Highlights
-
•
Infection resurgence might occur during a simultaneous circulation of COVID-19 and flu.
-
•
Viral cooperation takes place to boost the reproductive probabilities.
-
•
Stratification of people at a very high risk of death is crucial for public health.
Keywords: Influenza, COVID-19, Simultaneous circulation, Viral cooperation, High risk of death
Abstract
Based on data updated to 20 May 2020, the total recorded number of patients who died due to COVID-19-related reasons in Italy was 31,851. Demographic and clinical characteristics of patients who have died (including the number of comorbidities) are extremely relevant, especially to define those with a higher risk of mortality. Health authorities recommend influenza (flu) vaccinations in a number of categories at risk of serious medical complications, including: people aged ≥65 years, or patients with diabetes, cardiovascular diseases, chronic obstructive pulmonary disease (COPD), renal failure, cancer, immunodeficiencies, chronic hepatopathies, and chronic inflammatory bowel diseases. The seasonal flu peak certainly preceded that of the pandemic; however, it would seem clear that the two viruses have been simultaneously circulating in Italy for a while. Hence, after the peak of seasonal flu, influenza-like illness-related (ILI) deaths started to grow again. While some of the excess mortality reported in the ILI group may be attributable to COVID-19, a question arises: do we have to consider this observation as a result of a random sequence of events or a potential relationship between the two viruses play a role? A cooperation mechanism intended at establishing an absolute advantage over the host could also be assumed; this system often takes place to boost the reproductive probabilities. A characterization of those who died due to virus-related reasons can be performed by cross-linking data (stored in different warehouses) from the same geographical area and developing electronic health records. It would be of great relevance to identify people at very high risk of mortality as a result of an overlapping or combination of risk factors that were separately reported in patients who died from COVID-19 or influenza. A description of the subgroup of people at higher risk of mortality will be crucial for prioritizing and implementing future public health prevention and treatment programs.
Introduction
Social distancing and other forms of precautionary public health measures have led to an effective control of beta coronavirus circulation in China, Italy, other European countries, and the USA. The blood toll paid in Italy was early in the pandemic and particularly high. At present, a ‘ceasefire’ has been successfully achieved, but the war against COVID-19 may not yet be over. Indeed, a resurgence of infections due to beta coronavirus could be observed at the time of possible simultaneous circulation of COVID-19 and influenza. Such an event could be expected between late autumn and early winter this year.
Based on data updated to 20 May 2020, the total recorded number of patients who came into contact with COVID-19 and died in Italy was 31,851 (National Institute of Health (Istituto Superiore di Sanità, 2020a). The preliminary case-fatality rate (CFR) among patients with COVID-19 was estimated by Onder et al. (2020); they calculated the overall crude CFR to be 7.2%, which is a much higher value than the average reported in China (2.3%) (Wu and McGoogan, 2020). Demographic and clinical characteristics of patients who died (including the number of comorbidities) are extremely relevant, especially to define those with a higher risk of mortality. Age was recognized as one of the main risk factors for death associated with COVID-19. The median age of mortality was 82 years (interquartile range: 74–88). This is 20 years older compared with the median age of infected patients (patients who died 82 years, and infected 62 years) (National Institute of Health (Istituto Superiore di Sanità, 2020b). Italy is one of the countries with the highest mean age in the world, along with Japan and Germany. Pre-existing chronic diseases (diagnosed before the COVID-19 infection) is an additional and substantial risk factor. The most frequently reported comorbidities are high blood pressure (67.9%), type 2 diabetes (30.0%), ischemic heart disease (28.2%), and renal failure (20.4%) (National Institute of Health (Istituto Superiore di Sanità, 2020b). This comorbidity assessment was derived from a careful analysis of medical records from 3,602 of 33,532 patients who died (about 10% of the overall number) (National Institute of Health (Istituto Superiore di Sanità, 2020b); the average number of diseases observed in this population was 3.3 ± 1.9.
A study that was recently performed by the Italian National Institute of Health (ISS) has shown that the excess mortality associated with influenza-like illness (ILI) was between 11.6–41.2 per 100,000, reaching a total of >68,000 deaths in a 4-year time period (Rosano et al., 2019). In other words, the estimated excess of mortality in the aforementioned period was 7,027, 20,259, 15,801 and 24,981 patients in 2013–2014, 2014–2015, 2015–2016 and 2016–2017, respectively (Rosano et al., 2019). Unluckily, no clinical or demographic features were investigated in this paper. The State-Region Conference (a government institution), in agreement with the Ministry of Health, recommends a free flu vaccination for a number of categories at risk of serious medical complications, including: people aged >65 years, or patients with diabetes or other metabolic diseases (including those with a BMI > 30), cardiovascular diseases, COPD, renal failure, cancer, immunodeficiencies, chronic hepatopathies, and chronic inflammatory bowel diseases (Ministry of Health, 2020).
A comparison between individuals at risk of mortality associated with both diseases (i.e. COVID-19 and influenza) is of the utmost importance. A sub-stratification of people at very high risk of mortality might be predictable, in particular when the circulation of both viruses is supposed to occur in the same period.
Source of data and methodological issues
Although attempts are being made to develop unique data collection platforms in Italy, data needed for accurate mortality risk estimation are commonly located in different places. This is true for both flu and COVID-19. The national sentinel influenza surveillance system (known as InfluNet) is a network of general practitioners and pediatricians working as sensors and providing healthcare to at least 2% of the whole Italian population (Rosano et al., 2019). As a consequence, estimating the role of flu in the overall mortality calculation is quite complex, since the diagnosis is not always confirmed by laboratory tests and most deaths are due to complications. For these reasons, although the death is flu-related, flu may not be officially reported on all death certificates or registries. An indirect measure of its impact on mortality is given by the attributable excess of mortality, which can be calculated as the differential between the number of deaths observed during the flu season and the expected baseline value (in the absence of flu). Several statistical regression models have been adopted to estimate the excess mortality attributable to influenza, considering the potential confounding effect of variables such as temperature, viral genotypes and age distribution patterns of the population (Rosano et al., 2019, Viboud et al., 2005, Dushoff et al., 2006, Rizzo et al., 2007).
As far as COVID-19-related deaths are concerned, the ISS has set up a specific web platform (similar to InfluNet) for epidemiological and immunological data collection, including recommended procedures for molecular diagnostics. However, as not all COVID-19-related deaths are assumed to have been hospitalized (i.e. some deaths occurred at home or often in healthcare homes), mortality data are presumed to be underestimated. Nonetheless, it is very important to note that the cause of death may not be purely virus-dependent. This circumstance might be more frequently detected in patients with multiple comorbidities (>80% of people who died were affected by ≥2 comorbidities) (National Institute of Health (Istituto Superiore di Sanità, 2020a). Moreover, data pertaining to drugs previously prescribed in patients with chronic clinical conditions (such as high blood pressure, diabetes, ischemic heart disease or COPD) may not be detailed enough to identify those at higher risk of death or having factors increasing the risk itself.
Finally, the frequency of people who are eligible for the flu vaccination who have actually been vaccinated is also missing. However, and most importantly, the National Institute of Statistics (ISTAT) and ISS, in cooperation with the Home Affairs Department (for data retrieved from the National Registry of Resident Population) and the Ministry of Economy and Finance (for deaths data reported in the Tax Registry), are closely working together to disseminate basic information about the COVID-19 pandemic.
Preliminary observations
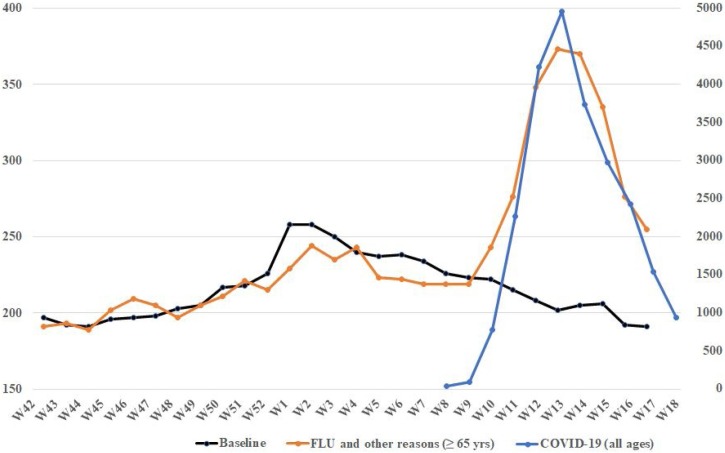
Based on initial integrated data provided by the ISTAT (National Institute of Statistics (ISTAT), 2020) and ISS (National Institute of Health (Istituto Superiore di Sanità, 2020c), the mortality curves associated with ILI (and other causes in people aged ≥65 years) and Covid-19 between October 2019 (week 42) and April 2020 (week 18) were processed (Figure 1). When the shape and trend of curves are examined over time, something immediately jumps out: the two death curves show a completely overlapping profile, although the magnitude of numbers associated with COVID-19 is remarkably greater. This is not surprising. ILI-related deaths have also started growing again. The peak of seasonal flu certainly preceded the pandemic one; however, it would seem clear that the two viruses have been simultaneously circulating in Italy for a while. While some of the excess mortality reported in the ILI group (in those aged ≥65 years) may be attributable to COVID-19 infection, a question arises: do we have to consider this observation as a result of a random sequence of events or a potential relationship between the two viruses play a role?
Figure 1.
Average number of deaths per week in the period October 2019–April 2020.
The measurement scale for COVID-19 is shown on the right of the diagram, for influenza-like illness (ILI) and other death-related reasons on the left. COVID-19 group included all-age patients with a diagnosis confirmed by reverse transcriptase-polymerase chain reaction. The group of deaths associated with ILI and other causes included patients aged ≥65 years. The respective scales for both viruses have been adapted to allow a visual comparative assessment of the curves.
Although they show a different binding affinity for their own specific redundant receptors (i.e. sialic acids of glycoproteins or glycolipids and ACE2 for influenza and COVID-19, respectively) (Matrosovich et al., 1997, Paules et al., 2020), a cellular access pattern with different effectiveness and consequently a diverse pathophysiology, both have an elective tropism for the respiratory tract. Taking viral signaling into consideration, a cooperation mechanism intended at establishing an absolute advantage over the host could also be envisaged (Erez et al., 2017). This system often takes place between different viral strains sharing the same interest in boosting their own reproductive probabilities. An example of effective viral cooperation was found in several oncogenic genotypes of high-risk and low-risk human papillomavirus (Krawczyk et al., 2008). Moreover, virulence also has usually turned out to be based on a cooperative effect between different genes encoding for specific viral capabilities such as transmissibility and fatality (Burrel et al., 2017). Hence, it has become increasingly clear that many viruses actively work together, teaming up to co-infect hosts and neutralize antiviral immune procedures. Although at this stage it is merely a hypothesis that needs further investigation, the simultaneous or sequential infection of both viruses (COVID-19 and influenza) leading to strengthening the effectiveness of the single infection cannot be excluded.
Conclusions
Several data are still missing, data that are needed to estimate the real impact of influenza and COVID-19 on overall mortality. In addition, assuming the potential simultaneous circulation of the two viruses (during the flu season), it is suggested that health authorities undertake a study (or perform a full assessment) specifically aimed at establishing the predominant risk factors of virus-related death.
It would be of great relevance to identify people at very high risk of mortality as a result of an overlapping or combination of risk factors that were separately reported in patients who died from COVID-19 or influenza. Subjects aged ≥65–70 years and suffering from hypertension or diabetes and especially those with metabolic syndrome or with 2–3 comorbidities (mentioned above in the two groups) are likely to be the elective target of prevention programs. This is the reason why a similar investigation is mandatory.
A detailed characterization of patients who died due to virus-related reasons can be performed by cross-linking data stored in different archives or warehouses of the same geographical area (classically a Region). This guarantees high intrinsic quality of the analysis and at the same time reduces the need to use alternative proxies or sources that could introduce a systematic bias. Administrative and demographic databases can be retrospectively queried using a unique code referring to a single individual in order to develop a longitudinal electronic health record with a predetermined time interval. A description of the subgroup of people at higher risk of mortality will be crucial for prioritizing and implementing future public health prevention and treatment programs, such as a mass flu vaccination campaign.
Italy was not as ready to manage such a rapid and dramatic pandemic as many other countries were. The gathering of data and assessment of consequences associated with COVID-19 infections in Italy took some time and appropriate responses may have been slightly delayed (i.e. quarantine and social distancing measures). This may also have contributed to smoothing the viral transmission and worsening some patients clinical conditions. Hopefully, nothing will happen in late autumn, but an integrated pandemic plan for Italy must be prepared as soon as possible.
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding source
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval
This manuscript was submitted in accordance and acceptation of instructions for authors and other conditions posed by the International Journal of Infectious Diseases. The submitted material is original and it has been neither published elsewhere nor submitted for publication simultaneously. Moreover, if accepted, the paper will not be published elsewhere in the same form, in English or in any other language, without written consent of the copyright holder.
References
- National Institute of Health (Istituto Superiore di Sanità) 2020. Characteristics of SARS-CoV-2 patients dying in Italy. Report based on available data on May 28th, 2020. https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_28_May_2020.pdf [last accessed 03 June 2020] [Google Scholar]
- Onder G., Rezza G., Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;23(2020) doi: 10.1001/jama.2020.4683. Published online March. [DOI] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- National Institute of Health (Istituto Superiore di Sanità) 2020. Characteristics of COVID-19 patients dying in Italy.https://www.epicentro.iss.it/coronavirus/sars-cov-2-decessi-italia#2 [Google Scholar]
- Rosano A., Bella A., Gesualdo F., Acampora A., Pezzotti P., Marchetti S. Investigating the impact of influenza on excess mortality in all ages in Italy during recent seasons (2013/14–2016/17 seasons) Int J Infect Dis. 2019;88:127–134. doi: 10.1016/j.ijid.2019.08.003. [DOI] [PubMed] [Google Scholar]
- Ministry of Health . 2020. Prevenzione e controllo dell’influenza: raccomandazioni per la stagione 2019-2020.http://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2019&codLeg=70621&parte=1%20&serie=null [Google Scholar]
- Viboud C., Grais R.F., Lafont B.A., Miller M.A., Simonsen L. Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis. 2005;192:233–248. doi: 10.1086/431150. [DOI] [PubMed] [Google Scholar]
- Dushoff J., Plotkin J.B., Viboud C., Earn D.J., Simonsen L. Mortality due to influenza in the United States - an annualized regression approach using multiple-cause mortality data. Am J Epidemiol. 2006;163:181–187. doi: 10.1093/aje/kwj024. [DOI] [PubMed] [Google Scholar]
- Rizzo C., Bella A., Viboud C., Simonsen L., Miller M.A., Rota M.C. Trends for Influenza-related deaths during Pandemic and Epidemic Seasons, Italy, 1969-2001. Emerg Infect Dis. 2007;13(5):694–699. doi: 10.3201/eid1305.061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Statistics (ISTAT) 2020. Impatto dell’epidemia COVID-19 sulla mortalità totale della popolazione residente. Primo quadrimestre 2020.https://www.istat.it/it/files//2020/06/Rapp_Istat_Iss_3Giugno.pdf Full text available for download from the ISTAT website: [Google Scholar]
- National Institute of Health (Istituto Superiore di Sanità) 2020. FluNews – Italia. Rapporto della sorveglianza integrata dell’influenza. Stagione 2019/2020.https://www.epicentro.iss.it/influenza/flunews#mortalita [Google Scholar]
- Matrosovich M.N., Gambarayan A.S., Teneberg S., Piskarev V.E., Yamnikova S.S., Lvov D.K. Avian influenza A viruses differ from human viruses by recognition of sialyloligosaccharides and gangliosides and by a higher conservation of the HA receptor-binding site. Virology. 1997;233(1):224–234. doi: 10.1006/viro.1997.8580. [DOI] [PubMed] [Google Scholar]
- Paules C.I., Marston H.D., Fauci A.S. Coronavirus Infections - More Than Just the Common Cold. JAMA. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- Erez Z., Steinberger-Levy I., Shamir M., Doron S., Stokar-Avihail A., Peleg Y. Communication between viruses guides lysis-lysogeny decisions. Nature. 2017;541(7638):488–493. doi: 10.1038/nature21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk E., Suprynowicz F.A., Liu X., Dai Y., Hartmann D.P., Hanover J. Koilocytosis: a cooperative interaction between the human papillomavirus E5 and E6 oncoproteins. Am J Pathol. 2008;173:682–688. doi: 10.2353/ajpath.2008.080280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrel C.J., Howard C.R., Murphy F.A. 5th ed. Elsevier; London: 2017. Fenner and White’s Medical Virology; pp. 101–102. [Google Scholar]