field gradient and the magnetic flux density [31]. This delivery approach has been used for various applications including drug delivery and gene delivery, and has shown efficient therapeutic effects on various diseases [32]. Our group has shown enhanced BBB transmigration of various magnetic nanoparticles using the magnetic targeting approach [33–35]. We have also conducted in vitro and in vivo toxicity studies of these nanoparticles and shown minimum toxicity in CNS cells and small animals. In addition to the brain delivery across the BBB, targeting specificity in CNS diseases can be introduced by functionalizing IONPs with the ligands specific to the surface receptors of the diseases. For the detection and treatment of AD, some molecules that specifically bind to amyloid plaques have been used as targeting ligands such as D-enantiomeric peptide [36], curcumin [37] and β-sheet breaker peptide LPFFD [38]. The targeting approach for brain tumors has been actively studied because of the poor outcome of conventional chemotherapy. The focus of brain-tumor-targeting studies includes ligands specific to glioma cells and angiogenic cells, such as RGD (arginine-glycine-aspartic acid) peptide, interleukin (IL)-13 peptide [39], lactoferrin [21] and an antibody against vascular endothelial growth factor (VEGF) [40].
Biomedical applications of multimodal magnetic nanoparticles
IONPs and CF have great potential as contrast agents for medical imaging systems including MRI and MPI. MRI uses hydrogen protons as the signal source, and images are generated based on the proton relaxation in tissues. Although MRI provides high-resolution anatomical features especially for soft tissues, the sensitivity is relatively poor and contrast agents are required to enhance the contrasts in some cases. IONPs and CF mainly contribute as negative contrast agents (or T2 contrast agents) that shorten the transverse relaxation time of protons nearby [15,41]. MPI is a new imaging modality that uses nonlinear magnetization responses of IONPs under a time-varying magnetic field as the signal source [42]. Contrary to MRI, MPI directly images IONP distribution without background signals from surrounding tissues. MPI has the potential to perform quantitative 3D imaging with high sensitivity and spatial resolution in real time, which provides promise for molecular imaging applications [43]. IONPs show great promise not only in medical imaging but also in a wide range of therapeutic applications (Figure 1). Drug delivery systems using IONPs allow efficient treatment with minimum side effects by delivering drugs specifically to the disease sites and releasing the drugs at the desired time. The targeted delivery is achieved either by passive targeting or active targeting. Passive targeting uses passive accumulation of nanoparticles in tumor or inflammation sites [44]. Active targeting uses the magnetic guidance capacity of IONPs or the specificity of ligands functionalized on the surface of IONPs [45]. Similarly, IONPs have been explored as a gene delivery vector. Owing to the safety concern of conventional viral vectors, IONPs were utilized as nonviral vectors that facilitate cellular internalization of DNA molecules [46]. Moreover, IONPs are known to generate heat under alternating magnetic fields. This local heat generation is beneficial for heat-triggered drug release in drug delivery systems [47] and hyperthermia. Hyperthermia is a thermal treatment for cancer based on the heat sensitivity of cells, treating cancer cells by increasing the local temperature at tumor sites. This minimally invasive approach provides a treatment with fewer side effects compared with traditional cancer treatments, such as surgical operation, chemotherapy and radiation therapy. Owing to the active targeting capacity and externally controlled heat induction, IONPs are promising heat sources for local hyperthermia treatment [48].
Figure 1.

Biomedical applications of magnetic nanoparticles and surface engineering for each application. Imaging applications include MRI and magnetic particle imaging (MPI). Multimodal imaging with a combination of MRI or MPI and other imaging modalities such as X-ray computed tomography (CT), positron emission tomography (PET) and photoacoustic imaging can be achieved by labeling magnetic nanoparticles with contrast agents. Therapeutic applications include drug delivery, gene delivery (magnetofection), hyperthermia and photodynamic therapy. Disease- and cell-specific targeting capacity can be obtained by functionalizing magnetic nanoparticles with specific ligands.
Functionalized magnetic nanoparticles to manage CNS diseases
AD is the most common neurodegenerative disorder identified by cognitive impairment. It is characterized by the accumulation of extracellular amyloid plaques which are composed of aggregates of amyloid-β peptides and neurofibrillary tangles (aggregates of hyperphosphorylated tau proteins) [49]. Various ligands specific to amyloid plaques have been found and utilized to achieve early detection and treatment for AD. Cheng etcil. functionalized IONPs with curcumin, which is known to bind to amyloid-β plaques and iron, and successfully visualized amyloid plaques in transgenic mouse brains using MRI [50]. Anti-ferritin antibody-conjugated IONPs were also developed as contrast agents to detect amyloid plaques. The enhanced expression of ferritin and the corresponding accumulation of anti-ferritin IONPs were observed in the subiculum area of transgenic mouse brains [51]. Moreover, the heat generation property of IONPs under alternating magnetic fields were proved to be beneficial to treat AD. Loynachan et al. developed amyloid^-targeting IONPs by grafting leucine-proline-phenylalanine-phenylalanine-aspartic acid (LPFFD) on IONPs, and demonstrated the disaggregation of amyloid-β aggregates by applying a remotely triggered local heating from IONPs (Figure 2a) [38].
Figure 2.

(a) Transmission electron microscope (TEM) images of amyloid-β aggregates decorated with LPFFD-grafted IONPs before and after 3 h exposure to an alternating magnetic field (AMF). Disaggregation of amyloid-β aggregates was observed after local heating. Reproduced, with permission, from [38]. (b) DNA damage assessment for animals after treatment with (i) 5% glucose, (ii) polymer nanoparticles containing IONPs, siTGF-β and TMZ (LBTA), and (iii) LBTA functionalized with angiopep-2 (ALBTA). (iv) Relative γH2A.X foci number in tumor. (c) In vivo T2*-weighted MRI images of brains in intracranial glioblastoma mice before and after injection of LBTA and ALBTA. Reproduced, with permission, from [63].
Parkinson’s disease is the second-most common neurodegenerative disorder, characterized by a selective loss of dopaminergic neurons of the substantia nigra in the brain [52]. It appears to be linked to the misfolding and aggregation of α-synuclein, a presynaptic neuronal protein [53]. Gene therapy that reduces α-synuclein gene expression has been proposed as a therapeutic approach for Parkinson’s disease. Niu et al. developed nanocarriers consisting of shRNA plasmids and IONPs functionalized with nerve growth factor (NGF). The functionalization with NGF achieved specific cell targeting through NGF receptor-mediated endocytosis. Downregulation of α-synuclein expression was observed after treatment with the nanocarriers in in vitro and in vivo Parkinson’s disease models [54].
Epilepsy is one of the most common chronic neurological diseases. It is caused by an abnormal electrical activity within the brain, which leads to unpredictable seizures [55]. Although antiepileptic drugs are the first choice of treatment for epilepsy, current drugs fail to suppress seizures in some patient populations [56]. In the case of drug-resistant epilepsy, surgical resection of the epileptogenic tissue is another option to ease seizures. To achieve accurate and precise resection of epileptogenic tissues, IONPs have been used for the mapping of epileptogenic regions within the brain. Portnoy etal. demonstrated a selective accumulation of fluorescently labeled IONPs within myeloid cells in the hippocampus of the chronic temporal lobe epilepsy rat model in association with neuroinflammation [57]. Enhanced contrasts of IONP aggregates, which were formed by high electrical and magnetic activities at epileptic regions, have also been suggested as a mapping approach for epilepsy [58]. Additionally, Long et al. demonstrated MRI tracking capacity of IONPs for bone marrow mesenchymal stem cell (BMSC) transplantation, which reduced the number of epileptiform waves in a temporal lobe epilepsy model [59].
Glioblastoma is the most common malignant primary brain tumor with poor prognosis [60]. Various IONPs have been developed for glioblastoma diagnostic and therapeutic applications including MRI, MPI, drug delivery, gene delivery and hyperthermia. Magnetosomes – biomineralized IONPs present in magnetotactic bacteria [61] – have been used as contrast agents for MR-based molecular imaging of glioblastoma. The magnetosomes functionalized with RGD peptides showed higher accumulation at the tumor sites compared with nonfunctionalized magnetosomes after injection into glioma-bearing mice [62]. Highly monodispersed IONPs functionalized with lactoferrin have also been developed as MPI tracers for glioblastoma diagnostics. The lactoferrin conjugation increased cellular uptake of IONPs into C6 glioma cells and enhanced the in vitro MPI signal after the cell-specific internalization [21].
Moreover, the development of IONP-based smart nanoformulations facilitated advanced therapies with synergistic effects from multiple therapeutic approaches. Qiao et al. developed reactive oxygen species (ROS)-responsive polymer nanoparticles containing IONPs, siRNA against tumor growth factor β (TGF-β) and a chemotherapy drug temozolomide (TMZ). The nanoparticles were functionalized with angiopep-2, which promotes BBB transmigration and glioblastoma-cell-targeting through low-density lipoprotein receptor-related protein-1 (LRP-1) overexpressed on the cells [63]. The nanoparticles successfully targeted the glioblastoma cells and downregulated TGF-β expression, which improved the immunosuppressive microenvironment and enhanced the therapeutic effect of TMZ (Figure 2b, c). Yin etal. Applied zinc-doped IONPs bound with a tumor suppressor lethal-7a miRNA for the dual function of miRNA delivery and hyperthermia [64]. The synergistic effect of miRNA and magnetic hyperthermia induced an enhanced apoptosis after the uptake of the zinc-doped IONPs into brain cancer cells.
The multifunctionality of IONPs toward molecular imaging and various therapeutic applications offer potential for emerging approaches such as magnetically controlled drug-release systems, multimodal imaging, image-guided therapy and theranostics. By combining more than two imaging modalities, the multimodal imaging technologies have evolved significantly, and improved the detection sensitivity and precision in disease diagnosis and treatments. Compared with a single imaging modality, this technology can provide far better comprehensive information on accurate disease progressions and real-time tracking of therapeutic agents [15,25,65]. The capability of theranostics to diagnose, monitor biodistribution and quantify therapeutic agents at a target site has a significant benefit in achieving optimal therapeutic effects. Arange of IONPs conjugated with anticancer drugs has been developed for image-guided drug delivery targeting brain tumors [63,66]. Fan et al. developed IONPs and doxorubicin (DOX)-conjugated microbubbles which enhanced local opening of the BBB by applying a focused ultrasound. The local BBB penetration enhanced drug delivery efficiency to the tumor site, and the distribution of DOX was visualized within the brain in a rat glioma model using MRI [67]. Sun et al. formulated gold and IONP-loaded micelles as a glioblastoma theranostic agent. The tumor borders were detected in a glioblastoma mouse model using MRI and X-ray computed tomography (CT), and gold nanoparticles showed a radiosensitizing effect in a glioblastoma cell line [68].
Our group has developed a novel on-demand drug release system using magneto-electric nanoparticles (MENPs) for the treatment of neuroAIDS. MENPs are composed of a CF core and barium titanate shell; and possess ferrimagnetic and ferroelectric properties in a single nanostructure. The magnetic property of MENPs allows magnetically guided delivery to the brain, and the magneto-electric effect enables manipulation of polarization in the ferroelectric shell by applying an external magnetic field [69]. This polarization change triggers the release of therapeutic agents from the nanoparticle surface by breaking the electrostatic interaction between MENPs and the therapeutic agents. We have demonstrated brain delivery of MENPs in mice and evaluated the biodistribution of MENPs and neuronal behavior of the mice [33]. On-demand release of an anti-HIV drug: 3′-azi do-3′-deoxythymidine-5′-triphosphate (AZTTP), from MENPs was demonstrated by applying an external low-frequency magnetic field [70]. Moreover, we have developed another type of core-shell material: magneto-plasmonic nanoparticles, for an image-guided drug delivery system. Magneto-plasmonic nanoparticles consisting of an IONP core and a gold shell demonstrated excellent multimodal imaging properties including MRI, MPI, X-ray CT and photoacoustic imaging owing to their magnetic and plasmonic features and great X-ray attenuation [26,34]. Magnetic targeting using magneto plasmonic nanoparticles enhanced the BBB transmigration when guided with an external magnetic field [26,34]. We have demonstrated a reduction of p24 (HIV capsid protein) level by treating HIV-infected microglia cells with a liposomal formulation containing magneto-plasmonic nanoparticles and an anti-HIV drug: tenofovir disoproxil fumarate [34].
Multimodal magnetic nanoparticles for measuring drug biodistribution
In the past ten years, the concept of personalized and precision medicine based on multimodal imaging technology has created huge interest in the biomedical sector [71]. Recently, the multimodal imaging technology has been widely accepted in clinical diagnosis to overcome restrictions of single-imaging modalities [72,73]. MRI is well known for soft tissue T1 or T2 contrasts as well as its very high spatial resolution and low sensitivity. Single-photon emission computed tomography (SPECT) is a nuclear medicine imaging technique based on the emission of gamma rays by radioisotopes. Although SPECT is highly sensitive, it has low spatial resolution and lacks anatomical data [15]. SPECT can typically work with CT and MRI to obtain anatomical or structural information. The multimodal theranostic nanoparticles with MRI and SPECT imaging capabilities can generate complementary information of drug biodistribution and disease progression with high resolution and high sensitivity. The real-time tracking of drug biodistribution using MRI and SPECT will allow successful design of personalized medicine. Therefore, MRI and SPECT theranostic nanoparticles such as radiolabeling of IONPs and fluorescent-tagged CF have been fascinating in biomedical research [15,25].
The application of theranostic-nanoparticle-loaded macrophages in targeted and image-guided drug delivery and antiretroviral therapy at residual latent virus sites has been reported [15,25]. As the prevention, treatment and cure of HIV/AIDS gain momentum, the need for effective platforms for the screening of drug biodistribution has also increased. The tracking of nanoparticle therapies at the cellular and tissue levels is of the utmost importance as we move forward with the development in HIV therapy. Recently, Kevadiya et al. designed multimodal nanoparticles that were utilized for fluorescence imaging, MRI and antiretroviral therapy. These nanoparticles were composed of a lipid-encapsulated polycaprolactone (PCL) core that incorporated europium-doped CF (EuCF) particles as well as the HIV integrase inhibitor dolutegravir (DTG). The synthesized EuCF-DTG nanoparticles were then coated with a lipid shell containing folic acid (FA) to serve as a ligand for the targeting of FA receptors on macrophages. FA-decorated EuCF-DTG nanoparticles demonstrated high uptake and antiretroviral activity in macrophages. Furthermore, these particles were tested in rats and rhesus macaques infected with simian immunodeficiency virus (SIV) to assess the real-time drug biodistribution by MRI T2 imaging, iron and cobalt level quantification, and drug analysis (Figure 3a,b). The results demonstrated that MRI quantification of iron values were closely coordinated with the drug levels in tissue biodistribution sites. These results were also validated with co-localization testing of nanoparticles within macrophages in liver and spleen tissues using confocal and electron microscopies. However, EuCF-DTG demonstrated only bimodality imaging by MRI and fluorescent images. Despite its high resolution, the sensitivity is limited to seeing early time-point changes of nanoparticle biodistribution trends in vital organs such as lymph nodes and the spleen. Recently, Ottemann et al. further modified EuCF nanoparticles to a more advanced imaging modality with SPECT/CT, MRI and fluorescence capabilities. They used 111indium (111In) radiolabeled multimodal EuCF particles encapsulated in PCL and a lipid core shell structure coated with the antiretroviral drug rilpivirine (111InEuCF-PCL/RPV particles) to impart therapeutic capabilities. The SPECT/CT images showed that the particles were taken up by the lymph nodes, liver and spleen. Autoradiographic images of the liver and spleen tissues post injection of the theranostic nanoparticles showed nanoparticle distribution in the liver and spleen. In the spleen, the particles were found in heterogeneous distributions related to macrophage-rich red pulp areas and the marginal zone where latent HIV virus persists. These particles were injected into small animals to confirm that the multimodal component of nanoparticles correlated with drug biodistribution by function of time (Figure 3c,d) [15]. The sensitivity, stability and specificity of these particles to SPECT/CT and MRI make them a robust platform for further modification and long-term pharmacokinetic and pharmacodynamic studies. These particles not only allow real-time visualization of drug particle distribution but also enable quantification of drug particle accumulation in various tissues.
Figure 3.

Drug biodistribution with multimodal magnetic nanoparticles. (a) Schematic diagram of macrophage-based biodistribution of EuCF-DTG nanoparticles in rhesus macaques. (b) Representative T2-weighted images of a single macaque after EuCF-DTG nanoparticle administration. (c) Top: representative pre-scan and post-scan T2-weighted images of mice 2 and 5 days after intravenous injection administration of EuCF-RPV nanoparticles. Bottom: 2D image representations of 3D SPECT/CT scan of mice treated intravenously with 111InEuCF-PCL nanoparticles. (d) Autoradiographic images of liver and spleen sections from mice sacrificed 2 and 5 days post injection with 111InEuCF-PCL nanoparticles. (a,b) Reproduced, with permission, from [25]. (c,d) Reproduced, with permission, from [15].
In addition to MRI, SPECT/CT provides imaging of tissues such as the lymph nodes that cannot be imaged by MRI. The fact that a very high correlation was seen between metal and drug levels in tissues of the spleen and lymph nodes within the first week of drug particle biodistribution shows that this platform can be used effectively to study the biodistribution of long-acting formulations. To conclude, the highly stable 111InEuCF particles display versatility, high sensitivity and, more importantly, high efficacy in monitoring real-time drug biodistribution using SPECT/CT and MRI. They can be translated for applications in various disease conditions owing to a wide range of possible modifications that can be applied to the nanoparticles. Furthermore, multimodal theranostic technology can be a quick noninvasive assessment tool for drug biodistribution in deep tissues and designing of treatments. This technology will benefit the evolution of personalized nanomedicine. Finally, theranostic nanoparticles can help in accurately and effectively delivering therapeutic agents to the target sites as well as designing doses for personalized nanomedicine with multimodal imaging techniques.
Concluding remarks: translation challenges and vision
Bionanotechnology holds considerable promise in diagnostic and therapeutic applications. To manage CNS diseases, multifunctional surface-engineered magnetic nanoparticles possess favorable properties for nanomedicine applications in comparison with other nanomaterials. The unique magnetic properties of magnetic nanoparticles show great potential in CNS disease diagnostics and treatment using magnetically guided drug delivery across the BBB and hyperthermia as well as image-guided therapy and theranostics. Clinical studies using IONPs, which have been performed for diagnosis and treatment of CNS diseases, are summarized in Table 1. Although some IONPs have been clinically approved as MRI contrast agents, the majority of them have been discontinued with a few exceptions such as ferumoxytol [74,75]. To bring functionalized IONPs to the clinical setting and achieve early diagnosis and effective treatment of CNS diseases, it is crucial to bridge the gap between basic research and clinical practice.
Table 1.
Clinical studies for CNS disorders using IONPs reported over the past decade
| Types of nanoparticles | Target disease | Application | Refs and/or ClinicalTrials.gov identifier |
|---|---|---|---|
| Ferumoxytola | Epilepsy | Neuroinflammation imaging in epilepsy using MRI | NCT02084303 |
| Ferumoxytol | Brain glioblastoma | Imaging in patients with glioblastoma multiforme using MRI | NCT00660543 [73] |
| Ferumoxytol | Brain neoplasm | Imaging of primary or metastatic brain tumors using MRI | NCT00659126 [73] |
| Ferumoxytol | Brain glioblastoma | Steady-state blood volume maps in glioblastoma using MRI | NCT02359097 [74] |
| NanoTherm®b | Brain glioblastoma | Intratumoral thermotherapy in patients with recurrent glioblastoma | [75] |
| Ferumoxytol | CNS lymphoma, Malignant glioma, Metastatic malignant neoplasm in the brain | Imaging of high-grade brain tumors and/or cerebral metastases using MRI | NCT00103038 |
| Ferumoxytol Ferumoxtran-10c | CNS inflammatory diseases CNS lymphoma | Imaging of CNS inflammatory diseases and primary CNS lymphoma using MRI | [76] |
| Ferumoxytol | Multiple sclerosis | Inflammation imaging in multiple sclerosis using MRI | NCT02511028 |
| Ferumoxtran-10 | Multiple sclerosis | Imaging of multiple sclerosis lesions using MRI | [77] |
| Ferucarbotran | Multiple sclerosis | Tracking of macrophage infiltration in patients with multiple sclerosis using MRI | [78] |
| Ferumoxided (Feridex®) | Multiple sclerosis, Amyotrophic lateral sclerosis | Transplantation of iron oxide labeled mesenchymal stem cells | [79] |
| Ferumoxytol | Migraine headache | Vascular inflammation imaging in unilateral migraine using MRI | NCT02549898 |
Ferumoxytol (Feraheme®): superparamagnetic iron oxide coated with polyglucose sorbitol carboxymethylether
NanoTherm®: aminosilane-coated superparamagnetic iron oxide nanoparticles.
Ferumoxtran-10: dextran-coated ultrasmall superparamagnetic iron oxides.
Ferumoxide: dextran-coated superparamagnetic iron oxide nanoparticles.
One of the crucial factors that needs to be addressed is the lack of standardization and regulatory guidelines of nanomaterials. Serious efforts must be made by national agencies to prepare such guidelines to classify methods of characterization to explore size, surface properties, reproducibility and nanotoxicity. The introduction of such authentic guidelines will help the researchers to develop multimodal magnetic nanoparticles for targeted applications and to explore future therapy [76]. Among various factors, the toxicity evaluation is crucial to propose nanomaterials for any in vivo or health-wellness-related applications. Although IONPs have been reported biocompatible, free iron ions resulting from degradation of IONPs can lead to oxidative stress, DNA damage and an inflammatory response [77]. Therefore, examination of its homeostatic condition in in vivo application must be explored. To make IONPs more suitable for biomedical applications, capping agents and ligands conjugated on the core IONPs also need to be considered because this can affect toxicity and pharmacokinetics. Extensive studies on short-term toxicity as well as the long-term fate of IONPs are essential to ensure the safe usage of IONPs in clinical applications. In addition, it is crucial to establish interdisciplinary collaborations between different types of professionals for the translation of nanomedicine to the clinical setting. We summarize the systematic approaches to bringing magnetic nanotheranostics to the clinic in Figure 4. Strong interdisciplinary collaborations and the standardization will be the key to bridging the gap and leading to the successful translation from the lab to the clinical setting.
Figure 4.
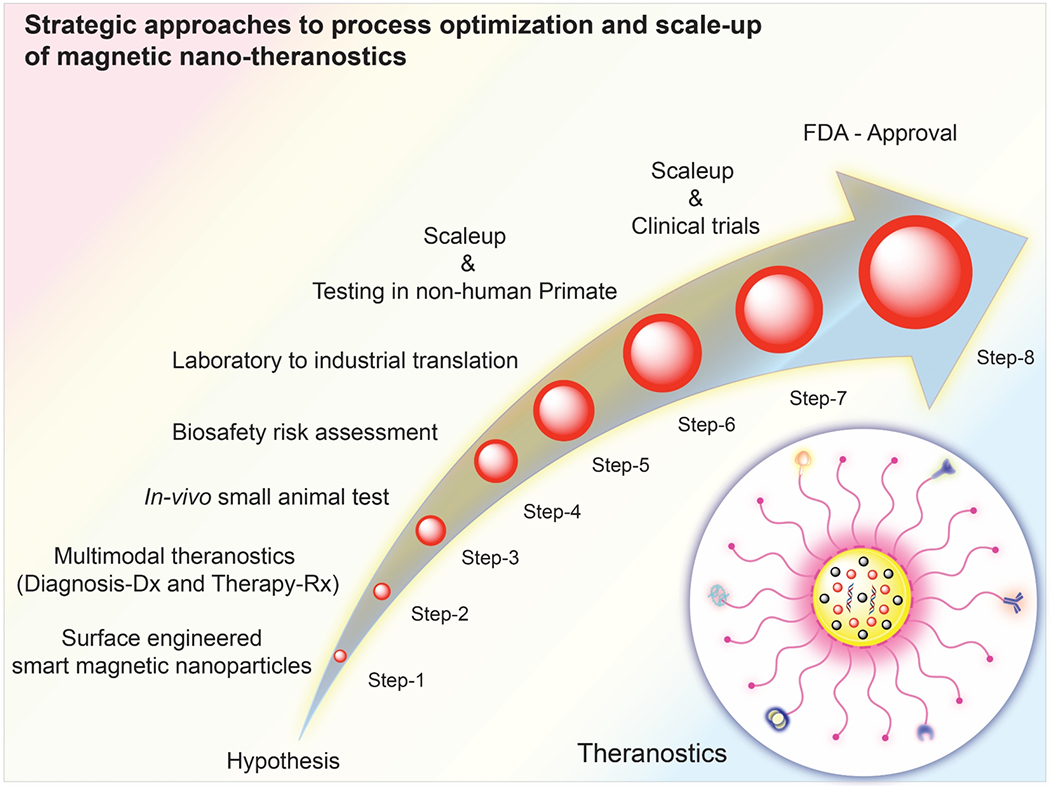
Systematic approaches to achieve multifunctional surface-engineered magnetic nanotheranostics.
In this review, we have discussed the recent advances in the development of surface-engineered multimodal magnetic nanoparticles for effective CNS diagnostics and therapeutics, image-guided drug delivery and theranostics. We have also addressed the challenges for the translation of multimodal magnetic nanoparticles from bench to bedside. Successful development of magnetic theranostics and translation to the clinical setting will allow precise and accurate diagnostics in the early stages of diseases and highly efficient therapeutics to manage CNS diseases.
Highlights:
CNS diseases affect millions of people globally and cause economic burden
Development of an effective delivery system to the brain is urgently required for CNS disease diagnostics and therapeutics
Surface-engineered multifunctional magnetic nanoparticles are promising for image-guided therapy and theranostics
Translation of multimodal magnetic nanoparticles from bench to bedside is crucial to manage CNS diseases
Acknowledgments
This work was supported by National Institute of Health Grants NIH grants (R01DA037838, R01DA040537, R01DA034547 and R01DA042706), the Major State Basic Research Development Program of China (2017YFA0205201, 2014CB744503 and 2013CB733802), the National Natural Science Foundation of China (81422023, 81371596, 81672023, U1705281 and U1505221), the Fundamental Research Funds for the Central Universities (20720160065 and 20720150141) and the Program for New Century Excellent Talents in University, China (NCET-13-0502). The authors also acknowledge the research facilities of Institute of NeuroImmune Pharmacology (INIP) and Advanced Materials Engineering Research Institute (AMERI) of Florida International University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Teaser: This review discusses the advances in multifunctional magnetic nanoparticles for theranostic applications for the management of CNS diseases. Surface-engineered magnetic-nanoparticle-based nanomedicine can be promoted as a future CNS therapy.
Conflicts of interest
The authors declare no conflicts of interest.
References
- [1].GBD 2015 Neurological Disorders Collaborator Group (2017) Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 16, 877–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ballabh P et al. (2004) The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol. Dis 16, 1–13 [DOI] [PubMed] [Google Scholar]
- [3].Pardridge WM (2005) The blood-brain barrier: bottleneck in brain drug development. J. Am. Soc. Exp. Neurother 2, 3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wilhelm Conrad Röntgen GGS and Thomson JJ (1899) Röntgen rays; memoirs. In (Barker GF, ed.), pp. 1835–1910, New York: Harper & brothers [Google Scholar]
- [5].Pollack HM (1999) History of lodinated contrast media. Trends Contrast Media 1999, 3–19 [Google Scholar]
- [6].Wei H et al. (2017) Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc. Natl. Acad. Sci. U. S. A 114, 2325–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tong L et al. (2011) Synthesis and application of superparamagnetic iron oxide nanoparticles in targeted therapy and imaging of cancer. Front. Med 5, 379–387 [DOI] [PubMed] [Google Scholar]
- [8].Thakor AS et al. (2016) Clinically approved nanoparticle imaging agents. J. Nucl. Med 57, 1833–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Arami H et al. (2015) In vivo multimodal magnetic particle imaging (MPI) with tailored magneto/optical contrast agents. Biomaterials 52, 251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang J et al. (2016) Imaging-guided delivery of RNAi for anticancer treatment. Adv. Drug Deliv. Rev 104, 44–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dobson J (2006) Magnetic nanoparticles for drug delivery. Drug Dev. Res 60, 55–60 [Google Scholar]
- [12].Tomitaka A and Takemura Y (2015) Measurement of specific loss power from intracellular magnetic nanoparticles for hyperthermia. J. Pers. NanoMedicine 1, 33–37 [Google Scholar]
- [13].Benezra M et al. (2011) Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Invest 121, 2768–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lin G et al. (2018) Photo-excitable hybrid nanocomposites for image-guided photo/TRAIL synergistic cancer therapy. Biomaterials 176, 60–70 [DOI] [PubMed] [Google Scholar]
- [15].Ottemann BM et al. (2018) Bioimaging predictors of rilpivirine biodistribution and antiretroviral activities. Biomaterials 185, 174–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kevadiya BD et al. (2017) Development of europium doped core-shell silica cobalt ferrite functionalized nanoparticles for magnetic resonance imaging. Acta Biomater. 49, 507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mascolo MC et al. (2013) Nanoparticles in a large pH window with different bases. Materials 6, 5549–5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hufschmid R et al. (2015) Synthesis of phase-pure and monodisperse iron oxide nanoparticles by thermal decomposition. Nanoscale 7, 11142–11154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Laurent S et al. (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev 108, 2064–2110 [DOI] [PubMed] [Google Scholar]
- [20].Hyeon T et al. (2001) Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process. J. Am. Chem. Soc 123, 12798–12801 [DOI] [PubMed] [Google Scholar]
- [21].Tomitaka A et al. (2015) Lactoferrin conjugated iron oxide nanoparticles for targeting brain glioma cells in magnetic. Nanoscale 7, 16890–16898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Paterson J and Webster CI (2016) Exploiting transferrin receptor for delivering drugs across the blood-brain barrier. Drug Discov. Today Technol 20, 49–52 [DOI] [PubMed] [Google Scholar]
- [23].Stalmans S et al. (2015) Cell-penetrating peptides selectively cross the blood-brain barrier in vivo. PLoS One 10, e0139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cheng Y et al. (2014) Blood-brain barrier permeablegold nanoparticles: an efficient delivery platform for enhanced malignant glioma therapy and imaging. Small 10, 5137—5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kevadiya BD et al. (2018) Multimodal theranostic nanoformulations permit magnetic resonance bioimaging of antiretroviral drug particle tissue-cell biodistribution. Theranostics 8, 256–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tomitaka A et al. (2017) Development of magneto-plasmonic nanoparticles for multimodal image-guided therapy to the brain. Nanoscale 9, 764–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nair M et al. (2013) Externally controlled on-demand release of anti-HIV drug using magneto-electric nanoparticles as carriers. Nat. Commun 4, 1707. [DOI] [PubMed] [Google Scholar]
- [28].Dilnawaz F and Sahoo SK (2015) Therapeutic approaches of magnetic nanoparticles for the central nervous system. Drug Discov Today 20, 1256–1264 [DOI] [PubMed] [Google Scholar]
- [29].Shapiro B et al. (2015) Open challenges in magnetic drug targeting. Wiley Interdiscip. Rev Nanomedicine Nanobiotechnology 7, 446–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].D’Agata F et al. (2018) Magnetic nanoparticles in the central nervous system: targeting principles, applications and safety issues. Molecules 23, 1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Asmatulu R et al. (2005) Synthesis, characterization and targeting of biodegradable magnetic nanocomposite particles by external magnetic fields. J. Magn. Magn. Mater 292, 108–119 [Google Scholar]
- [32].Mody VV et al. (2014) Magnetic nanoparticle drug delivery systems for targeting tumor. Appl. Ncmosci 4, 385–392 [Google Scholar]
- [33].Kaushik A et al. (2016) Magnetically guided central nervous system delivery and toxicity evaluation of magneto-electric nanocarriers. Sci. Rep 6, 25309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tomitaka A et al. (2018) Hybrid magneto-plasmonic liposomes for multimodal image-guided and brain-targeted HIV treatment. Nanoscale 10, 184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jayant RD et al. (2015) Sustained-release nanoART formulation for the treatment of neuroAIDS. Int.J. Nanomedicine 10, 1077–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhang C et al. (2014) Dual-functional nanoparticles targeting amyloid plaques in the brains of Alzheimer’s disease mice. Biomaterials 35, 456–465 [DOI] [PubMed] [Google Scholar]
- [37].Cheng KK et al. (2013) Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. AAPS J. 15, 324–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Loynachan CN et al. (2015) Targeted magnetic nanoparticles for remote magnetothermal disruption of amyloid-β aggregates. Adv. Healthc. Mater 4, 2100–2109 [DOI] [PubMed] [Google Scholar]
- [39].Gao H et al. (2014) RGD and interleukin-13 peptide functionalized nanoparticles for enhanced glioblastoma cells and neovasculature dual targeting delivery and elevated tumor penetration. Mol. Pharm 11, 1042–1052 [DOI] [PubMed] [Google Scholar]
- [40].Abakumov MA et al. (2015) VEGF-targeted magnetic nanoparticles for MRI visualization of brain tumor. Nanomedicine Nanotechnology Biol. Med 11, 825–833 [DOI] [PubMed] [Google Scholar]
- [41].Na HB et al. (2009) Inorganic nanoparticles for MRI contrast agents. Adv. Mater 21, 2133–2148 [Google Scholar]
- [42].Pablico-Lansigan MH et al. (2013) Magnetic particle imaging: advancements and perspectives for real-time in vivo monitoring and image-guided therapy. Nanoscale 5, 4040–4055 [DOI] [PubMed] [Google Scholar]
- [43].Arami H et al. (2017) Tomographic magnetic particle imaging of cancer targeted nanoparticles. Nanoscale 9, 18723–18730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Maeda H et al. (2009) Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur.J. Pharm. Biopharm 71, 409–419 [DOI] [PubMed] [Google Scholar]
- [45].Estelrich J et al. (2015) Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci 16, 8070–8101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Al-Dosari MS and Gao X (2009) Nonviral gene delivery: principle, limitations, and recent progress. AAPS J. 11, 671–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hayashi K et al. (2014) Magnetically responsive smart nanoparticles for cancer treatment with a combination of magnetic hyperthermia and remote-control drug release. Theranostics 4, 834–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Périgo EA et al. (2015) Fundamentals and advances in magnetic hyperthermia. Appl. Phys. Rev 2, 041302 [Google Scholar]
- [49].Spires-Jones TL and Hyman BT (2014) The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 82, 756–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cheng KK et al. (2015) Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials 44, 155–172 [DOI] [PubMed] [Google Scholar]
- [51].Fernández T et al. (2018) Functionalization and characterization of magnetic nanoparticles for the detection of ferritin accumulation in Alzheimer’s disease. ACS Chem. Neurosci 9, 912–924 [DOI] [PubMed] [Google Scholar]
- [52].Barzilai A and Melamed E (2003) Molecular mechanisms of selective dopaminergic neuronal death in Parkinson’s disease. Trends Mol. Med 9, 126–132 [DOI] [PubMed] [Google Scholar]
- [53].Breydo L et al. (2012) α-Synuclein misfolding and Parkinson’s disease. Biochim. Biophys. Acta 1822, 261–285 [DOI] [PubMed] [Google Scholar]
- [54].Niu S et al. (2017) Inhibition by multifunctional magnetic nanoparticles loaded with alpha-synuclein RNAi plasmid in a Parkinson’s disease model. Theranostics 7, 344–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bennewitz MF and Saltzman WM (2009) Nanotechnology for delivery of drugs to the brain for epilepsy. Nenrother. J. Am. Soc. Exp. Nenrother 6, 323–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tellez-Zenteno JF et al. (2014) A validation of the new definition of drug-resistant epilepsy by the International League Against Epilepsy. Epilepsia 55, 829–834 [DOI] [PubMed] [Google Scholar]
- [57].Portnoy E et al. (2016) Tracking inflammation in the epileptic rat brain by bi-functional fluorescent and magnetic nanoparticles. Nanomedicine Nanotechnology Biol. Med 12, 1335–1345 [DOI] [PubMed] [Google Scholar]
- [58].Pedram MZ et al. (2015) Toward epileptic brain region detection based on magnetic nanoparticle patterning. Sensors 15, 24409–24427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Long Q et al. (2015) MRI tracking of bone marrow mesenchymal stem cells labeled with ultra-small superparamagnetic iron oxide nanoparticles in a rat model of temporal lobe epilepsy. Neurosci. Lett 606, 30–35 [DOI] [PubMed] [Google Scholar]
- [60].Cloughesy TF et al. (2014) Glioblastoma: from molecular pathology to targeted treatment. Annn. Rev. Pathol. Mech. Dis 9, 1–25 [DOI] [PubMed] [Google Scholar]
- [61].Yan L et al. (2012) Magnetotactic bacteria, magnetosomes and their application. Microbiol. Res 167, 507–519 [DOI] [PubMed] [Google Scholar]
- [62].Boucher M et al. (2017) Genetically tailored magnetosomes used as MRI probe for molecular imaging of brain tumor. Biomaterials 121, 167–178 [DOI] [PubMed] [Google Scholar]
- [63].Qiao C et al. (2018) Traceable nanoparticles with dual targeting and ROS response for RNAi-based immunochemotherapy of intracranial glioblastoma treatment. Adv. Mater doi: 10.1002/adma.201705054 [DOI] [PubMed] [Google Scholar]
- [64].Yin PT. et al. (2014) Combined magnetic nanoparticle-based microRNA and hyperthermia therapy to enhance apoptosis in brain cancer cells. Small 10, 4106–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lee D et al. (2012) Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev 41, 2656–2672 [DOI] [PubMed] [Google Scholar]
- [66].Richard S et al. (2016) Anti oxidative theranostic iron oxide nanoparticles toward brain tumors imaging and ROS production. ACS Chem. Biol 11, 2812–2819 [DOI] [PubMed] [Google Scholar]
- [67].Fan C et al. (2016) Ultrasound/magnetic targeting with SPIO-DOX-microbubble complex for image-guided drug delivery in brain tumors. Theranostics 6, 1542–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sun L et al. (2016) Theranostic application of mixed gold and superparamagnetic iron oxide nanoparticle micelles in glioblastoma multiforme. J. Biomed. Nanotechnol 12, 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bird T et al. (2012) The magnetoelectric effect in transition metal oxides: insights and the rational design of new materials from first principles. Carr. Opin. Solid State Mater. Sci 16, 227–242 [Google Scholar]
- [70].Nair M et al. (2013) Externally controlled on-demand release of anti-HIV drug using magneto-electric nanoparticles as carriers. Nat. Commun 4, 1707. [DOI] [PubMed] [Google Scholar]
- [71].Dominietto MD and Capobianco E (2016) Expected impacts of connected multimodal imaging in precision oncology. Front. Pharmacol 7, 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Marques JS and Pinto FJ (2015) Clinical use of multimodality imaging in the assessment of dilated cardiomyopathy. Heart 101, 565–572 [DOI] [PubMed] [Google Scholar]
- [73].Yankeelov TE et al. (2016) Quantitative multimodality imaging in cancer research and therapy. Nat. Rev. Clin. Oncol 11, 670–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Anselmo AC and Mitragotri S (2015) A review of clinical translation of inorganic nanoparticles. AAPS J. 17, 1041–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Anselmo AC and Mitragotri S (2016) Nanoparticles in the clinic. Bioeng. Tran si. Med doi: 10.1002/btm2.10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mulvaney P et al. (2016) Standardizing nanomaterials. ACS Nano 10, 9763–9764 [DOI] [PubMed] [Google Scholar]
- [77].Singh N et al. (2010) Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 1, 5358. [DOI] [PMC free article] [PubMed] [Google Scholar]


