Abstract
Purpose
To longitudinally evaluate vision-related quality of life (VRQoL) in geographic atrophy (GA) secondary to age-related macular degeneration (AMD) and define its relation to visual function and structural biomarkers.
Methods
Patients with GA secondary to AMD were recruited in the context of the prospective, non-interventional, natural-history Directional Spread in Geographic-Atrophy study (NCT02051998). Fundus autofluorescence and infrared reflectance images were semi-automatically annotated for GA. Linear mixed-effects models were applied to investigate the association of putative determinants with the National Eye Institute Visual Function Questionnaire 25 (NEI VFQ-25) VRQoL.
Results
A total of 87 patients with a mean age ± SD of 77.07 ± 7.49 years were included in the analysis. At baseline, median (IQR) best-corrected visual acuity (BCVA) was 0.3 (0.51) for the better eye and 0.89 (0.76) for the worse eye; 46% of the patients showed binocular and 25.3% monocular non-central GA. The VRQoL composite score was impaired: 69.96 (24.03). Sixty-six patients with a median of 2 (2) follow-up visits after 1.08 (0.78) years were examined longitudinally.
In the multivariable cross-sectional analysis, predictors of the VRQoL composite score were BCVA, GA size, and low-luminance visual acuity (LLVA) for the better eye and BCVA, foveal sparing status, and LLVA for the worse eye (cross-validated R2 = 0.32).
In the longitudinal analysis, a similar prediction accuracy for VRQoL was determined (cross-validated R2 = 0.28). Prediction accuracy for VRQoL did not improve when follow-up time was added as an independent variable.
Conclusions
Vision-related quality of life is significantly impaired in patients with GA secondary to AMD. The cross-sectional and longitudinal association of VRQoL with visual functional and structural biomarkers supports the validity of the NEI VFQ-25 VRQoL.
Keywords: geographic atrophy, quality of life, vision-related quality of life, patient reported outcomes, age-related macular degeneration
Age-related macular degeneration (AMD) is one of the most common causes of central vision loss worldwide, especially in industrial countries.1–3 Around 28 million people are affected by AMD, and a drastic increase to 288 million by 2040 is expected.4 In contrast to neovascular AMD, there is no therapy currently available for patients with geographic atrophy (GA). Its high prevalence, the expected increase due to demographic trends, and the lack of treatment options make the dry late form of AMD an important study subject.2 Therapeutic trials are currently emerging.
The non-exudative late-stage manifestation GA is hallmarked by atrophy of the retinal pigment epithelium and concurrent atrophy of the outer neuroretina.5–8 Typically, foci of GA manifest initially in the parafovea and may spare the fovea, a phenomenon known as foveal sparing.5,9–11 Over time, these foci tend to slowly expand in size and coalesce with eventual involvement of the fovea.5,11 Foveal sparing may persist for a considerable time due to the significantly faster centrifugal compared with centripetal GA spread.11 Although the best-corrected visual acuity (BCVA) may be preserved in patients with foveal sparing,12–14 patients typically experience visual impairments such as reading difficulties, as has been recently demonstrated quantitatively.15–17 This complex monocular relationship between structure and function, as well as the binocular nature of the disease, suggests that monocular enlargement of atrophy as detected by fundus autofluorescence (FAF), an accepted endpoint by the U.S. Food and Drug Administration (FDA),18 may not necessarily correlate strongly with patient-reported outcomes.
Both the FDA and the European Medicines Agency (EMA) strongly advocate the use of patient-reported outcomes as endpoints in clinical trials.19–21 The 25-item National Eye Institute Visual Function Questionnaire (NEI VFQ-25)22 may constitute a feasible patient-reported outcome measure (PROM) to quantify vision-related function in patients with GA secondary to AMD.23 Of note, strong internal consistency, reproducibility, and convergent validity with binocular reading speed were recently demonstrated for the NEI VFQ-25 by Sivaprasad et al.23 However, in ongoing and former clinical trials aiming to slow the progression of GA secondary to AMD with intravitreal injection of therapeutic agents, treatment is typically confined to the worse eye (i.e., monocular). Precise understanding of the monocular contribution of visual function and structural biomarkers to the overall (binocular) vision-related quality of life (VRQoL) in patients is a prerequisite for designing clinical trials testing for efficacy in terms of PROMs.24
Accordingly, the purpose of this study was to prospectively evaluate the VRQoL as assessed by the NEI VFQ-25 in patients with GA secondary to AMD, to assess it longitudinally, and to define its relation to monocular visual function and structural biomarkers in patients with binocular GA. We hypothesized that VRQoL is primarily dependent on visual function and structural biomarkers of the better eye, both in a cross-sectional and longitudinal setting. Further, we hypothesized that the association of VRQoL is overall stronger with visual function biomarkers as compared to with structural biomarkers. Last, we hypothesized that some degree of decline in VRQoL over time may occur beyond the association with the herein evaluated structural and functional biomarkers.
Methods
Patients
Patients were recruited in the context of the non-interventional, prospective, natural history Directional Spread in Geographic Atrophy study (DSGA, NCT02051998) at the Department of Ophthalmology at the University Hospital in Bonn, Germany.11,25 This study adhered to the tenets of the Declaration of Helsinki and was approved by the University of Bonn institutional review board (approval ID 197/12). The patients included in this study had GA in both eyes and were 55 years of age or older at the baseline visit. We excluded patients with the presence of choroidal neovascular membrane or any other ocular disease that could confound assessment of the retina in the study eye, as well as patients with any systemic disease with a limited survival prognosis or any other condition that would make adherence to the examination schedule of once every 6 months for up to 24 months difficult.
Clinical Assessment
Age, gender, medical history, best-corrected visual acuity (BCVA), low-luminance visual acuity (LLVA), reading acuity, foveal involvement, and the NEI VFQ-25 visual and socioemotional function scales were collected for this study. BCVA, LLVA (2.0 log neutral density filter), and reading acuity were assessed using the Early Treatment Diabetic Retinopathy Study (ETDRS) and Radner Reading Charts.26,27 The NEI VFQ-25 includes visual and socioemotional function scales. The base questionnaire consists of 25 items comprising a composite score and 12 subscales addressing general health and different aspects of vision-related functioning. NEI VFQ-25 scores range from 0 to 100, with a higher score representing better visual function.22,23 Changes of four to six points have been judged to represent a clinically meaningful change, corresponding to a ≥15-letter change in BCVA in patients with neovascular AMD.28
Imaging
Following pupil dilatation with 0.5% tropicamide and 2.5% phenylephrine, all patients underwent 30° × 30° fundus autofluorescence imaging (λ excitation, 488 nm; λ emission, 500–700 nm), 30° × 30° infrared reflectance (λ, 815 nm) imaging, and 30° × 25° spectral-domain optical coherence tomography imaging (121 B-scans, ART 25) using a Spectralis HRA-OCT 2 (Heidelberg Engineering, Heidelberg, Germany).
Image Grading
Each visit, all eyes were graded for the presence of GA. The size of GA was semi-automatically annotated using the RegionFinder software for FAF and infrared reflectance (Heidelberg Engineering), as previously described.29,30 The grading task was performed by two readers and subsequent arbitration by a third reader if the GA size deviated by more than 0.3 mm2 between the first and second reader.
Statistical Analyses
Statistical analyses were performed using the software environment R and the add-on packages lme4, glmnet, stepwise, and glmmLasso. BCVA and LLVA were assessed using the ETDRS charts, and confirmed to the base-10 logarithm of minimal angle of resolution (logMAR) scale.27 GA size was square root transformed. The better eye and worse eye were defined by the respective values in each individual determinant. Variables were assessed for normality using the Shapiro–Wilk test. For normally distributed variables, the mean and standard deviation are presented. For non-normally distributed variables, the median and interquartile range (IQR) are presented.
Univariable linear regression was applied to analyze the associations between the individual putative determinants and the dependent variable VRQoL.
Multicollinearity (two or more explanatory variables with high bivariate correlation) was evident (Supplementary Fig. S1), which can lead to instability in model coefficients and variable selection when using conventional multivariable least-squares regression.
To address this issue, we applied least absolute shrinkage and selection operator (LASSO) regression with the VRQoL as the dependent variable for the cross-sectional multivariable analysis at baseline. LASSO regression is designed to handle multicollinearity and carries out variable selection by performing regularization and shrinking coefficient estimates toward zero. This usually enhances the prediction accuracy and interpretability by providing a parsimonious model (i.e., model with few predictors).31 It is specifically designed for predictive modeling (the aim of our analysis), in contrast to conventional (unregularized) least-squares regression for explanatory modeling and statistical inference.
Nested cross-validation with patient-wise splits was applied to estimate the prediction accuracy of the model (outer leave-one-out cross-validation [LOOCV]) and to optimize the tuning parameter λ of the LASSO regression (nested inner LOOCV). For comparison, we present the results of a conventional (least-squares) cross-sectional multivariable regression analysis in Supplementary Table S1 and Supplementary Figure S2. Variables were selected through stepwise forward section based on Akaike information criterion.
For the longitudinal multivariable analysis, we applied LASSO regression (with nested cross-validation) for linear mixed-effects models with the VRQoL as the dependent variable in consideration of the multicollinear and longitudinal data.32 Patients were considered as random effect. For comparison, we present the results of a conventional linear mixed-effects model analysis in Supplementary Table S2.
Results
Baseline Data
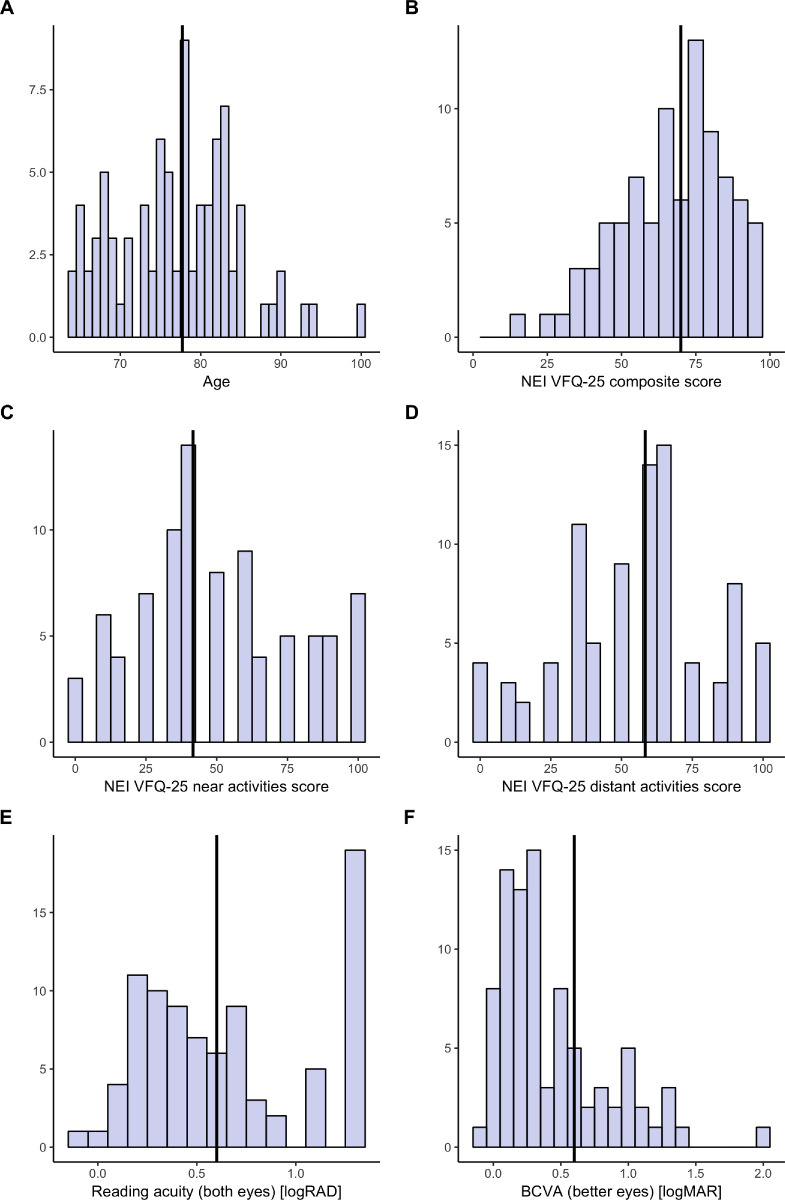
A total of 87 patients with a mean age ± SD of 77.07 ± 7.49 years at baseline (Fig. 1A; Table 1) were included in this study. The median (IQR) values for BCVA (Fig. 1F) and LLVA were 0.30 (0.51) logMAR and 0.79 (0.60) logMAR in the better eye and 0.89 (0.76) logMAR and 1.1 (0.51) logMAR in the worse eye, respectively. The mean square-root-transformed GA size was 2.73 ± 1.31 mm in the better eye and 3.25 ± 1.22 mm in the worse eye at baseline. Both, BCVA (P < 0.05) and GA size (P < 0.05) were correlated between the better and worse eye, with correlation coefficients of 0.70 and 0.93, respectively (Supplementary Fig. S1).
Figure 1.
Data distribution. The histograms show the cross-sectional distribution of age (A), VRQoL composite score (B), near vision subscore (C), distant vision subscore (D), binocular reading acuity (E), and BCVA of the better eye (F). The solid vertical line denotes the median value. Please note, the Radner reading acuity (B) score exhibits a marked floor effect and that, due to the design of the questionnaire, some values in C and D could not be attained.
Table 1.
Cohort Characteristics
| Cross-Sectional Cohort | Baseline Characteristics, Longitudinal Cohort | |
|---|---|---|
| Number of patients | 87 | 66 |
| Gender | 40 male, 47 female | 32 male, 34 female |
| Age (y), mean ± SD | 77.07 ± 7.49 | 77.48 ± 7.43 |
| Square root transformed geographic atrophy area | ||
| Better eye (mm), mean ± SD | 2.73 ± 1.31 | 2.94 ± 1.25 |
| Worse eye (mm), mean ± SD | 3.25 ± 1.22 | 3.40 ± 1.20 |
| Progression (mm/y), median (IQR) | NA | 0.21 (0.19) |
| Vision-related quality of life, median (IQR) | ||
| Composite score | 69.96 (24.03) | 73.96 (23.80) |
| Distant-vision subscore | 58.33 (33.33) | 50.57 (30.47) |
| Near-vision subscore | 41.67 (37.50) | 41.67 (33.33) |
| Best-corrected visual acuity (logMAR), median (IQR) | ||
| Better eye | 0.30 (0.51) | 0.30 (0.51) |
| Worse eye | 0.89 (0.76) | 0.89 (0.82) |
| Reading acuity (logRAD), median (IQR) | ||
| Binocular | 0.60 (0.8) | 0.51 (0.59) |
| Better eye | 0.51 (0.8) | 0.51 (0.62) |
| Worse eye | 1.30 (0.54) | 1.30 (0.50) |
| Low-luminance visual acuity (logMAR), median (IQR) | ||
| Better eye | 0.79 (0.60) | 0.80 (0.73) |
| Worse eye | 1.10 (0.51) | 1.10 (0.58) |
| Follow-up time (y), median (IQR) | NA | 1.08 (0.78) |
| Follow-up visits (n), median (IQR) | NA | 2 (2) |
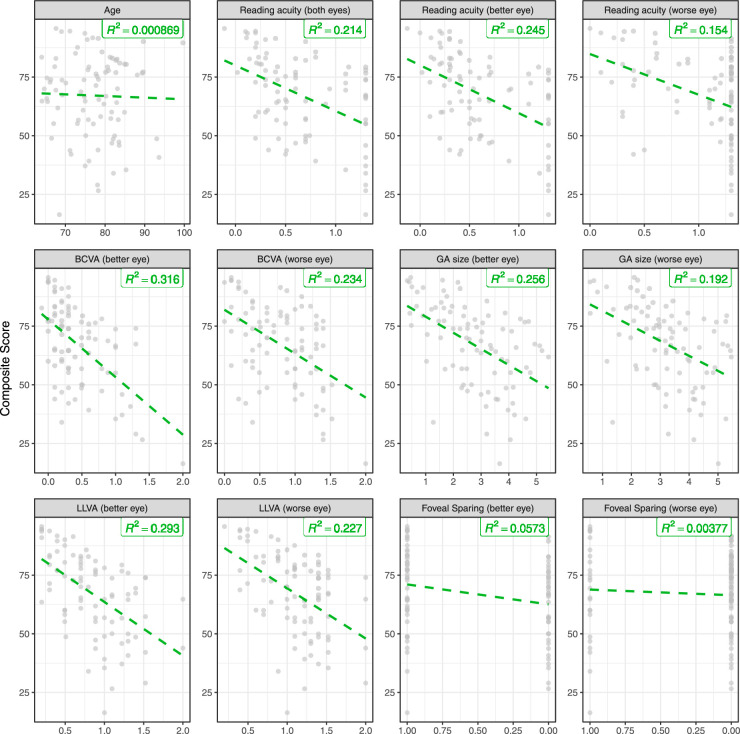
Figure 2.
Cross-sectional determinates of vision-related quality of life. All plots show VRQoL composite scores on the y-axis and the corresponding determinants on the x-axis. The dashed green lines show the univariable linear regression lines. The shown R2 estimates were obtained from models fit to the complete data (cross-validated R2 estimates are provided in Table 2).
The patients had impaired vision-related functioning and quality-of-life scores, as evidenced by a median NEI VFQ-25 composite score of 69.96 (24.03) at baseline (Fig. 1B), with a near vision subscale score of 41.67 (37.50) (Fig. 1C) and with a distant vision subscale score of 58.33 (33.33) (Fig. 1D), representing the worst affected subscores. Patients had a median reading acuity of 0.60 (0.80) logarithm of the reading acuity determination (logRAD) binocular (Fig. 1E), with monocular 0.51 (0.80) logRAD in the better eye and 1.30 (0.54) logRAD in the worse eye, respectively. In this study, 25.3% of the patients had a monocularly and 46% a binocularly non-center-involving GA (foveal sparing or non-central GA). Spearman correlation analysis revealed strong multicollinearity among the features (Supplementary Fig. S1).
Cross-Sectional Univariable Analysis of Determinants of Vision-Related Quality-of-Life at Baseline
In the univariable analysis (Fig. 2; Table 2), binocular and monocular reading acuity, visual acuity, low luminance visual acuity, and GA size for both eyes exhibited a significant association with the VRQoL composite score (all P < 0.0001). The same holds for the near and distant vision subscores (all P < 0.0001). The presence of a preserved central visual field (foveal sparing or non-central GA) in at least one eye was significant for the near vision subscore (P = 0.0002) but not for the composite or distant vision scores.
Table 2.
Determinants of VRQoL Composite Score (Univariable Cross-Sectional Analysis)
| Coefficient | LOOCV R2 | |
|---|---|---|
| Reading acuity (logRAD) | ||
| Binocular | –19.41 | 0.17 |
| Better eye | –20.43 | 0.21 |
| Worse eye | –17.35 | 0.12 |
| Best-corrected visual acuity (logMAR) | ||
| Better eye | –24.55 | 0.28 |
| Worse eye | –18.70 | 0.19 |
| Square root transformed geographic atrophy area (mm) | ||
| Better eye | –6.87 | 0.22 |
| Worse eye | –6.39 | 0.15 |
| Low-luminance visual acuity (logMAR) | ||
| Better eye | –22.90 | 0.26 |
| Worse eye | –21.44 | 0.19 |
| Foveal sparing | ||
| Better eye | 8.49 | 0.01 |
| Worse eye | 2.40 | –0.05 |
The different biomarkers are listed with their coefficient obtained from linear least-squares regression, and coefficient of determination (R2) determined by LOOCV of the univariable cross-sectional analysis.
The distribution of reading acuity (Figs. 1E, 2) revealed a negative skew due to frequent recordings of the worst possible reading acuity of 1.3 logRAD, indicating a marked floor effect of the Radner Reading Charts in the setting of GA.
Cross-Sectional Multivariable Analysis of Determinants of Vision-Related Quality-of-Life at Baseline
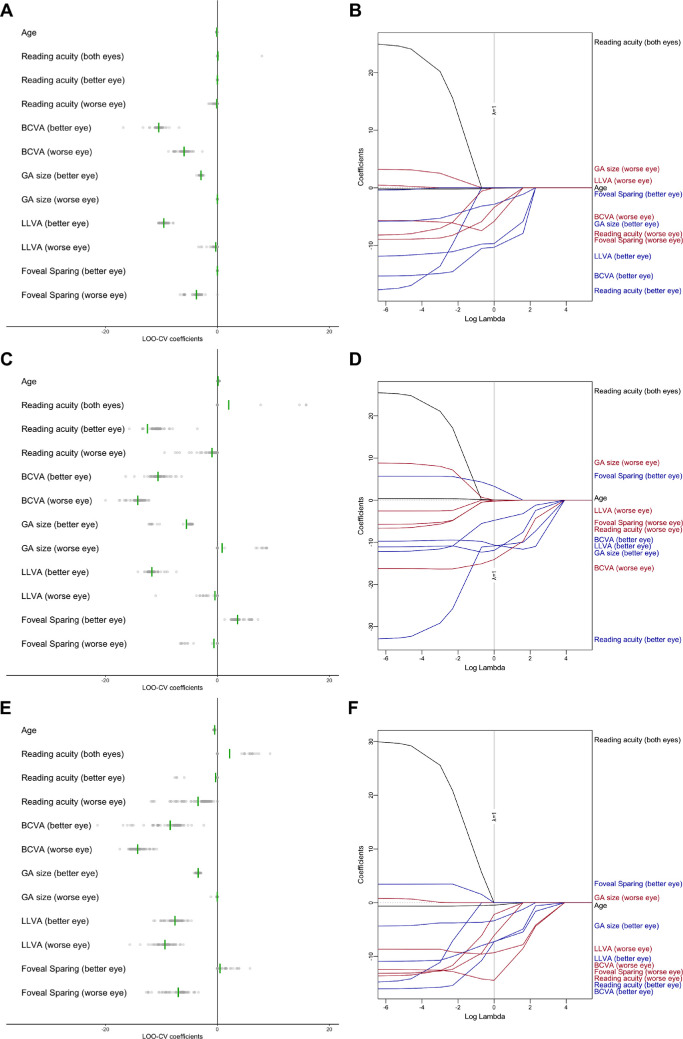
In Figure 3, we present the coefficient estimates for cross-validation (Figs. 3A, 3C, 3E), as well as the magnitude of the coefficient estimates dependent on the regularization parameter (Figs. 3B, 3D, 3F). With regard to the composite score, in Figure 3B, moving from left to right (i.e., increasing values for the regularization parameter), age and foveal sparing of the better eye were the first variables eliminated from the model. Functional characteristics of the worse eye including BCVA, LLVA, and reading acuity were among the next variables to be eliminated. In contrast, LLVA, BCVA, and GA size of the better eye were the last variables to be eliminated. This ranking of the features is also reflected in in the coefficient estimates in the cross-validation provided in Figure 3A and Table 3.
Figure 3.
Cross-sectional multivariable analysis of determinants of VRQoL. Dot plots of regression coefficients derived from the training splits for each of the outer cross-validation folds are shown for the composite score (A), near vision subscore (C), and distant vision subscore (E). Cross-validation within these training splits (inner resampling) was used to determine the optimal tuning parameter λ of the LASSO regression model. Note that the points were plotted semitransparently to avoid overplotting. The green vertical lines indicate the mean coefficient. A zero coefficient estimate effectively implies exclusion of the respective variable from the LASSO regression model. The coefficients dependent on tuning parameter λ for the complete dataset are shown for the composite score (B), near vision subscore (D), and distant vision subscore (F). The dashed gray line indicates the optimal value of tuning parameter λ derived from the inner resampling. Biomarkers of the better eye are highlighted in blue and biomarkers of the worse eye in red.
Table 3.
Cross-Sectional Multivariable Analysis of Determinants of VRQoL
| Coefficient | 95% Confidence Interval | |
|---|---|---|
| Best-corrected visual acuity (better eye) | –10.72 | –15.24 to 91.95 |
| Best-corrected visual acuity (worse eye) | –9.63 | –169.56 to –6.20 |
| Square root transformed geographic atrophy area (better eye) | –3.50 | –7.76 to –1.35 |
| Low-luminance visual acuity (better eye) | –9.71 | –312.18 to –9.37 |
| Low-luminance visual acuity (worse eye) | –0.34 | 2.91 to 443.50 |
| Foveal sparing (worse eye) | –8.13 | –18.96 to –1.32 |
LASSO regression was applied for variable selection and shrinkage of coefficient estimates. The selected biomarkers are listed with their coefficient obtained from the LASSO and the corresponding 95% confidence interval.
The final cross-sectional multivariable model was obtained by fitting a linear model to the complete dataset at baseline using LASSO regression with the optimal regularization parameter determined by cross-validation. With composite score as the dependent variable, the most important predictors were BCVA of the better eye (regularized effect estimate on the composite score, –11 units/logMAR), LLVA of the better eye (–10 units/logMAR), and BCVA of the worse eye (–10 units/logMAR), yielding a cross-validated R2 = 0.32 (Table 3). Similar coefficients were obtained with conventional (least-squares) multivariable analysis (Supplementary Table S1); however, cross-validation revealed more instability in model coefficients and variable selection for the least-squares multivariable analysis (Supplementary Fig. S2).
In Figure 3D, for the near vision subscore, the age as well as the LLVA of the worse eye were the first variables eliminated from the model (when increasing regularization parameter). In contrast, BCVA, LLVA, reading acuity, and GA size of the better eye and the BCVA of the worse eye were the last variables to be eliminated.
With the near vision subscore as the dependent variable, the most important determinants in the resulting cross-sectional multivariable model were BCVA of the worse eye (–15 units/logMAR), LLVA of the better eye (–13 units/logMAR), and reading acuity of the better eye (–11 units/logMAR), resulting in a cross-validated R2 = 0.58.
In Figure 3F, for the distant vision subscore, the reading acuity of the better eye, foveal sparing of the better eye, and binocular reading acuity were the first variables eliminated from the model (when increasing the regularization parameter). In contrast, LLVA and BCVA of the better and worse eyes were the last variables to be eliminated. With the distant vision subscore as the dependent variable, the most important determinants in the resulting cross-sectional multivariable model were BCVA of the worse eye (–17 units/logMAR), foveal sparing of the worse eye (–11 units/eye), and LLVA of the worse eye (–9 units/logMAR), resulting in a cross-validated R2 = 0.27.
Longitudinal Determinants of Vision-Related Quality-of-Life
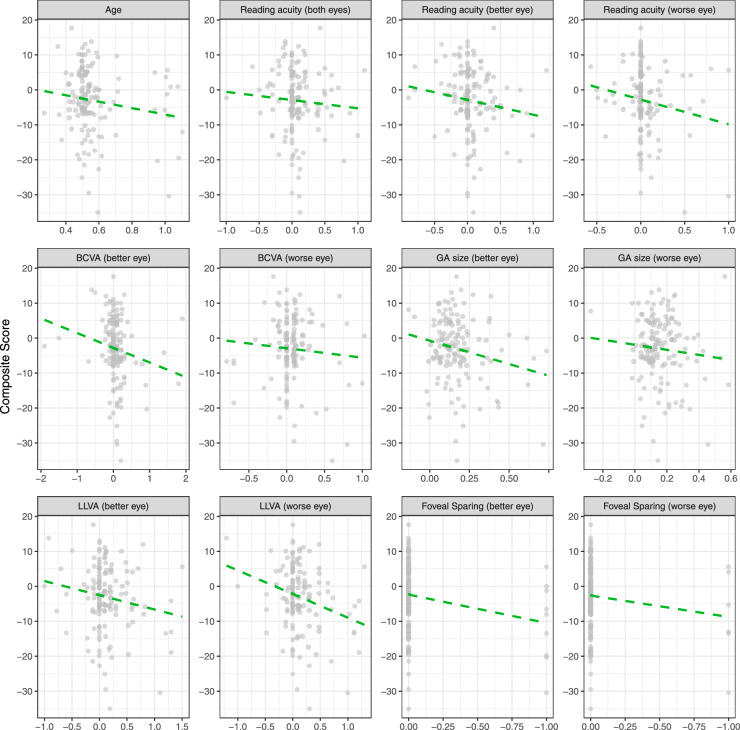
The longitudinal data contained 66 patients with a median (IQR) of 2 (2) follow-up visits spanning a median of 1.08 (0.78) years. The patients in our study had an average square-root GA progression rate (mean change ± SD) of 0.21 ± 0.19 mm/year per eye. There was no significant change in VRQoL composite (–3.07 ± 9.55/year) or near (–3.44 ± 16.13/year) and distant (–3.56 ± 20.97/year) vision subscores over time (Fig. 4). BCVA (better eye, 0.07 ± 0.39; worse eye, 0.07 ± 0.27 logMAR/year), LLVA (better eye, 0.12 ± 0.38; worse eye, 0.12 ± 0.35 logMAR/year), and binocular and monocular reading acuity (binocular, 0.07 ± 0.39; better eye, 0.06 ± 0.29; worse eye, 0.04 ± 0.22 logRAD/year) also did not change significantly.
Figure 4.
Longitudinal change of VRQoL. All plots show changes in VRQoL composite scores on the y-axis and changes in the corresponding determinants on the x-axis. The dashed green lines show the fitted linear regression lines derived from mixed-effects models with consideration of patients as a random factor. None of the features exhibited a significant change over the given follow-up time.
Longitudinal Multivariable Analysis of Determinants of Vision-Related Quality-of-Life
To determine whether longitudinal assessments in VRQoL are paralleled in the above-mentioned structural and functional biomarkers or whether decline in VRQoL may be observed independently of these associations, we repeated the analysis on the longitudinal data, including the follow-up time as a new covariate. The longitudinal multivariable model was obtained by fitting the linear mixed-effects model to the complete dataset using LASSO regression with the optimal regularization parameter determined in the cross-validation.
The multivariable longitudinal analysis revealed that the LLVA of both eyes, the reading acuity of both eyes, and the BCVA, GA size, and foveal sparing status of the better eye constituted important predictors of the VRQoL composite score, explaining 27.8% of the variability in VRQoL (cross-validated R2). Importantly, age and follow-up time were eliminated by the LASSO regression as prognostic features. Again, the variable selection was similar for the LASSO regression and conventional mixed-effects model analysis (Supplementary Table S2).
Similar results were obtained for the near and distant vision subscores. For near vision, the binocular reading acuity and reading acuity of the better eye and the worse eye, the BCVA and GA size of both the better eye and the worse eye, and the LLVA and foveal sparing status of the better eye constituted important predictors of VRQoL, explaining 52.4% of the variability in the near vision subscore (cross-validated R2). Again, age and follow-up time were eliminated by the LASSO regression as prognostic features.
For the distant vision subscore, BCVA and LLVA of both eyes, as well as binocular reading acuity, reading acuity, GA size, and foveal sparing status of the better eye, were significant predictors of VRQoL, explaining 20.2% of the variability in the near vision subscore (cross-validated R2). Again, age and follow-up time were eliminated by the LASSO regression as prognostic features.
Discussion
In this study, we demonstrated that VRQoL and near and distant vision subscores are associated with conventional structural and functional biomarkers in patients with GA secondary to AMD. The LLVA of the better eye and the BCVA of both eyes were the most important determinants of VRQoL. Moreover, the longitudinal analysis further underscored these results. The significant effect of presence of foveal sparing in at least one eye on the near vision subscore but not on the composite score or distant vision subscore suggests that the presence of GA in the fovea does not affect independently domains other than near vision in terms of VRQoL. No evidence for a decline in VRQoL or subscores over time beyond the aforementioned associations was identified. (Fig. 4) These results have important implications for the application of the VRQoL for PROMs in clinical trials for GA secondary to AMD as outlined below.
In view of the call for PROMs in clinical trials by regulatory agencies, including the FDA and EMA,19–21 PROMs such as the NEI VFQ-25 have been previously applied in GA secondary to AMD.23 In addition to the robust psychometric properties of PROMs, their validity in a disease-specific context is a prerequisite to their application. Sivaprasad et al.23 demonstrated strong internal consistency and reproducibility for the NEI VFQ-25 with regard to GA secondary to AMD. Moreover, they validated the binocular MNREAD Acuity Charts and patient-reported Functional Reading Independence Index.23,33 However, to the best of our knowledge, there are no published data regarding the monocular influence on VRQoL, a highly important aspect considering that the study eyes in clinical trials regarding GA are typically the structurally and functionally worse eyes.34
This motivated us to develop our first hypothesis, and this study indeed confirmed that VRQoL was primarily dependent on the better eye (Fig. 5), in both cross-sectional and longitudinal settings. Interestingly, LLVA exhibited the most stable coefficient in the model across a variety of values of the LASSO regularization parameter λ, highlighting its relevance for VRQoL. Although previous studies demonstrated that LLVA is significantly reduced and also prognostic for subsequent BCVA loss in eyes with GA secondary to AMD, its precise cellular correlate is not well understood.9,35 BCVA is primarily limited by the retinal peak cone density, but LLVA may be indicative of decreased cone dark-adaptation and postreceptoral cell dysfunction.36,37 For the near and distant vision subscores, the results were very similar.
Figure 5.
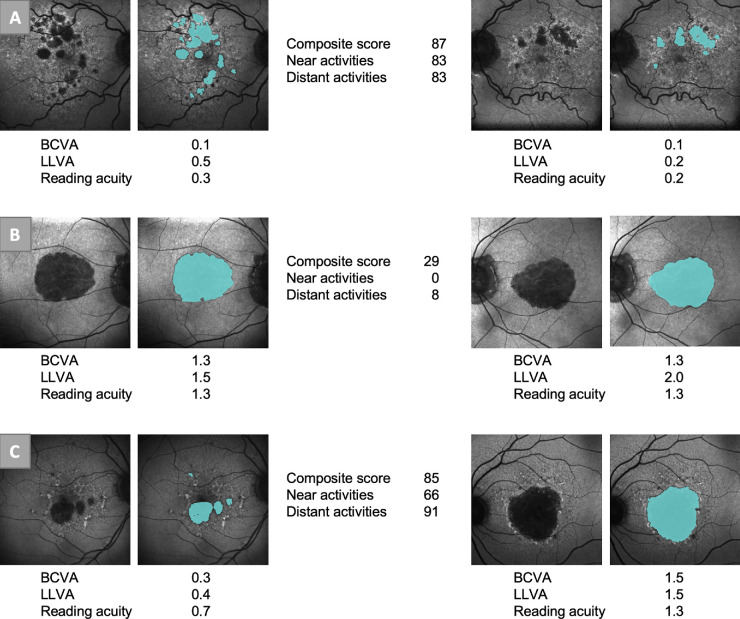
Example patients. The figure shows the FAF and semiautomatically graded GA (blue areas) of three patients. The BCVA and LLVA (logMAR) and the reading acuity (logRAD) are shown. Patient A had high VRQoL scores and good visual function in both eyes. Patient B had low VRQoL scores and poor visual function in both eyes. Patient C had high VRQoL scores but only one eye with good function.
In terms of the second hypothesis, we observed that functional biomarkers were indeed more prognostic for VRQoL and the subscores, as compared with structural biomarkers (GA size and status of foveal sparing). In the univariable analysis, structural variables (i.e., GA area of the better eye) could only explain up to 22% of the variability in VRQoL, highlighting the fact that these variables are rather unsuitable for demonstrating drug efficiency in patient-relevant terms, despite the obvious suitability to measuring biological effectiveness.
Finally, we expected that some degree of decline in VRQoL over time might be observable beyond the above-mentioned associations; however, based on the longitudinal analysis, we found that neither age nor follow-up time was able to improve predictions of VRQoL when added to the multivariable LASSO models. This finding highlights the close association between the evaluated functional and structural biomarkers and VRQoL, as well as the near and distant vision subscores. Further, this suggests that the residual unexplained variability in VRQoL among patients may be representative of rather unspecific factors such as retest variability and general patient attitudes rather than disease-specific factors, which would be correlated to follow-up time.
Multiple important implications can be drawn from these results. First, the results highlight the relevance of LLVA as a functional outcome measure in GA secondary to AMD. Second, for clinical trials designed to prove efficiency in terms of patients' VRQoL rather than safety and biological efficiency, treatment should be allocated to the better eye. This is very much in contrast to previous clinical trials, in which the eyes with the worse BCVA were typically allocated to treatment.34 To address this matter, one could use a model to indirectly infer the expected effect on VRQoL of a given treatment. For this purpose, a model would be trained only on the data of the better eyes from a natural-history study to infer VRQoL. This model could then be applied to infer the expected effect of a given treatment on VRQoL with the assumption that the treated (worse) eye would have been the better eye. Third, in clinical practice, the decision to refer patients to low-vision clinics or counseling via patient-led groups should be based primarily on the function of the better eye.
Strengths of this study include the large number of functional and structural biomarkers that were assessed in a monocular setting to evaluate the validity of the NEI VFQ-25 as a PROM. Moreover, the stringent analysis with nested resampling (inner resampling for model optimization and outer resampling for the evaluation of model performance) provided a rather unbiased estimate of the prognostic model accuracy. Limitations of the study include the limited sample size for the longitudinal cohort and limited follow-up time, as well as inherent test characteristics of some of the functional tests. Longer follow-up times may be advantageous to detect significant changes in VRQoL over time; however, current interventional trials feature similar follow-up times.34 For the Radner reading acuity distribution, an excess of the value of 1.3 logRAD (floor of the test) was observed. Accordingly, a revised version of the test allowing for larger optotypes or a longer maximal time per line may be warranted in the setting of GA. As highlighted by Owsley et al.,38 a target questionnaire such as the Low Luminance Questionnaire may have revealed further facets of loss of visual function in association with AMD.
In conclusion, this study underscores the validity of the NEI VFQ-25 for measuring the impact on patients’ VRQoL of GA secondary to AMD. The markedly higher association of VRQoL with visual function and structural biomarkers of the better eye compared to the worse eye has important implications for the design of future clinical trials. Specifically, treatment should be allocated to the better eye in trials aiming to evaluate efficiency in terms of patient VRQoL.
Supplementary Material
Acknowledgments
The authors thank Joanna Czauderna, Verena Bonn, and Ruth Hassenrik for patient coordination and data acquisition.
Supported by a dissertation grant from the German Ophthalmological Society (SHK), by grants from the German Research Foundation (PF 950/1-1 to MP, 658/4-1 and 658/4-2 to MF, and 2846/1-1 to ML), and by grants from the BONFOR GEROK Program of the Faculty of Medicine, University of Bonn (O-137.0022 and O-137.0025 to MP). CenterVue SpA (Padova, Italy) provided research equipment (Scotopic Macular Integrity Assessment device) for this study. CenterVue had no role in the design or conduct of the experiments. MF was supported in part by an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology & Visual Sciences, University of Utah.
Disclosure: S.H. Künzel, Heidelberg Engineering (F), Optos (F), Carl Zeiss Meditec (F), CenterVue (F), Novartis (F); P.T. Möller, Heidelberg Engineering (F), Optos (F), Carl Zeiss Meditec (F), CenterVue (F), Novartis (F); M. Lindner, Heidelberg Engineering (F), Optos (F), Carl Zeiss Meditec (F), CenterVue (F), Novartis (F); L. Goerdt, Heidelberg Engineering (F), Optos (F), Carl Zeiss Meditec (F), CenterVue (F), Novartis (F); J. Nadal, None; M. Schmid, None; S. Schmitz-Valckenberg, Acucela (F), Alcon/Novartis (C, F, R), Allergan (C, F, R), Bayer (F, R), Bioeq/Formycon (F, C), Carl Zeiss MedicTec (F, R), CenterVue (F), Galimedix (C), Genentech/Roche (F, R), Heidelberg Engineering (F), Katairo (F), Optos (F); F.G. Holz, Acucela (C, F, R), Allergan (F, R), Apellis (C, R), Bayer (C, F, R), Boehringer-Ingelheim (C), Bioeq/Formycon (F, C), CenterVue (F), Ellex (R), Roche/Genentech (C, F, R), Geuder (C), Grayburg Vision (C, R), Heidelberg Engineering (C, F, R), Kanghong (C, F), LinBioscience (C, R), NightStarX (F), Novartis (C, F, R), Optos (F), Pixium Vision (C, F, R), Oxurion (C, R), Stealth BioTherapeutics (C, R), Zeiss (F, R); M. Fleckenstein, Novartis (F, C), Heidelberg Engineering (F), STZ GRADE Reading Center (E), Genentech/Roche (C), Ophthalmo Update GmbH (C), pending patent US20140303013A1; M. Pfau, Heidelberg Engineering (F), Optos (F), Carl Zeiss Meditec (C, F), CenterVue (F), Novartis (F)
References
- 1. Cheung LK, Eaton A. Age-related macular degeneration. Pharmacother J Hum Pharmacol Drug Ther. 2013; 33: 838–855. [DOI] [PubMed] [Google Scholar]
- 2. Holz FG, Schmitz-Valckenberg S, Fleckenstein M. Recent developments in the treatment of age-related macular degeneration. J Clin Invest. 2014; 124: 1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rasmussen A, Sander B. Long-term longitudinal study of patients treated with ranibizumab for neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2014; 25: 158–163. [DOI] [PubMed] [Google Scholar]
- 4. Wong WL, Su X, Li X, et al.. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Heal. 2014; 2: e106–e116. [DOI] [PubMed] [Google Scholar]
- 5. Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye. 1988; 2: 552–577. [DOI] [PubMed] [Google Scholar]
- 6. Fleckenstein M, Issa PC, Helb H-MM, et al.. High-resolution spectral domain-OCT imaging in geographic atrophy associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008; 49: 4137–4144. [DOI] [PubMed] [Google Scholar]
- 7. Zanzottera EC, Ach T, Huisingh C, Messinger JD, Freund KB, Curcio CA. Visualizing retinal pigment epithelium phenotypes in the transition to atrophy in neovascular age-related macular degeneration. Retina. 2016; 36: S26–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sadda SR, Guymer R, Holz FG, et al.. Consensus definition for atrophy associated with age-related macular degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology. 2018; 125: 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sunness JS, Rubin GS, Applegate CA, et al.. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997; 104: 1677–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sunness JS, Margalit E, Srikumaran D, et al.. The long-term natural history of geographic atrophy from age-related macular degeneration. enlargement of atrophy and implications for interventional clinical trials. Ophthalmology. 2007; 114: 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lindner M, Böker A, Mauschitz MM, et al.. Directional kinetics of geographic atrophy progression in age-related macular degeneration with foveal sparing. Ophthalmology. 2015; 122: 1356–1365. [DOI] [PubMed] [Google Scholar]
- 12. Lindner M, Nadal J, Mauschitz MM, et al.. Combined fundus autofluorescence and near infrared reflectance as prognostic biomarkers for visual acuity in foveal-sparing geographic atrophy. Invest Ophthalmol Vis Sci. 2017; 58: BIO61–BIO67. [DOI] [PubMed] [Google Scholar]
- 13. Sayegh RG, Sacu S, Dunavolgyi R, et al.. Geographic atrophy and foveal-sparing changes related to visual acuity in patients with dry age-related macular degeneration over time. Am J Ophthalmol. 2017; 179: 118–128. [DOI] [PubMed] [Google Scholar]
- 14. Schmitz-Valckenberg S, Nadal J, Fimmers R, et al.. Modeling visual acuity in geographic atrophy secondary to age-related macular degeneration. Ophthalmologica. 2016; 235: 215–224. [DOI] [PubMed] [Google Scholar]
- 15. Lindner M, Pfau M, Czauderna J, et al.. Determinants of reading performance in eyes with foveal-sparing geographic atrophy. Ophthalmol Retin. 2019; 3: 201–210. [DOI] [PubMed] [Google Scholar]
- 16. Sunness JS. Reading newsprint but not headlines: pitfalls in measuring visual acuity and color vision in patients with bullseye maculopathy and other macular scotomas. Retin Cases Brief Rep. 2008; 2: 83–84. [DOI] [PubMed] [Google Scholar]
- 17. Sunness JS, Rubin GS, Zuckerbrod A, Applegate CA. Foveal-sparing scotomas in advanced dry age-related macular degeneration. J Vis Impair Blind. 2008; 102: 600–610. [PMC free article] [PubMed] [Google Scholar]
- 18. Csaky KG, Richman EA, Ferris FL. Report from the NEI/FDA ophthalmic clinical trial design and endpoints symposium. Invest Ophthalmol Vis Sci. 2008; 49: 479–489. [DOI] [PubMed] [Google Scholar]
- 19. US Food and Drug Administration. Patient-reported outcome measures: use in medical product development to support labeling claims. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims. Accessed May 8, 2020.
- 20. European Medicines Agency. EU Regulatory Workshop – Ophthalmology – Summary and Report. Available at: https://www.ema.europa.eu/en/documents/report/european-union-regulatory-workshop-ophthalmology-summary-report_en.pdf. Accessed May 8, 2020.
- 21. Csaky K, Ferris F 3rd, Chew EY, Nair P, Cheetham JK, Duncan JL. Report from the NEI/FDA endpoints workshop on age-related macular degeneration and inherited retinal diseases. Invest Ophthalmol Vis Sci. 2018; 58: 3456–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001; 119: 1050–1058. [DOI] [PubMed] [Google Scholar]
- 23. Sivaprasad S, Tschosik E, Kapre A, et al.. Reliability and construct validity of the NEI VFQ-25 in a subset of patients with geographic atrophy from the phase 2 Mahalo study. Am J Ophthalmol. 2018; 190: 1–8. [DOI] [PubMed] [Google Scholar]
- 24. Cheng QE, Gao J, Kim BJ, Ying G. Design characteristics of geographic atrophy treatment trials: systematic review of registered trials in ClinicalTrials.gov. Ophthalmol Retin. 2018; 2: 518–525. [DOI] [PubMed] [Google Scholar]
- 25. Pfau M, Lindner M, Goerdt L, et al.. Prognostic value of shape-descriptive factors for the progression of geographic atrophy secondary to age-related macular degeneration. Retina. 2019; 39: 1527–1540. [DOI] [PubMed] [Google Scholar]
- 26. Radner W. Reading charts in ophthalmology. Graefes Arch Clin Exp Ophthalmol. 2017; 255: 1465–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pelli DG, Bex P. Measuring contrast sensitivity. Vision Res. 2013; 90: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suñer IJ, Kokame GT, Yu E, Ward J, Dolan C, Bressler NM. Responsiveness of NEI VFQ-25 to changes in visual acuity in neovascular AMD: validation studies from two phase 3 clinical trials. Invest Ophthalmol Vis Sci. 2009; 50: 3629–3635. [DOI] [PubMed] [Google Scholar]
- 29. Schmitz-Valckenberg S, Brinkmann CK, Alten F, et al.. Semiautomated image processing method for identification and quantification of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 7640–7646. [DOI] [PubMed] [Google Scholar]
- 30. Pfau M, Goerdt L, Schmitz-Valckenberg S, et al.. Green-light autofluorescence versus combined blue-light autofluorescence and near-infrared reflectance imaging in geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017; 58: BIO121–BIO130. [DOI] [PubMed] [Google Scholar]
- 31. Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd ed. New York: Springer; 2009. [Google Scholar]
- 32. Groll A, Tutz G. Variable selection for generalized linear mixed models by L1-penalized estimation. Stat Comput. 2014; 24: 1–35. [Google Scholar]
- 33. Kimel M, Leidy NK, Tschosik E, et al.. Functional Reading Independence (FRI) index: a new patient-reported outcome measure for patients with geographic atrophy. Invest Ophthalmol Vis Sci. 2016; 57: 6298–6304. [DOI] [PubMed] [Google Scholar]
- 34. Holz FG, Sadda SR, Busbee B, et al.. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: Chroma and Spectri phase 3 randomized clinical trials. JAMA Ophthalmol. 2018; 136: 666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sunness JS, Rubin GS, Broman A, Applegate CA, Bressler NM, Hawkins BS. Low luminance visual dysfunction as a predictor of subsequent visual acuity loss from geographic atrophy in age-related macular degeneration. Ophthalmology. 2008; 115: 1480–1488, 1488.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu Z, Ayton LN, Guymer RH, Luu CD. Low-luminance visual acuity and microperimetry in age-related macular degeneration. Ophthalmology. 2014; 121: 1612–1619. [DOI] [PubMed] [Google Scholar]
- 37. Stockman A, Sharpe LT. Into the twilight zone: the complexities of mesopic vision and luminous efficiency. Ophthalmic Physiol Opt. 2006; 26: 225–239. [DOI] [PubMed] [Google Scholar]
- 38. Owsley C, McGwin G. Vision-targeted health related quality of life in older adults: patient-reported visibility problems in low luminance activities are more likely to decline than daytime activities. BMC Ophthalmol. 2016; 16: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.