Abstract
Purpose
The aim of this study was to determine the roles of collagen XII in the regulation of stromal hierarchical organization, keratocyte organization, and corneal mechanics.
Methods
The temporal and spatial expression of collagen XII at postnatal days 4, 10, 30, 90, and 150 were evaluated in wild-type (WT) mice. The role of collagen XII in hierarchical organization was analyzed by measuring fibril diameter and density, as well as stromal lamellar structure, within ultrastructural micrographs obtained from WT and collagen XII-deficient mice (Col12a1–/–). Keratocyte morphology and networks were assessed using actin staining with phalloidin and in vivo confocal microscopy. The effects of collagen XII on corneal biomechanics were evaluated with atomic force microscopy.
Results
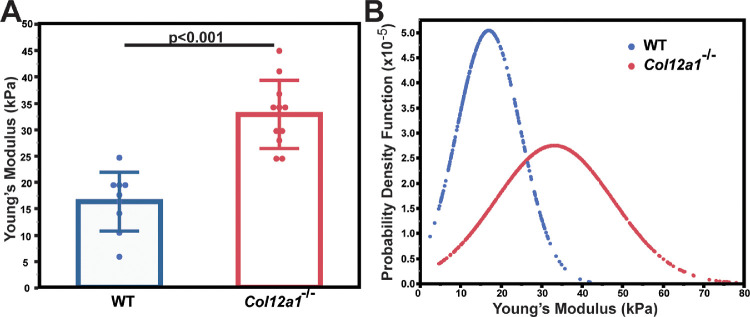
Collagen XII was localized homogeneously in the stroma from postnatal day 4 to day 150, and protein accumulation was shown to increase during this period using semiquantitative immunoblots. Higher fibril density (P < 0.001) and disruption of lamellar organization were found in the collagen XII null mice stroma when compared to WT mice. Keratocyte networks and organization were altered in the absence of collagen XII, as demonstrated using fluorescent microscopy after phalloidin staining and in vivo confocal microscopy. Corneal stiffness was increased in the absence of collagen XII. Young's modulus was 16.2 ± 5.6 kPa in WT and 32.8 ± 6.4 kPa in Col12a1–/– corneas. The difference between these two groups was significant (P < 0.001, t-test).
Conclusions
Collagen XII plays a major role in establishing and maintaining stromal structure and function. In the absence of collagen XII, the corneal stroma showed significant abnormalities, including decreased interfibrillar space, disrupted lamellar organization, abnormal keratocyte organization, and increased corneal stiffness.
Keywords: collagen XII, stroma, collagen fibril, development, keratocyte
The specialized extracellular matrix (ECM) organization of the corneal stroma is essential for vision.1–4 The stroma comprises 90% of the cornea and is mainly made of water, collagens, and proteoglycans.1,2,4,5 A highly organized hierarchical arrangement of collagen fibrils, as well as rigid control of stromal hydration, is necessary for transparency and vision. Stromal structure and function are dependent not only on ECM organization but also on cellular components. Between the orthogonal layers of collagen fibrils, networks of unique neural crest-derived cells known as keratocytes exist in a quiescent state.6
Fibril-associated collagens with interrupted triple helices interact with collagen fibrils as well as basement membranes and have been implicated in regulation of matrix organization and cell behavior.5 Collagen XII is a homotrimer composed of three Col12a1 chains; it has two major alternatively spliced variants, and the large variant has a glycosaminoglycan attachment site. Our Col12a1–/– model used here is deficient for all forms. The function of collagen XII is not well understood, but it has been implicated in the regulation of tissue structure and function, as well as cell organization.7 In addition, its expression is influenced by mechanical forces.8–11 Collagen XII is expressed in tissue regions of high mechanical stress, and its expression is upregulated by mechanical stimulation and injury.9,10,12
In the chicken cornea, collagen XII is expressed in interfacial regions at hatching, suggesting a major role in the integration of different corneal layers.13 In humans, collagen XII is upregulated in the matrix between the Descemet's layer and the stroma.14 Recent work has demonstrated the importance of collagen XII in establishing cell orientation, cell–cell interactions, and communication, as well as the translational effects of collagen XII dysfunction in human pathologies.7,15,16 In human and mouse corneas, collagen XII is upregulated during stromal wound healing and is present in scarred stroma.12,17
Interactions among fibrillar collagens, FACIT collagens, and proteoglycans and modifications via crosslinking enzymes are key regulators of tissue structure and function.18,19 We propose that collagen XII regulates intrinsic and essential properties of the stroma. Our hypothesis is that collagen XII plays a major role in stromal structure and function by regulating its hierarchical organization and intrinsic biomechanical properties.
Materials and Methods
Animals
Wild-type (WT) and collagen XII-deficient mice (Col12a1–/–) on C57BL/6 and 129/SvJ mixed backgrounds were used.7 Col12a1–/– mice are deficient for collagen XII, and no collagen XII protein was detected in these corneas.20 Corneas from mice at postnatal days 4, 10, 30, 60, 90, and 150 were included in this study. All animal studies were performed in compliance with the Institutional Animal Care and Use Committee approved animal protocols of the University of South Florida College of Medicine. Experiments conformed to the use of laboratory animals and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
RNA Isolation and Quantification of mRNA
Whole corneas were dissected from WT mice at days 4, 10, 30, 90, and 150 and treated with Dispase II (Roche Diagnostics, Indianapolis, IN, USA) to remove the cornea epithelium and endothelium. The corneal stroma was cut into small pieces, and total RNA was extracted using QIAzol Lysis Reagent (Qiagen, Hilden, Germany) and the Qiagen RNeasy MinElute Cleanup Kit. Reverse transcription and quantitative real-time PCR analyses were performed as described previously.21 The primer sequences were as follows: Col12a1 forward primer, TGACTACGGTGCAGATGAGC; Col12a1 reverse primer, AAGCGACGCAGAGAAAACAT; β-actin forward primer, AGATGACCCAGATCATGTTTGAGA; and β-actin reverse primer, CACAGCCTGGATGGCTACGT. Each sample was run in a duplicate PCR reaction, and statistical analysis was performed on three or four corneas from different mice at each time point.
Quantitation of Protein Expression
The stromas from WT and Col12a1–/– mice were cut into small pieces at days 4, 10, 30, 90, and 150. Stromas from two to seven mice were pooled, and protein was extracted using a radioimmunoprecipitation assay lysis buffer. Protein concentration was determined through a bicinchoninic acid assay (Thermo Fisher Scientific, Waltham, MA, USA). Collagen XII protein expression was analyzed using the Wes Western Assay (ProteinSimple, San Jose, CA, USA) following the manufacturer's instructions. Furthermore, a 12- to 230-kDa separation module, anti-rabbit detection module, and total protein detection module were applied. Briefly, samples diluted with 0.1× sample buffer (ProteinSimple) at a concentration of 0.125 µg/µl were loaded and hybridized with anti-collagen XII antibody (1:50 dilution; KR33).12,20 Total protein was used as a loading control.
Immunofluorescence Microscopy
Fresh eyes were harvested from WT mice at days 4, 10, 30, 90, and 150 and embedded in optimal cutting temperature compound, frozen with isopentane (Sigma-Aldrich, St. Louis, MO, USA) on dry ice. Corneal sections were cut at 5 µm using a HM 505E cryostat (GMI, Ramsey, MN, USA). Sections were blocked using 10% donkey serum (Sigma-Aldrich) and then incubated with rabbit anti-collagen XII antibody (1:100 dilution; KR33)12,20 for 2 hours at room temperature. The secondary antibody was Invitrogen Alexa Fluor 488 donkey anti-rabbit immunoglobulin G (Thermo Fisher Scientific), used at 1:200. VECTASHIELD mounting solution with 4′,6-diamidino-2-phenylindole (DAPI) as a nuclear marker (Vector Laboratories, Burlingame, CA, USA) was used. Images were captured using a fluorescence microscope (Leica DM5500B; Wetzlar, Germany). Identical conditions and negative controls, with no primary antibody use and Col12a1–/– corneas, were used to facilitate comparisons between samples.
Transmission Electron Microscopy
Cornea samples from WT and Col12a1–/– mice at days 4 and 30 were analyzed using transmission electron microscopy as previously described.22 Briefly, at least three corneas per group were dissected and fixed in 4% paraformaldehyde, 2.5% glutaraldehyde, and 0.1-M sodium cacodylate, pH 7.4, with 8.0-mM CaCl2, post-fixed in 1% osmium tetroxide. The corneas were dehydrated in graded ethanol series, followed by propylene oxide. The tissue samples were infiltrated and embedded in a mixture of Embed 812, nadic methyl anhydride, dodecenyl succinic anhydride, and DMP-30 (Electron Microscopy Sciences, Hatfield, PA, USA). Thin sections (∼90 nm) were cut with a Leica ultramicrotome and poststained with 2% aqueous uranyl acetate and 1% phosphotungstic acid, pH 3.2. The sections were examined at 80 kV with a JEOL JEM-1400 transmission electron microscope (Peabody, MA, USA) equipped with a Gatan UltraScan US1000 2K digital camera (Pleasanton, CA, USA).
Fibril Diameter and Fibril Spacing Distribution
Three corneas from three different mice at day 30 were analyzed from both WT and Col12a1–/– mice. Six digital images per cornea were taken from non-overlapping regions in the central stroma at a magnification of 100,000×. These images were randomly masked, and fibril diameter and density were measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA). A region of interest (ROI) of appropriate size was chosen within each image where fibrils were perpendicular/cross-sectional to the viewing plane. Two to four ROIs that included a minimum of 80 fibrils were measured for each image. MinFeret, which measures the minimum caliper diameter in ImageJ software, was chosen to represent the fibril diameters. Microsoft Excel 2013 (Redmond, WA, USA) was used for data analysis, to create histograms of the diameter distribution, and for Kolmogorov–Smirnov testing. Fibril density was obtained as the fibril number per unit area.
Histological and In Vivo Analysis of Keratocyte Organization
The organization of keratocytes was analyzed by evaluating the distribution of keratocytes within the corneal stroma. In fixed corneal tissue, actin staining in cross-sections was performed in WT and Col12a1–/– day 30 mice. Sections were stained for actin with phalloidin (Life Technologies, Carlsbad, CA, USA) for 2 hours at 4°C. Cell nuclei were stained with DAPI. Tissue sections were imaged on a confocal laser-scanning microscope (FV1000 MPE; Olympus Corporation, Tokyo, Japan). To avoid the effects of tissue sectioning, dehydration, and fixation on keratocyte organization, in vivo analysis of keratocyte organization was performed in Col12a1–/– and WT day 30 mice under general anesthesia using an in vivo confocal microscope (Heidelberg Retina Tomograph [HRT] III with Rostock Cornea Module; Heidelberg Engineering, Heidelberg, Germany), as previously described.21 A drop of GENTEAL gel (Novartis Pharmaceuticals, Basel, Switzerland) was applied to the tip of the HRT III objective as a lubricant. A series of images was collected throughout the entire thickness. Reconstructed images of the cornea were obtained with ImageJ software using the orthogonal image reconstruction function.
Tissue Stiffness and Compression Resistance in Adult Corneas
To analyze corneal stiffness, atomic force microscopy measurements were performed on nine adult male, day 50, corneas from five WT and 14 corneas from seven Col12a1–/– mice. The results from three Col12a1–/– mice were excluded from the analysis as outliers, because the values differed more than two standard deviations from the mean.
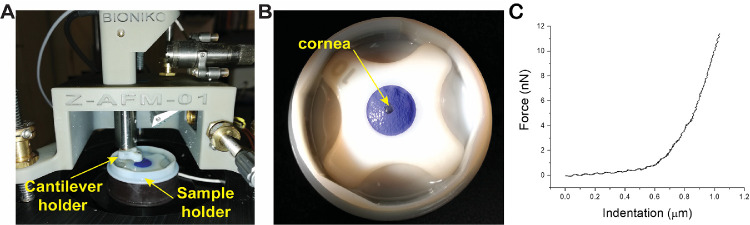
After euthanasia, the corneas were excised from the whole eyes beyond the limbus, placed in vials containing Optisol corneal preservation medium (Chiron Ophthalmics, Irvine, CA, USA), and shipped to the laboratory overnight. Just prior to elasticity measurements, the corneas, with epithelium and endothelium intact, were placed on the base of a custom holder consisting of a plano-convex lens. To ensure that the cornea was flat on the holder base, two or three incisions were made around the circumference of the corneal rim. The corneas were immobilized by placing a latex disk with a hole in the center on the top. The latex disk was then secured to the base of the holder with a Teflon ring (Figs. 1A, 1B). To maintain hydration and isotonic conditions that would not affect stromal hydration during experiments, the corneas were immersed in 15% dextran solution for at least 30 minutes, during which time the measurements were acquired.23
Figure 1.
A modified device for measurement of corneal stiffness. (A) Young's modulus of elasticity was measured using atomic force microscopy (AFM) adapted for corneal tissue. (B) A custom-designed modification was needed to overcome the challenge of the mouse cornea size. (C) Typical force versus indentation curve obtained during AFM testing after applying force, measured in nanonewtons versus corneal indentation in micrometers.
Elasticity measurements of the cornea were performed with a laboratory-built atomic force microscopy (AFM) system custom designed for mechanical characterization of mouse corneal tissues. Mouse cornea elasticity was quantified by indenting the samples with a silicon nitride triangle cantilever with a 2.5-µm silica bead affixed to the apex (k = 0.12 N/m; Novascan Technologies, Inc., Ames, IA, USA). A piezoelectric mechanism (60 µm maximal expansion, P-841.40; Physik Instrumente, Karlsruhe, Germany) was programmed to lower the cantilever onto the sample vertically at the rate of 15 µ/s. Upon reaching the maximum indentation force of 1 V (corresponding to ∼15 nN), the cantilever was retracted. According to the principle of AFM indentation, the cantilever would undergo a combination of bending and indentation of the sample during interaction. The cantilever bending was monitored and recorded with the reflection of a laser from the back of the cantilever to a position-sensitive photodiode. Measurements were repeated 10 times in at least three different locations around the cornea. Samples were indented approximately 1 µm with a maximal force of approximately 15 nN.
Young's modulus of elasticity of each cornea was calculated from each measurement using custom developed MATLAB software (MathWorks, Natick, MA, USA). The photodiode voltage versus piezoelectric displacement recorded during the measurement scans was converted to force versus indentation after accounting for the cantilever spring constant (0.12 N/m) and the cantilever bending response on a hard surface, assuming no indentation. The force versus indentation curves were analyzed using the Hertz model for a spherical indenter:
where F is the measured force (N); E is Young's modulus of elasticity (Pa), υ is Poisson's ratio (0.49), and D is the measured indentation (m). Each curve fit was verified visually.
Results
Collagen XII Is Homogeneously Expressed in the Stroma During Development, and Its Expression Is Maintained in the Adult
To assess the role of collagen XII in corneal development and stromal matrix homeostasis, temporal and spatial expression of collagen XII was examined in different stages: immature (days 4–10), maturing (day 30), and adult corneas (days 90 and 150). Col12a1 mRNA expression was measured in the cornea stroma using quantitative real-time PCR. Col12a1 mRNA was expressed at all stages studied, between days 4 and 150 (Fig. 2A). Temporal changes in expression were observed. Col12a1 mRNA expression peaked during maturation at day 10 and decreased to a stable level in the mature cornea. Corneal collagen XII protein also was present in the corneal stroma from day 4 to 150 when analyzed using the Wes Western Assay (Fig. 2B). In contrast to mRNA expression, collagen XII content gradually increased with age (days 4–30), reaching a relatively constant level in the mature cornea (days 90 and 150). This is consistent with the accumulation of collagen XII as the cornea developed and increased in size.
Figure 2.
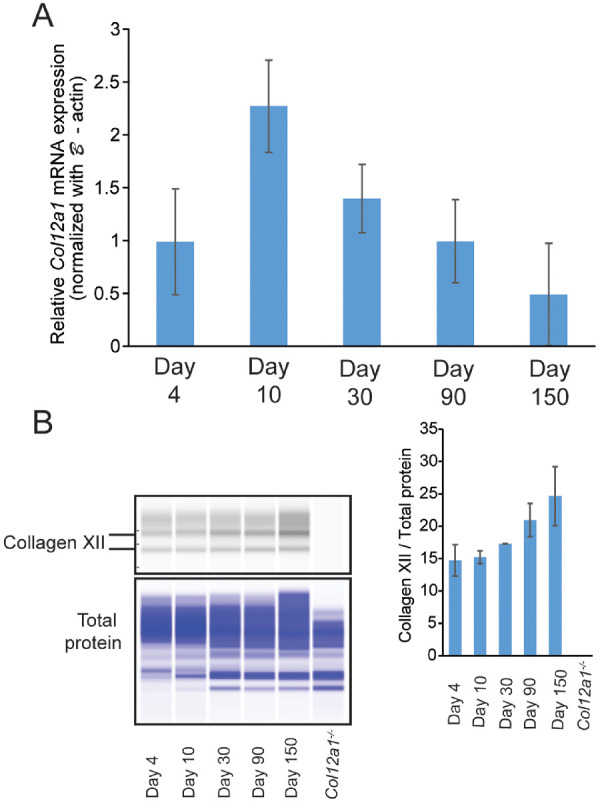
Temporal expression of collagen XII in wild-type mice. (A) Col12a1 mRNA expression was higher during stromal development but was maintained in the adult WT stromas. Relative Col12a1 mRNA expression was obtained by real-time PCR analysis using a comparative ΔCT method that used β-actin as an internal control and sample 1 of a WT day 4 cornea as the reference sample. The sample means and standard deviations were calculated from three or four corneal stromas of different mice. (B) Collagen XII protein was present in the maturing and mature stroma. Representative Wes image shows that the short form and long form of collagen XII were present and accumulated throughout the corneal development stages. Total protein was used as a protein loading control. The right-side panel shows the relative collagen XII protein quantitation that was normalized to total protein. The standard deviation was obtained by Wes analysis of two sets of independent protein samples.
The spatial localization of collagen XII demonstrated homogeneous localization throughout the corneal stroma using immunofluorescence microscopy. No changes in the spatial localization of collagen XII were observed from day 4 to day 150, and no collagen XII was noted in the epithelial layers (Figs. 3A–3E). Together, these data show that collagen XII expression is dynamic during stromal development. Collagen XII expression is increased during early development and maintains a high level of expression in the mature stroma. Continued accumulation of collagen XII within the stroma with aging favors the hypothesis that collagen XII plays a role in regulatory functions during development and in the adult cornea.
Figure 3.
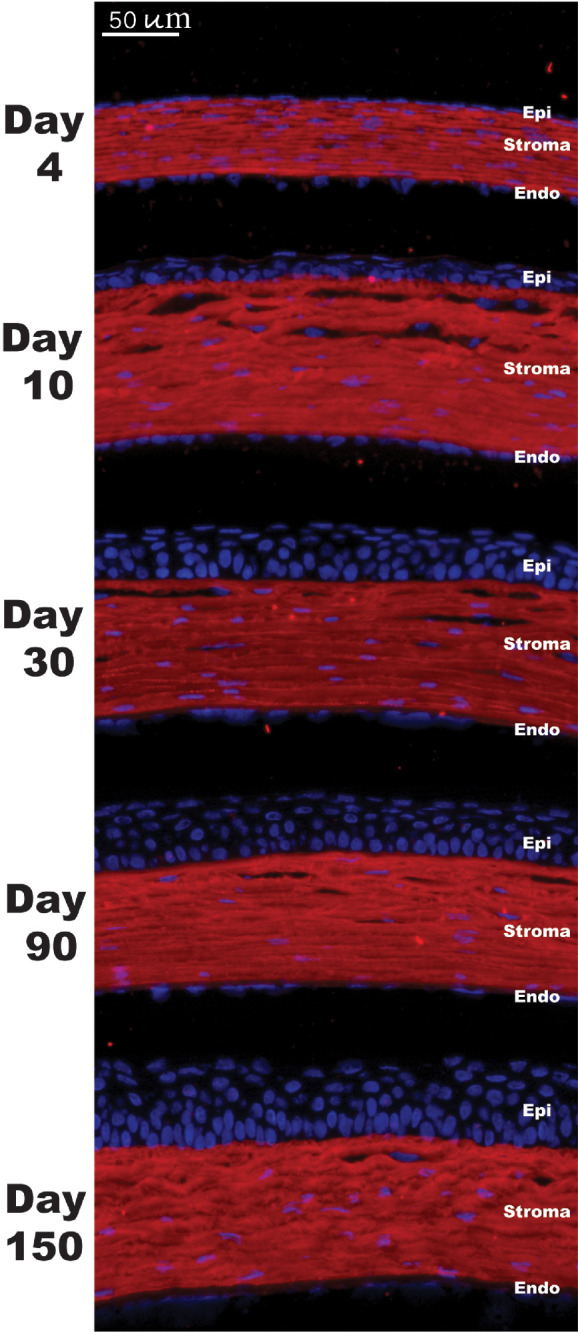
Spatial expression of collagen XII in WT mice. Immunofluorescence localization images showed homogeneous and persistent expression of collagen XII (green) in the WT corneal stroma at different ages. No corneal epithelial or endothelial staining was noted. Negative controls showed no reactivity. Nuclei were stained with DAPI (blue).
Collagen XII Regulates Stromal Fibril Diameter and Packing
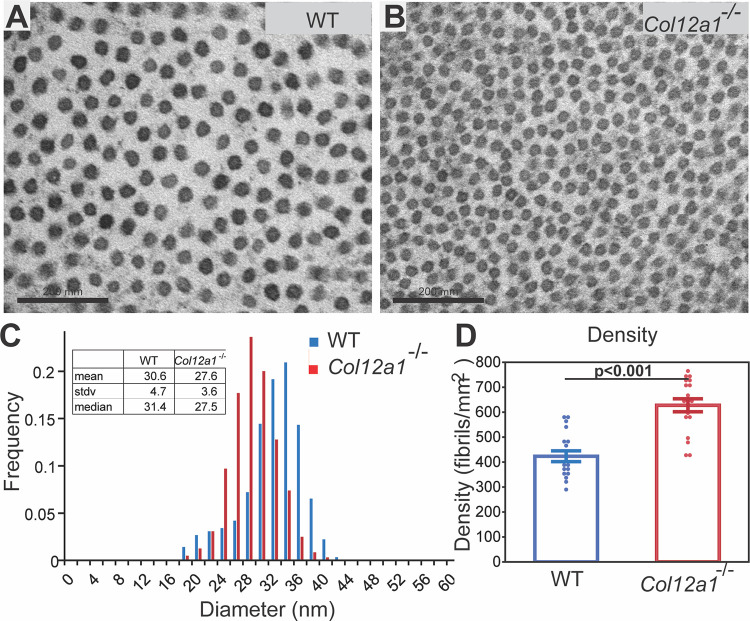
To define the regulatory role of collagen XII in fibrillogenesis, stromal structure and fibril packing in WT mice were compared to Col12a1–/– mice at day 30 using ultrastructural analysis in the central stroma. Collagen fibrils from the stroma of both WT and Col12a1–/– mice had relatively homogeneous diameters, and no aberrant fibrils were observed; however, differences in fibril diameter and density were present. In Col12a1–/– corneas, fibrils had smaller diameters and decreased interfibrillar spacing compared to WT control stromas (Figs. 4A, 4B).
Figure 4.
Abnormal fibril diameter and packing in Col12a1–/– corneal stromas. (A) Representative electron micrographs show normal interfibrillar space and fibril packing in the WT corneal stroma, day 30. (B) Compared to WT mice, the Col12a1–/– stroma had smaller diameter fibrils and decreased interfibrillar spacing. (C) The fibril diameter in the Col12a1–/– central corneal stromas shifted to smaller diameters compared to those for the WT. (D) In contrast, increased fibrillar density was noted in the absence of collagen XII. The increase in the fibril density of the Col12a1–/– stroma was significant based on the analysis of 18 images from each group. Bar 200 nm.
An analysis of fibril diameter distributions demonstrated a significant difference between fibrils in Col12a1–/– and WT stromas (Fig. 4C). Compared to the WT stroma, the fibril diameter distribution in the central stroma of Col12a1–/– mice showed a statistically significant shift to the smaller fibril diameter, as assessed by the Kolmogorov–Smirnov test. The mean fibril diameter in the day 30 Col12a1–/– was 27.6 ± 3.6 nm, compared to a mean diameter of 30.7 ± 4.8 nm in the WT corneal stroma. There was a statistically significant difference in mean fibril diameter (P < 0.001, t-test). The median of fibril diameters for Col12a1–/– and WT corneal stromas were 27.5 nm and 31.5 nm, respectively (Fig. 4C).
Associated with altered regulation of fibril diameter, there also was a statistically significant difference in fibril density with a higher fibril density found in the Col12a1–/– stroma compared to the WT stroma by paired t-test (P < 0.001). The Col12a1–/– stroma had 218 more fibrils per square micrometer than the WT stroma, a 51.5% increase (Fig. 4D). Overall, our data show that collagen XII regulates fibril size and packing. These findings demonstrate the regulation of stromal hierarchical organization by collagen XII.
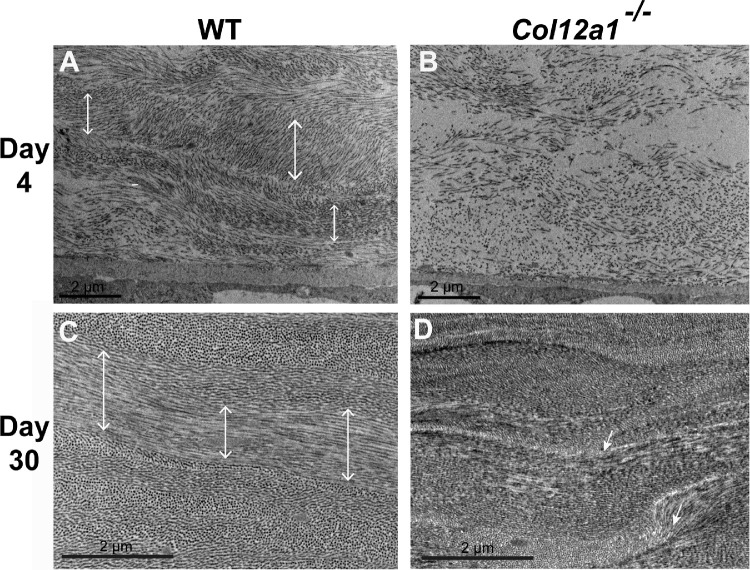
Disrupted Lamellar and Higher Order Matrix Organization in the Absence of Collagen XII
To determine if collagen XII is involved in the regulation of higher order organization in the developing stromal matrix, we analyzed lamella formation at day 4 during development and at day 30 in the maturing cornea. In the WT day 4 stroma, fibrils were organizing into distinct lamella that were becoming orthogonal (Fig. 5A). In contrast, poor fibril organization into lamella was noted in the Col12a1–/– stroma (Fig. 5B). Further analysis of stomal maturation at day 30 demonstrated a normal arrangement and organization of collagen fibrils into lamella that were roughly orthogonal in WT control stromas (Fig. 5C). In contrast, in the Col12a1–/– stroma, areas of abnormal lamellar organization and orientation were evident. The Col12a1–/– stroma had not reached the same level of matrix organization as WT corneas (Figs. 5C, 5D). In summary, these observations demonstrate that collagen XII regulates higher order matrix organization by directing lamellae formation and alignment.
Figure 5.
Higher order stromal disorganization in the absence of collagen XII during development. (A) Fibril space was decreasing and fibrils were organizing during development in WT day 4 corneas. (B) In contrast, the Col12a1–/– stroma had significantly increased interfibrillar space, and no lamella formation was noted at day 4. (C) Normal lamellar arrangement in the WT mature stroma; orthogonal lamellar organization was present at day 30. (D) In the Col12a1–/– mice, lamellar organization was disrupted and disorganized in comparison to WT. Double-head arrows, organized lamella; arrows, disorganized lamella.
Collagen XII Regulates Keratocyte Organization
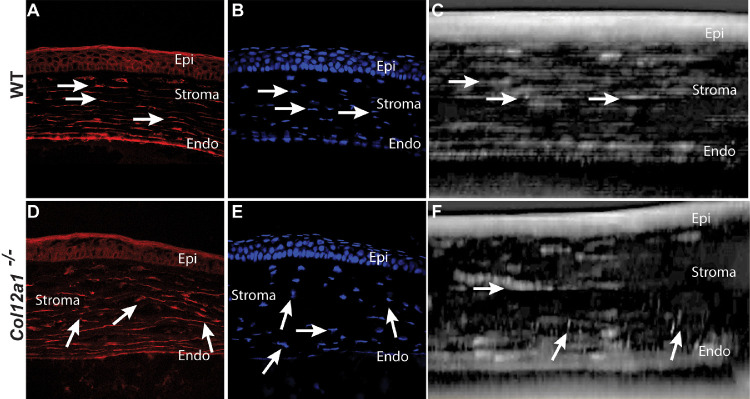
To define the role of collagen XII in keratocyte network organization, we analyzed keratocyte morphology in tissue sections and in vivo in Col12a1–/– and WT day 30 corneas. Morphological differences in keratocyte organization were observed using confocal microscopy of sectioned corneas. In WT corneas, keratocytes are regularly arranged in layers parallel to the corneal surface, demonstrated after actin staining of keratocytes using phalloidin (Fig. 6A), as well as DAPI staining (Fig. 6B) of keratocyte nuclei. Keratocytes were well aligned and organized in parallel. In contrast, Col12a1–/– corneas had poorly organized keratocytes networks (Figs. 6D, 6E). Comparison of keratocyte organization in WT and Col12a1–/– stromas demonstrated a significant disruption in keratocyte network organization in the absence of stromal collagen XII.
Figure 6.
Disorganized keratocyte distribution and networks in tissue sections and reconstructed stromal images from in vivo confocal microscopy. (A) Actin filaments showed well-aligned keratocytes oriented in the interlamellar space. (D) In contrast, keratocyte organization and alignment were disrupted in the absence of collagen XII. (B) DAPI showed keratocyte nuclei horizontally aligned between stromal lamellae. (E) DAPI nuclear staining showed disorganized nuclei orientation and arrangement in Col12a1–/– corneas. (C) Reconstructed in vivo confocal two-dimensional image also shows well-aligned keratocytes in the horizontal plane. (F) In contrast, disrupted organization and alignment were noted in a representative in vivo confocal two-dimensional image in Col12a1–/– corneas.
Three-dimensional reconstruction of in vivo confocal images confirmed the organized keratocyte networks and well-organized and polarized keratocytes embedded within the WT mouse stroma (Fig. 6C). In contrast, Col12a1–/– corneas showed poor keratocyte alignment and organization (Fig. 6F). Together, these findings suggest that collagen XII regulates keratocyte orientation and organization within the stroma. Control of cell organization may influence matrix organization during development and maturation.
Collagen XII Regulates Tissue Stiffness and Resistance to Compression
To determine whether or not alteration in the regulation of stromal structural hierarchy and keratocyte organization in the absence of collagen XII affects tissue mechanics, we tested corneal stiffness in adult stromas. Young's modulus was 16.2 ± 5.7 kPa for WT corneas and 32.8 ± 6.4 kPa for Col12a1–/– corneas. The lack of collagen XII resulted in a stiffer cornea that was more difficult to indent with the AFM cantilever. The difference between the two groups was significant (P < 0.001, t-test), indicating that collagen XII regulates corneal stiffness (Fig. 7A). The probability density function that describes the likelihood of occurrence for each value of Young's modulus also was analyzed. The distribution of Young's modulus in Col12a1–/– corneas was broader than that in WT corneas. It indicated that the stiffness of the Col12a1–/– corneas was more heterogeneous than for the WT corneas (Fig. 7B). These findings suggest that collagen XII regulates stromal resistance to compression and is a regulator of corneal mechanics.
Figure 7.
Collagen XII regulates tissue stiffness by decreasing resistance to tissue compression. (A) WT corneas were less resistant to compression than the Col12a1–/– model, as demonstrated by a significant increase in Young's modulus and indicating increased stromal stiffness in the absence of collagen XII. (B) The distribution of Young's modulus was broader in the Col12a1–/– model than in the WT corneas, indicating a larger variability of the resistance to compression.
Discussion
Precise assembly and alignment of collagen fibrils and interactions with extracellular matrix components are essential to establish the intrinsic properties of the corneal stroma necessary for proper corneal function (e.g., transparency, rigidity, avascularity, shape). How extracellular matrix components contribute to these intrinsic corneal properties is an evolving subject of investigation with significant translational potential. Here, we have established that collagen type XII is a molecule with a significant role in the regulation of stromal structure and function in the adult cornea.
The specific properties that collagen XII confers to the stroma during development and in the maintenance of stromal architecture are poorly understood. Previous studies evaluating collagen XII in the cornea are limited to expression patterns in different species, and the function of collagen XII remains mostly unexplored.14,24,25 A striking finding in our results is that collagen XII plays a major role in establishing the hierarchical organization of the stroma, as demonstrated by collagen XII upregulation at times of fibril organization, such as during development, as shown by our data at day 10, the highest mRNA expression period, and during wound healing,12 when newly assembled collagen fibrils are being organized into higher order structures.
Although the cornea in collagen XII null mouse models is transparent, we found significant reductions in interfibrillar space, fibril diameter in the Col12a1–/– corneas, and poor and disorganized stromal lamellar formation. We speculate, based on our data, that collagen XII is a potent regulator of fibril organization and therefore a major regulator of hierarchical stromal organization. How can collagen XII affect hierarchical organization in the stroma? Collagen fibril density can be regulated just by the physical separation of collagen fibrils. Collagen XII may act as a physical spacer between adjacent collagen fibrils, with the large non-collagenous domain projecting into the interfibrillar space, and contribute to the regular distribution and parallelism of fibrils necessary for corneal clarity. Proper alignment of collagen fibrils is essential to maintain the proper interfibrillar spacing and hierarchical organization necessary to avoid light scattering.
Maintaining the intricate balance between a keratocyte network and the surrounding specialized matrix is important for proper stromal structure and function. We found that keratocyte networks within the stroma were not evenly distributed and were poorly oriented within the stroma even at early development, day 10. Disrupted keratocyte organization does not favor proper organization of the extracellular matrix, and it is probable that disorganized keratocyte networks contribute to poor hierarchical disorganization during development and probably during corneal wound healing. The disorganized matrix and keratocyte networks, noted in the entire stroma, may hypothetically account for the increased corneal thickness we previously reported.20
An unexpected finding in this study is that adult corneas were stiffer and more resistant to tissue compression in the absence of collagen XII, indicating that collagen XII regulates the resistance of corneal tissue to compression, and it could be assumed that collagen XII is a regulator of corneal mechanics and stiffness. Our technique to estimate tissue compression, an indicator of stiffness, was performed using a custom-designed system for corneal tissue that performs bulk stromal tissue measurements in the micrometer range. We performed the measurements while the tissue was in 15% dextran, and we cannot discount that hydration changes may somehow have affected the results obtained in this study. We evaluated the hydration status of the cornea by measuring corneal thickness after 24 hours in Optisol and found no significant difference in the increased corneal thickness between the two groups. It is important to note that the Hertz model assumes that the sample is isotropic, homogeneous, linearly elastic, and infinitely thick, none of which accurately describes the mouse cornea. Although the use of the Hertz model will not provide absolute values for elasticity and viscosity, it will still give important relative values to compare differences between groups. Because of this, the use of the Hertz model and the associated variations for cantilever tip geometry has become standard among groups using AFM to characterize tissue mechanics, including the cornea. In the current study, all experimental conditions remained constant between groups, so any differences in Young's modulus are indicative of differences caused by collagen XII.
How does collagen XII regulate tissue stiffness? We could speculate that this large molecule acts as a stabilizing biomaterial between fibrils based on the location of collagen XII on the surface of the fibrils. Decreased fibril-to-fibril space translates to increased tissue stiffness or increased resistance to compression. This role as a regulator of corneal biomechanics can be accomplished either on its own by acting as a molecular interfibrillar spacer or spring between collagen fibrils or through the formation of a complex with other matrix components in the interfibrillar space. Collagen XII is known to interact with multiple matrix molecules through its large N-terminal non-collagenous domain. This domain has a large attached glycosaminoglycan chain26 and interacts with different matrix molecules: decorin,27 fibromodulin,28 tenascin X,29 cartilage oligomeric matrix protein,30 collagen VI,31 and keratoepithelin.32
These interactions of collagen XII with other matrix components may explain the decreased fibril size noted in the absence of collagen XII. Recently, it has been shown that human mutations in collagen XII in human skin are associated with decreased expression of tenascin X and decorin, both well-known regulators of fibril diameter.33,34 More research is needed regarding the relevance of collagen XII expression compared to proteoglycans expressed in the matrix. Collagen XII itself, by interacting with different matrix components, including collagen I, or by regulating tissue mechanics, may have multiple regulatory functions in the stroma.
From a translational view, the finding that collagen XII is relevant in regulating tissue compression in the stroma confirms the suspected biomechanical functions of collagen XII, and suggests that dysregulation of collagen XII may play a role in the etiology of corneal disorders with altered biomechanics, such as ectasia. Overexpression or downregulation of collagen XII in localized areas of the cornea could hypothetically result in islands of softer or stiffer stroma that could predispose to ectasia. It is interesting that floppy eyelid syndrome, a condition where eyelids are lax and probably create less compressive forces against the cornea with each blink, is associated with keratoconus. Does less stimulation, meaning less compressive forces acting on the cornea, create a dysregulation of collagen XII leading to ectasia? It is also tempting to speculate that collagen XII can regulate wound healing by regulating matrix stiffness and organization of newly synthesized matrix. Collagen XII may affect wound healing, by regulating tissue stiffness, which might influence myofibroblast behavior and matrix deposition. Will cells in a soft matrix heal better than in a stiff matrix, and will regulation of matrix stiffness affect myofibroblast differentiation, apoptosis, and function? That is an interesting question in relation to the cornea, where matrix deposition means scar formation and poor vision.
Future studies are needed to investigate the role that collagen XII plays during pathological conditions such as corneal edema and wound healing. Does collagen XII interact with different growth factors that activate keratocytes or bind antiangiogenic factors that are docked to this large molecule during homeostasis? The recapitulation of collagen XII expression during injury is an interesting research topic. It is known that collagen XII production plays a role during stromal wound healing. Massoudi et al.12 showed that collagen XII deposition in the stroma increases during wound healing and in scars in human and mouse corneas. The interactions of collagen XII with other stromal molecules and its role in fibrosis and scar formation remain poorly studied.35,36 Can collagen XII be manipulated during wound healing to regulate wound healing, myofibroblast phenotype conversion, and corneal scarring? Could collagen XII genetic or chemical manipulation enhance fibril organization to the point of improving vision during stromal repair?
Acknowledgments
Supported by National Institutes of Health/National Eye Institute Grant EY029395.
Disclosure: M. Sun, None; N. Zafrullah, None; F. Devaux, None; C. Hemmavanh, None; S. Adams, None; N.M. Ziebarth, None; M. Koch, None; D.E. Birk, None; E.M. Espana, None
References
- 1. Maurice DM. The structure and transparency of the cornea. J Physiol. 1957; 136: 263–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010; 91: 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen S, Mienaltowski MJ, Birk DE. Regulation of corneal stroma extracellular matrix assembly. Exp Eye Res. 2015; 133: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meek KM, Knupp C. Corneal structure and transparency. Prog Retin Eye Res. 2015; 49: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mienaltowski MJ, Birk DE. Structure, physiology, and biochemistry of collagens. Adv Exp Med Biol. 2014; 802: 5–29. [DOI] [PubMed] [Google Scholar]
- 6. Poole CA, Brookes NH, Clover GM. Keratocyte networks visualised in the living cornea using vital dyes. J Cell Sci. 1993; 106: 685–691. [DOI] [PubMed] [Google Scholar]
- 7. Izu Y, Sun M, Zwolanek D, et al.. Type XII collagen regulates osteoblast polarity and communication during bone formation. J Cell Biol. 2011; 193: 1115–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gordon MK, Gerecke DR, Olsen BR. Type XII collagen: distinct extracellular matrix component discovered by cDNA cloning. Proc Natl Acad Sci USA. 1987; 84: 6040–6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiquet M, Mumenthaler U, Wittwer M, Jin W, Koch M. The chick and human collagen alpha1(XII) gene promoter–activity of highly conserved regions around the first exon and in the first intron. Eur J Biochem. 1998; 257: 362–371. [DOI] [PubMed] [Google Scholar]
- 10. Arai K, Nagashima Y, Takemoto T, Nishiyama T. Mechanical strain increases expression of type XII collagen in murine osteoblastic MC3T3-E1 cells. Cell Struct Funct. 2008; 33: 203–210. [DOI] [PubMed] [Google Scholar]
- 11. Chiquet M, Birk DE, Bonnemann CG, Koch M. Collagen XII: protecting bone and muscle integrity by organizing collagen fibrils. Int J Biochem Cell Biol. 2014; 53: 51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Massoudi D, Malecaze F, Soler V, et al.. NC1 long and NC3 short splice variants of type XII collagen are overexpressed during corneal scarring. Invest Ophthalmol Vis Sci. 2012; 53: 7246–7256. [DOI] [PubMed] [Google Scholar]
- 13. Gordon MK, Foley JW, Lisenmayer TF, Fitch JM. Temporal expression of types XII and XIV collagen mRNA and protein during avian corneal development. Dev Dyn. 1996; 206: 49–58. [DOI] [PubMed] [Google Scholar]
- 14. Wessel H, Anderson S, Fite D, Halvas E, Hempel J, SundarRaj N. Type XII collagen contributes to diversities in human corneal and limbal extracellular matrices. Invest Ophthalmol Vis Sci. 1997; 38: 2408–2422. [PubMed] [Google Scholar]
- 15. Hicks D, Farsani GT, Laval S, et al.. Mutations in the collagen XII gene define a new form of extracellular matrix-related myopathy. Hum Mol Genet. 2014; 23: 2353–2363. [DOI] [PubMed] [Google Scholar]
- 16. Zou Y, Zwolanek D, Izu Y, et al.. Recessive and dominant mutations in COL12A1 cause a novel EDS/myopathy overlap syndrome in humans and mice. Hum Mol Genet. 2014; 23: 2339–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chaerkady R, Shao H, Scott SG, Pandey A, Jun AS, Chakravarti S. The keratoconus corneal proteome: loss of epithelial integrity and stromal degeneration. J Proteomics. 2013; 87: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014; 15: 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol. 2014; 15: 771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hemmavanh C, Koch M, Birk DE, Espana EM. Abnormal corneal endothelial maturation in collagen XII and XIV null mice. Invest Ophthalmol Vis Sci. 2013; 54: 3297–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun M, Chen S, Adams SM, et al.. Collagen V is a dominant regulator of collagen fibrillogenesis: dysfunctional regulation of structure and function in a corneal-stroma-specific Col5a1-null mouse model. J Cell Sci. 2011; 124: 4096–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ansorge HL, Meng X, Zhang G, et al.. Type XIV collagen regulates fibrillogenesis: premature collagen fibril growth and tissue dysfunction in null mice. J Biol Chem. 2009; 284: 8427–8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dias J, Ziebarth NM. Impact of hydration media on ex vivo corneal elasticity measurements. Eye Contact Lens. 2015; 41: 281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anderson S, SundarRaj S, Fite D, Wessel H, SundarRaj N. Developmentally regulated appearance of spliced variants of type XII collagen in the cornea. Invest Ophthalmol Vis Sci. 2000; 41: 55–63. [PubMed] [Google Scholar]
- 25. Young BB, Zhang G, Koch M, Birk DE. The roles of types XII and XIV collagen in fibrillogenesis and matrix assembly in the developing cornea. J Cell Biochem. 2002; 87: 208–220. [DOI] [PubMed] [Google Scholar]
- 26. Koch M, Bernasconi C, Chiquet M. A major oligomeric fibroblast proteoglycan identified as a novel large form of type-XII collagen. Eur J Biochem. 1992; 207: 847–856. [DOI] [PubMed] [Google Scholar]
- 27. Font B, Eichenberger D, Rosenberg LM, van der Rest M. Characterization of the interactions of type XII collagen with two small proteoglycans from fetal bovine tendon, decorin and fibromodulin. Matrix Biol. 1996; 15: 341–348. [DOI] [PubMed] [Google Scholar]
- 28. Font B, Eichenberger D, Goldschmidt D, Boutillon MM, Hulmes DJ. Structural requirements for fibromodulin binding to collagen and the control of type I collagen fibrillogenesis–critical roles for disulphide bonding and the C-terminal region. Eur J Biochem. 1998; 254: 580–587. [DOI] [PubMed] [Google Scholar]
- 29. Veit G, Hansen U, Keene DR, et al.. Collagen XII interacts with avian tenascin-X through its NC3 domain. J Biol Chem. 2006; 281: 27461–27470. [DOI] [PubMed] [Google Scholar]
- 30. Agarwal P, Zwolanek D, Keene DR, et al.. Collagen XII and XIV, new partners of cartilage oligomeric matrix protein in the skin extracellular matrix suprastructure. J Biol Chem. 2012; 287: 22549–22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Izu Y, Ezura Y, Koch M, Birk DE, Noda M. Collagens VI and XII form complexes mediating osteoblast interactions during osteogenesis. Cell Tissue Res. 2016; 364: 623–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Runager K, Klintworth GK, Karring H, Enghild JJ. The insoluble TGFBIp fraction of the cornea is covalently linked via a disulfide bond to type XII collagen. Biochemistry. 2013; 52: 2821–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Delbaere S, Dhooge T, Syx D, et al.. Novel defects in collagen XII and VI expand the mixed myopathy/Ehlers-Danlos syndrome spectrum and lead to variant-specific alterations in the extracellular matrix. Genet Med. 2019; 22: 112–123. [DOI] [PubMed] [Google Scholar]
- 34. Chen S, Sun M, Meng X, Iozzo RV, Kao WW, Birk DE. Pathophysiological mechanisms of autosomal dominant congenital stromal corneal dystrophy: C-terminal-truncated decorin results in abnormal matrix assembly and altered expression of small leucine-rich proteoglycans. Am J Pathol. 2011; 179: 2409–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ljubimov AV, Burgeson RE, Butkowski RJ, et al.. Extracellular matrix alterations in human corneas with bullous keratopathy. Invest Ophthalmol Vis Sci. 1996; 37: 997–1007. [PubMed] [Google Scholar]
- 36. El-Shabrawi Y, Kublin CL, Cintron C. mRNA levels of alpha1(VI) collagen, alpha1(XII) collagen, and beta ig in rabbit cornea during normal development and healing. Invest Ophthalmol Vis Sci. 1998; 39: 36–44. [PubMed] [Google Scholar]