Highlights
-
•
Molecular detection of SARS-CoV-2 is critical in the diagnosis of COVID-19.
-
•
The persistence of SARS-CoV-2 RNA shedding in the context of clinical features and comorbidities is understudied.
-
•
The cumulative cessation of viral shedding rate at 3 weeks from symptom-onset is 44%.
-
•
Repeating a SARS-CoV-2 PCR test within 21 days of a laboratory-confirmed COVID-19 diagnosis is considered unnecessary.
Keywords: Viral RNA shedding, COVID-19, SARSCo-V-2, PCR testing
Abstract
Background
Molecular detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is key in the diagnosis of coronavirus disease 2019 (COVID-19) and has been widely used for followup of cases as a proxy for contagiousness. The persistence of SARS-CoV-2 RNA shedding in the context of clinical features and comorbidities is understudied.
Methods
We retrospectively reviewed laboratory-confirmed COVID-19 adult symptomatic cases at Mayo Clinic, eventually achieving cessation of viral RNA shedding (CVS), defined as two consecutive negative SARS-CoV-2 PCR results on nasopharyngeal swabs collected at least 24 h apart.
Results
A total of 251 patients were included, median age was 53 years and 59 % female. The most common symptoms at diagnosis were cough, myalgia, dyspnea, fever and chills. Myalgia, cough, anosmia, ageusia and sore throat were common at CVS, but fever and dyspnea were not observed. The median time from symptom onset to CVS was 23 days, and did not differ by symptoms. The weekly cumulative CVS rate was 2, 14, 44, 73, 91 and 95 % at 1–6 weeks from symptom onset, respectively. Cough and fever were associated with a positive PCR test if tested within 2 weeks of symptoms (P < 0.05). Patients with asthma or immunosuppression were less likely to achieve CVS if tested 3 weeks into symptoms (P < 0.04).
Conclusions
The cumulative CVS rate at 3 weeks from symptom-onset is 44 % in our entire cohort. The findings of our study highlight the low yield of repeating a SARS-CoV-2 NP PCR test within 21 days of a laboratoryconfirmed COVID-19 diagnosis.
1. Background
The COVID-19 pandemic, caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), continues to expand after first being reported in China in December 2019 [1]. Clinical presentation is heterogeneous, with fever, cough, and fatigue being the most common clinical symptoms [2,3]. Given the challenges in defining this clinically, the World Health Organization has defined COVID-19 as any individual with a positive polymerase chain reaction (PCR) test regardless of symptoms [4] and this has led to reliance on PCR for the diagnosis and management of COVID-19.
There is growing evidence demonstrating that viral shedding begins before a patient is symptomatic, peaks at or shortly after symptom onset, and persists beyond symptom resolution [5,6]. It is known that asymptomatic viral shedding enhances the transmissibility of SARS-CoV-2 [7]. Studies on SARS-CoV-2 persistence report a median time from symptom onset to persistently negative SARS-CoV-2 PCR tests ranging from 11 to 31 days [6,[8], [9], [10], [11], [12], [13]] with positive tests found as late as 55 days from symptom onset [14]. Severe disease, in particular, has been associated with prolonged viral RNA detection in several reports [[15], [16], [17], [18]], and the presence of dyspnea, hypoxia, and tachypnea has also been associated with prolonged shedding in respiratory samples [11]. There have been questions as to whether this persistent RNA detection represents viable virus, and therefore, transmission risk.
Understanding viral dynamics of SARS-CoV-2 shedding in relation to symptom evolution and other underlying clinical factors has direct implications in diagnostic stewardship, implementation of effective infection prevention measures, and the development of public health strategies. Here, we aimed to evaluate clinical features as predictors for the cessation of SARS-CoV-2 RNA detection in a cohort of laboratory-confirmed COVID-19 cases.
2. Methods
We retrospectively reviewed laboratory-confirmed COVID-19 adult symptomatic cases who had a positive initial SARS-CoV-2 PCR nasopharyngeal (NP) swab and two subsequent negative SARS-CoV-2 PCR NP swab test results at Mayo Clinic, Rochester from February 1, 2020, through May 15, 2020. We excluded deceased patients to capture those who achieved viral RNA clearance. Other sample sources (e.g., bronchoalveolar lavage, other respiratory samples) were excluded to avoid testing variability. Cessation of viral RNA shedding (CVS) was considered to have been achieved on the date of the first of two consecutive negative SARS-CoV-2 PCR results on NP swabs collected at least 24 h apart.
Demographic, clinical, and laboratory data from each patient were extracted from the electronic health record. Date of symptom onset, symptoms at diagnosis, date of first and subsequent positive SARS-CoV-2 PCR tests, and symptoms at the time of CVS was recorded. Symptoms were recorded based on the current Centers for Disease Control and Prevention (CDC) case definition [19]. Immunocompromised patients were defined as solid organ transplant recipients, patients who had an active hematologic malignancy, and/or who were on anti-neoplastic chemotherapy, immunomodulators, or other immunosuppressive drugs. Fever was defined as a temperature > 38.5 °C. Ambulatory cases were defined as those cases that were not hospitalized during the course of the illness. The study was approved by the Mayo Clinic Institutional Review Board (IRB ID: 20-002629).
SARS-CoV-2 PCR testing was performed using SARS-CoV-2 qualitative assays using the cobas 6800 / 8800 systems (Roche Molecular Systems), the Abbott m2000 system (Abbott) or a SARS-CoV-2 laboratory-developed test that has received emergency use authorization from the Food and Drug Administration [20].
Categorical variables were reported as counts and percentages using Chi-square or Fisher’s exact test for comparison, as appropriate. Continuous variables were reported as mean and standard deviations, or medians and interquartile ranges (IQR) from the 25th to the 75th percentiles and compared using t-test or the Wilcoxon rank-sum test, as deemed appropriate. The difference in symptom prevalence at diagnosis and at CVS was tested using McNemar’s test. Logistical regression was applied to assess for associations between symptoms, risk factors, and CVS. A log-rank test was applied for time-to-event analysis for CVS and clinical features. Statistical tests were two-tailed with p < 0.05 considered significant. All analyses were performed using the R Project (R Foundation for Statistical Computing, Austria).
3. Results
A total of 1641 patients had a positive SARS-CoV-2 PCR test at our institution during the study period. Of these, 251 (15.2 %) were adults with a positive SARS-CoV-2 PCR on an NP swab followed by CVS. For this group of patients, a total of 1101 SARS-CoV-2 PCR tests were performed and reviewed, with a median of 4 tests (IQR 2) performed per patient.
3.1. Baseline characteristics
Patient demographics and comorbidities are summarized in Table 1 . The median patient age was 53 (IQR 27) years; 59 % were female. The most common underlying medical conditions were obesity (29.9 %), coronary artery disease (CAD, 27.9 %), asthma (18.3 %), diabetes mellitus (DM, 13.5 %), chronic obstructive pulmonary disease (COPD, 8.8 %) and chronic kidney disease (CKD, 8%). Twenty-two (8.8 %) patients were categorized as immunocompromised, and 89 (35.5 %) had no clinically relevant medical history.
Table 1.
Clinical characteristics and timing of PCR testing for patients with COVID-19.
| Variables | Ambulatory (N = 189) | Hospitalized (N = 62) | Total (N = 251) | P-value |
|---|---|---|---|---|
| Age, years | ||||
| Median (IQR) | 47 (27) | 60 (16.8) | 53 (27) | < 0.001 |
| Sex | ||||
| Male | 73 (38.6 %) | 30 (48.4 %) | 103 (41.0 %) | 0.175 |
| Female | 116 (61.4 %) | 32 (51.6 %) | 148 (59.0 %) | |
| Comorbidities and risk factors | ||||
| Obesity | 43 (22.8 %) | 32 (51.6 %) | 75 (29.9 %) | < 0.001 |
| Coronary artery disease | 38 (20.1 %) | 32 (51.6 %) | 70 (27.9 %) | < 0.001 |
| Asthma | 34 (18.0 %) | 12 (19.4 %) | 46 (18.3 %) | 0.809 |
| Diabetes mellitus | 13 (6.9 %) | 21 (33.9 %) | 34 (13.5 %) | < 0.001 |
| Chronic obstructive pulmonary disease | 11 (5.8 %) | 11 (17.7 %) | 22 (8.8 %) | 0.004 |
| Chronic kidney disease | 10 (5.3 %) | 10 (16.1 %) | 20 (8.0 %) | 0.006 |
| Use of anti-neoplastic chemotherapy, immunomodulators, or immunosuppressive drugs | 7 (3.7 %) | 9 (14.5 %) | 16 (6.4 %) | 0.002 |
| Congestive heart failure | 4 (2.1 %) | 5 (8.1 %) | 9 (3.6 %) | 0.029 |
| Solid organ transplant recipient | 0 (0.0 %) | 3 (4.8 %) | 3 (1.2 %) | 0.002 |
| Active hematologic malignancy or peripheral blood stem cell transplant recipient | 1 (0.5 %) | 2 (3.2 %) | 3 (1.2 %) | 0.09 |
| Cystic fibrosis | 1 (0.5 %) | 0 (0.0 %) | 1 (0.4 %) | 0.566 |
| None | 83 (43.9 %) | 6 (9.7 %) | 89 (35.5 %) | < 0.001 |
| Median time in days from symptoms to first positive PCR (IQR) | 3 (6) | 3 (5.8) | 3 (6) | 0.254 |
| Median time in days from symptoms to cessation of viral shedding (IQR) | 22 (12) | 24 (11.8) | 23 (12) | 0.663 |
| Median time in days from first positive PCR to cessation of viral shedding (IQR) | 17 (11) | 18 (11) | 17 (11) | 0.343 |
Abbreviations: IQR, interquartile range; PCR, polymerase chain reaction.
A total of 62 (24.7 %) patients were hospitalized, and comprised an older group of patients when compared to ambulatory cases (60 [IQR 16.8] years vs. 47 [IQR 27] years [P < 0.001]). They also had a significantly higher prevalence of comorbidities, namely obesity (51.6 vs 22.8 %; P < 0.001), CAD (51.6 vs 20.1 %; P < 0.001), DM (33.9 vs 6.9 %; P < 0.001), COPD (17.7 vs 5.8 %; P = 0.004), CKD (16.1 vs 5.3 %; P = 0.006), CHF (8.1 vs 2.1 %; P = 0.029) and immunocompromised status (22.6 vs 4.2 %; P < 0.001). Only 6 (9.7 %) hospitalized patients had no medical comorbidities compared to the ambulatory cases (43.9 %; P < 0.001).
3.2. Symptoms at initial laboratory diagnosis and at CVS
Symptoms at initial laboratory diagnosis and at CVS are summarized in Table 2 . At the time of laboratory diagnosis, the most prevalent symptoms among all patients were cough (84.1 %), myalgia (66.1 %), dyspnea (53.0 %), fever (50.2 %), and chills (49.0 %).
Table 2.
Prevalence of symptoms at diagnosis and at cessation of viral RNA shedding (CVS) for patients with COVID-19.
| Variables | Symptoms at diagnosis (N = 251) | Symptoms at CVS (N = 251) | P-valuea | Median time to CVS (IQR) |
|---|---|---|---|---|
| Cough | 154 (81.5 %) | 39 (20.6 %) | 0.0376 | 24 (17−29.5) |
| Dyspnea | 80 (42.3 %) | 0 (0.0 %) | <0.0001 | 23 (17−29) |
| Fever (T > 38.5C) | 92 (48.7 %) | 0 (0.0 %) | <0.0001 | 23 (19−29) |
| Subjective fever | 37 (19.6 %) | 0 (0.0 %) | <0.0001 | 21 (15−28.5) |
| Chills | 86 (45.5 %) | 1 (0.5 %) | <0.0001 | 23 (17−29) |
| Shivering | 1 (0.5 %) | 0 (0.0 %) | <0.0001 | 43 (43−43) |
| Muscle pain | 114 (60.3 %) | 48 (25.4 %) | 0.58 | 23 (17−29.8) |
| Diarrhea | 45 (23.8 %) | 1 (0.5 %) | <0.0001 | 24 (17−30) |
| Headache | 72 (38.1 %) | 12 (6.3 %) | <0.0001 | 23 (17−30) |
| Sore throat | 79 (41.8 %) | 34 (18.0 %) | <0.0001 | 23 (17−29) |
| Ageusia | 41 (21.7 %) | 38 (20.1 %) | <0.0001 | 24 (18−28.5) |
| Anosmia | 45 (23.8 %) | 41 (21.7 %) | <0.0001 | 23 (17−27.5) |
| None | 0 (0%) | 56 (29.6 %) | – | – |
| Unknown | 0 (0%) | 11 (5.8 %) | – | – |
| Dizziness | 28 (14.8 %) | 10 (5.3 %) | <0.0001 | 24 (17−30) |
McNemar's test for dependent proportions.
There was a higher prevalence of dyspnea (85.5 vs. 42.3 %; P=<0.001), myalgia (83.9 vs. 60.3 %; P=<0.001), diarrhea (43.5 vs 23.8 %; P = 0.003) and dizziness (46.8 vs. 14.8 %; P=<0.001) in the hospitalized compared to the ambulatory cases. The presence of cough (91.9 vs. 81.5 %; P = 0.051), fever (54.8 vs. 48.7 %; P = 0.4), and chills (59.7 vs. 45.5 %; P = 0.053) was also higher, but this difference was not statistically significant.
At the time of CVS, 56 (29.6 %) patients were asymptomatic. The most common symptoms that persisted at CVS were myalgia (25.4 %; P = 0.58), cough (20.6 %; P = 0.03), anosmia (21.7 %; P=<0.0001), ageusia (20.1 %; P=<0.0001), and sore throat (18 %; P=<0.0001). The prevalence of all symptoms was significantly lower at the time of CVS compared to the time of diagnosis, with the exception of myalgia, prevalence of which was lower but with a difference that did not reach statistical significance. Notably, none of the 251 patients reported objective fever, subjective fever, or dyspnea at the time of CVS, and only one patient reported chills.
3.3. Temporal relationship between PCR test results and symptoms
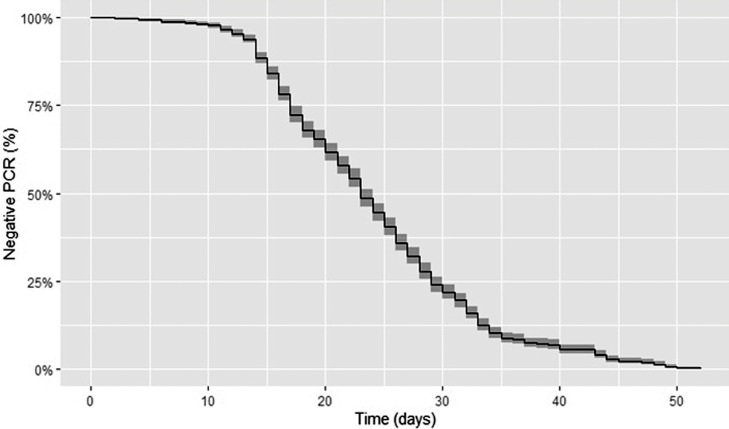
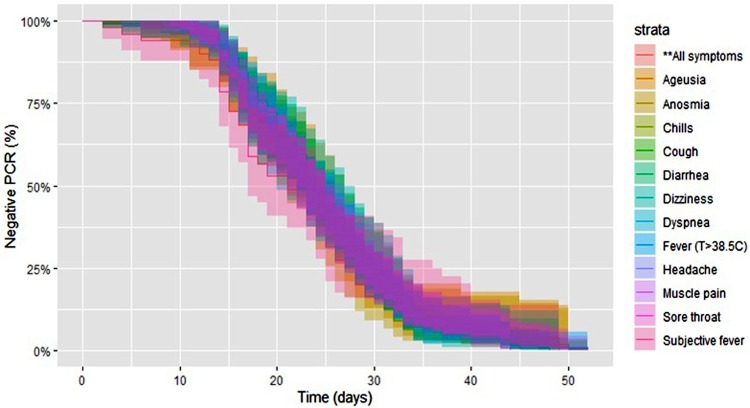
The median time from symptom onset to the first positive PCR test result was 3 (IQR 6) days. The median time from symptom onset to CVS was 23 (IQR 12) days, and this did not differ significantly when stratified by symptom (P = 0.76; Fig. 1, Fig. 2 ). The median time from the first positive PCR to CVS was 17 (IQR 11) days, and this also did not differ significantly by symptom. The cumulative CVS rate at different intervals from symptom onset in all patients was 1.6 % at 1 week, 13.5 % at 2 weeks, 43.8 % at 3 weeks, 73.3 % at 4 weeks, 90.8 % at 5 weeks and 94.8 % at 6 weeks. The cumulative CVS rate for patients tested within 1 week of symptom onset (n = 190) was 2.1 % at the first week, 16.8 % at 2 weeks, 53.2 % at 3 weeks, 81.6 % at 4 weeks, 96.3 % at 5 weeks and 97.9 % at 6 weeks.
Fig. 1.
Kaplan Meier curve of negative PCR test results after initial symptoms.
Fig. 2.
Kaplan Meier curve of negative PCR test results after symptom onset by symptoms.
3.4. Initial symptoms as predictors of CVS
Patients with cough were less likely to achieve CVS when a PCR test was repeated within 1 week of symptom onset (OR 0.0746, 95 % CI 0.00363 to 0.602 [P = 0.026]) and similarly, patient with fever at the time of diagnosis were less likely to achieve CVS when a PCR was repeated within 2 weeks of symptom onset (OR 0.41, 95 % CI 0.169 to 0.958 [P = 0.043]).
In contrast, patients with dyspnea at the time of diagnosis were more likely to achieve CVS if tested at week 3 of symptoms onset (OR 2.21, 95 % CI 1.178–4.203 [P = 0.014]). Anosmia at the time of COVID-19 diagnosis was also associated with CVS if PCR was performed at week 4 post symptom onset (OR 3.43, 95 % CI 1.181–12.5 [P = 0.036]). Patients with fever, chills, and dizziness at the time of diagnosis were more likely to achieve CVS at week 5 of symptoms (OR 5.17, 95 % CI 1.673–17.62 [P = 0.0057]; OR 3.68, 95 % CI 1.184–13.07 [P = 0.031]; OR 8.4, 95 % CI 1.49–158.6 [P = 0.048], respectively)
3.5. Risk factor as predictors for CVS
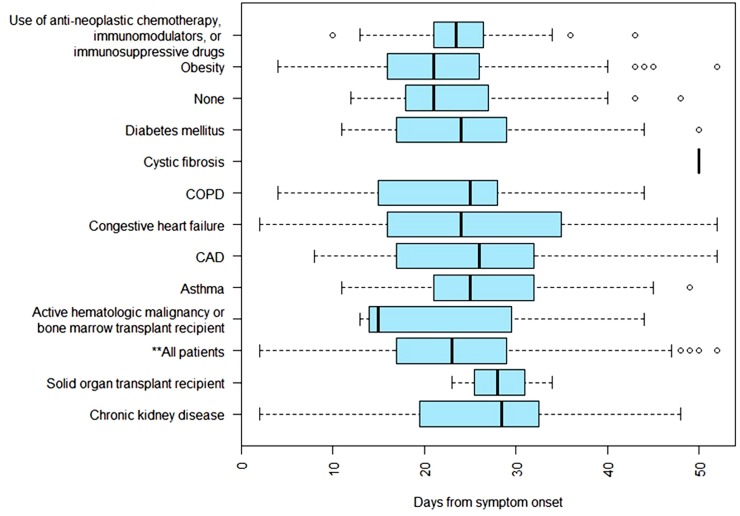
Patients with asthma or those receiving immunosuppressive drugs were less likely to achieve CVS if tested at week 3 post symptom onset (OR 0.11, 95 % CI 0.00587–0.605 [P = 0.038]; OR 0.289, 95 % CI 0.1–0.73 [P = 0.013], respectively). Obesity was associated with CVS if tested at week 3 of symptoms (OR 2.563, 95 % CI 1.233–5.51 [P = 0.013]). No other association between risk factors and CVS was found (Fig. 3 ).
Fig. 3.
Box Plot of median time to cessation of viral RNA shedding from symptom onset by risk factors.
4. Discussion
In our study, the cumulative CVS rate at 2 weeks from symptom onset was 13.5 %, and increased to 43.8 % at 3 weeks post symptom onset, suggesting that testing after 3 weeks of symptoms might have a greater CVS yield. Patients with cough and fever at diagnosis were more likely to have a persistently positive SARS-CoV-2 PCR result if tested within 2 weeks of symptom onset. Similarly, patients with asthma and immunosuppression were less likely to achieve CVS is tested within 3 weeks from symptom onset, indicating an association between viral RNA shedding in these populations within 3 weeks of symptoms. It is not known, however, if this persistent viral RNA shedding translates into persistent infectivity.
Symptoms associated with higher odds for CVS included dyspnea and anosmia if tested at 3 and 4 weeks from symptom onset, respectively. Similar results were demonstrated when initial symptoms of fever, chills, and dizziness were tested at week 5 from symptom onset. There were no symptoms or comorbidities associated with CVS before 3 weeks of symptom onset, suggesting that testing for CVS before 3 weeks of symptom onset might not be warranted, regardless of symptoms or comorbidities.
Cough, sore throat, and myalgia were the most prevalent persistent symptoms at CVS, all of which are the most commonly reported symptoms associated with the post-infectious syndrome (PIS) [21,22], a phenomenon that was first described by Bannister et.al. PIS is thought to be a clinical manifestation of persistent local and systemic immunologic derangements following the complex molecular interplay between a respiratory virus and the host immune system [23]. We speculate that the persistence of symptoms in our cohort at the time of CVS may be, at least in part, explained by the PIS.
De Chang et al. reported half of their cases (8/16) remained PCR positive even after the resolution of symptoms (median 2.5 days); furthermore, the timeline of SARS-CoV-2 PCR positivity and negativity is different in specimens other than NP swabs [5]. Wang W. et al. reported that PCR test positivity was higher in bronchoalveolar lavage specimens (93 %), followed by sputum (72 %), nasal (63 %), and pharyngeal swabs (32 %) [24]. In our study, all SARS-CoV-2 PCR tests were obtained from NP swabs; false-negative follow-up test results that may have occurred due to inappropriate timing of sample collection from symptom onset. Deficiencies in sampling technique cannot be ruled out.
Viral infections in an immunocompromised host are associated with severe disease at higher rates with a significant impact on the morbidity and mortality than in the healthy population [25]. Disease manifestations are dependent on the specific virus, and the type and duration of immunosuppression. Our study demonstrated that patients with risk factors including solid organ transplant recipients, those with active hematologic malignancy, and/or those receiving anti-neoplastic chemotherapy, immunomodulators, or other immunosuppressive drugs were less likely to achieve CVS if tested within 3 weeks from symptom onset than those without these conditions.
Although we did not have data on viral shedding before symptom onset in our cohort, viral RNA became detectable as early as the same day of symptom onset (median of 3 days) and became undetectable at a median time of 23 days. Patients had a median time from positive to negative PCR of 17 days. Our findings are consistent with previous reports, where for most individuals with symptomatic COVID-19 infection, viral RNA became detectable as early as day 1 of symptoms and peaked within the first week of symptom onset. Zhou, F. et al. reported on the persistence of viral RNA for a median of 20 days after symptom onset [13]. Sethuraman et al. reported that SARS-CoV-2 PCR test positivity starts to decline by week three and eventually becomes undetectable [26]. Many individualized, institution-specific testing protocols require follow-up PCR testing beginning day 7 from symptom onset for disease tracking purposes, and the CDC indicates that health care workers can return to work if at least 10 days have passed since symptom onset. On the contrary, Gombar, et al. suggested that return to work and contact precaution guidelines should assume viral shedding for 33 days following symptom onset [27]. In our study, patients achieved CVS at a median of 23 days (IQR 12). This finding presumably correlates with the decline of infectivity by week 3, validating the CDC recommendations and the results of Sethuraman et al. and Gombar et al. Of note, 70.4 % of our patient population was symptomatic at CVS.
In a rapidly expanding epidemic, the approach to contact tracing and quarantine is challenging. The majority of our patient population did not require hospitalization, and this significant difference may be due to the patients’ age and absence of significant comorbidities as risk factors when compared to the hospitalized cases. The current data is derived from mostly young, healthy, and non-immunocompromised subjects, which is consistent with the previous report from Chang D. et al. [28] With a substantial proportion of undefined pre-symptomatic transmission, measures such as enhanced personal hygiene and social distancing are likely to be key instruments for community disease control.
5. Limitations
The primary limitation is the retrospective nature of this study and the symptom onset relying on patient recall upon confirmation of COVID-19, thus making it difficult to establish a clear timeline for CVS. All specimens were obtained from NP swabs; having a negative result does not rule out the presence of viral RNA. Finally, PCR tests can detect non-viable virus, and thus the prolonged RNA shedding may not correlate to persistence of infectious virus.
6. Conclusion
Patients with cough and/or fever at the time of laboratory-confirmed COVID-19 disease are more likely to have a persistent SARS-CoV-2 PCR test result if tested within 2 weeks of symptom onset than those without cough and/or fever. Those with asthma or receiving immunosuppression are less likely to achieve CVS within 3 weeks of symptom onset than those without these conditions. Patients with fever, chills and cough are more likely to achieve CVS at week 5 of symptom onset than those without the aforementioned symptoms. The findings of our study highlight the low yield of repeating a SARS-CoV-2 PCR test on an NP swab within 21 days of a laboratory-confirmed COVID-19 diagnosis to document CVS.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Cristina Corsini Campioli: Visualization, Conceptualization, Investigation, Methodology, Data curation, Writing - original draft. Edison Cano Cevallos: Conceptualization, Data curation, Writing - original draft, Writing - review & editing, Software. Mariam Assi: Conceptualization, Investigation. Robin Patel: Writing - review & editing, Supervision. Matthew J. Binnicker: Writing - review & editing, Supervision. John C. O’Horo: Visualization, Data curation, Writing - review & editing, Supervision.
Declaration of Competing Interest
None.
References
- 1.Organization WH . 2020. Novel Coronavirus Situation Report. [Google Scholar]
- 2.Addi R.A., Benksim A., Amine M., Cherkaoui M. Asymptomatic COVID-19 infection management: the key to stop COVID-19. J. Clin. Exp. Investig. 2020;11 [Google Scholar]
- 3.Fu L., Wang B., Yuan T., Chen X., Ao Y., Fitzpatrick T. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J. Infect. 2020;80:656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organization WH . 2020. Global Surveillance for Human Infection with Coronavirus Disease (COVID-19) [Google Scholar]
- 5.Chang D., Mo G., Yuan X., Tao Y., Peng X., Wang F.-S. Time kinetics of viral clearance and resolution of symptoms in novel coronavirus infection. Am. J. Respir. Crit. Care Med. 2020;201:1150–1152. doi: 10.1164/rccm.202003-0524LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles’ heel of current strategies to control COVID-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J., Qi T., Liu L., Ling Y., Qian Z., Li T. Clinical progression of patients with COVID-19 in Shanghai. China. J Infect. 2020;80:e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Y., Han P., Zhu R., Bai T., Yi J., Zhao X. Risk factors for viral RNA shedding in COVID-19 patients. Eur. Respir. J. 2020 doi: 10.1183/13993003.01190-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo I.L., Lio C.F., Cheong H.H., Lei C.I., Cheong T.H., Zhong X. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int. J. Biol. Sci. 2020;16:1698–1707. doi: 10.7150/ijbs.45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou B., She J., Wang Y., Ma X. The duration of viral shedding of discharged patients with severe COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y., Chen S., Yang Z., Guan W., Liu D., Lin Z. SARS-CoV-2 viral load in clinical samples of critically ill patients. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202003-0572LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin A., He Z.-B., Zhang J.-G., Zhang X., Yan W.-H. Early risk factors for the duration of SARS-CoV-2 viral positivity in COVID-19 patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi L., Yang Y., Jiang D., Tu C., Wan L., Chen X. Factors associated with duration of viral shedding in adults with COVID-19 outside of Wuhan, China: a retrospective cohort study. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu Y.-H., Wei Y.-Y., Zhang D.-W., Chen C.-S., Hu X.-W., Fei G. 2020. Analysis of Factors Affected the SARS-CoV-2 Viral Shedding Time of COVID-19 Patients in Anhui, China: a Retrospective Study. [Google Scholar]
- 18.Xu K., Chen Y., Yuan J., Yi P., Ding C., Wu W. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prevention CfDCa . 2020. Symptoms of Coronavirus. [Google Scholar]
- 20.Administration USFaD . 2020. Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency (Revised) [Google Scholar]
- 21.Braman S.S. Postinfectious cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:138S–146S. doi: 10.1378/chest.129.1_suppl.138S. [DOI] [PubMed] [Google Scholar]
- 22.Yamato M., Kataoka Y. Fatigue sensation following peripheral viral infection is triggered by neuroinflammation: who will answer these questions? Neural Regeneration Res. 2015;10:203–204. doi: 10.4103/1673-5374.152369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bannister B.A. Post-infectious disease syndrome. Postgrad. Med. J. 1988;64:559–567. doi: 10.1136/pgmj.64.753.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Englund J., Feuchtinger T., Ljungman P. Viral infections in immunocompromised patients. Biol. Blood Marrow Transplant. 2011;17:S2–S5. doi: 10.1016/j.bbmt.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020 doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 27.Gombar S., Chang M., Hogan C.A., Zehnder J., Boyd S., Pinsky B.A. Persistent detection of SARS-CoV-2 RNA in patients and healthcare workers with COVID-19. J. Clin. Virol. 2020;129 doi: 10.1016/j.jcv.2020.104477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang D., Lin M., Wei L., Xie L., Zhu G., Dela Cruz C.S. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323:1092. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]