Abstract
Background
An increased rate of thrombotic events has been associated to Coronavirus Disease 19 (COVID-19) with a variable rate of acute stroke. Our aim is to uncover the rate of acute stroke in COVID-19 patients and identify those cases in which a possible causative relationship could exist.
Methods
We performed a single-center analysis of a prospective mandatory database. We studied all patients with confirmed COVID-19 and stroke diagnoses from March 2nd to April 30th. Demographic, clinical, and imaging data were prospectively collected. Final diagnosis was determined after full diagnostic work-up unless impossible due to death.
Results
Of 2050 patients with confirmed SARS-CoV-2 infection, 21 (1.02%) presented an acute ischemic stroke 21 and 4 (0.2%) suffered an intracranial hemorrhage. After the diagnostic work-up, in 60.0% ischemic and all hemorrhagic strokes patients an etiology non-related with COVID-19 was identified. Only in 6 patients the stroke cause was considered possibly related to COVID-19, all of them required mechanical ventilation before stroke onset. Ten patients underwent endovascular treatment; compared with patients who underwent EVT in the same period, COVID-19 was an independent predictor of in-hospital mortality (50% versus 15%; Odds Ratio, 6.67; 95% CI, 1.1-40.4; p 0.04).
Conclusions
The presence of acute stroke in patients with COVID-19 was below 2% and most of them previously presented established stroke risk factors. Without other potential cause, stroke was an uncommon complication and exclusive of patients with a severe pulmonary injury. The presence of COVID-19 in patients who underwent EVT was an independent predictor of in-hospital mortality.
Keywords: COVID-19, Acute stroke, Critical care, Pandemic
Introduction
Since December 2019, when the first case of Coronavirus Disease 2019 (COVID-19), caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was identified in Wuhan (China), an increasing number of people have been diagnosed with the disease (https://covid19.who.int/). This prompted World Health Organization to declare the pandemic on March 11th and several countries have established quarantine policies.
In COVID-19 hospitalized patients, acute stroke has been observed in 1% to 2.5% of cases with high in-hospital mortality rate.1, 2, 3 Although COVID-19 pandemic has produced an enormous collateral damage over stroke systems of care leading to a drop of mild strokes admissions and late arrival of severe strokes, only incidental cases of large vessel occlusion (LVO) in young adults infected by SARS-CoV-2 have been reported without a clear causative relationship.4 The presence of antiphospholipid antibodies5 and the endothelial cell dysfunction6 have been proposed as possible mechanisms that could induce a stroke in COVID-19 patients.
An increased rate of thrombotic events,7 , 8 mainly venous thromboembolism and acute pulmonary embolism have been associated with COVID-19. The infection may cause an hypercoagulable state supported by the presence of disseminated intravascular coagulation in most deaths9 and the results of autopsy reports.10
The presence of SARS-CoV-2 infection has been associated with worse functional outcome and higher mortality among patients with acute stroke;11 in parallel, history of stroke has also been associated with more severe clinical symptoms and poorer outcomes in patients with COVID-19.12
Our aim is to uncover the rate of acute stroke in COVID-19 patients admitted in a high-volume center and identify those cases in which a possible causative relationship could exist.
Methods
Ethics approval was obtained from Hospital Universitari Vall d'Hebron institutional review board (PR(AG)237/2020). No specific investigational measures were applied for the purpose of this study. Written informed consent was waived due the retrospective nature of the study. The data that support the findings of this study are available from the corresponding author on reasonable request.
Starting March 2nd 2020, all patients admitted to our institution were clinically screened for COVID-19 and a respiratory sample was obtained depending on clinical suspicion. We performed a single-center retrospective analysis of a prospective mandatory database that includes all stroke patients diagnosed in our institution. We studied all patients with confirmed COVID-19 and stroke diagnoses from March 2nd to April 30th. Demographic, clinical, and imaging data were prospectively collected. Among stroke data, National Institutes of Health Stroke Scale (NIHSS) score and prestroke modified Rankin Scale (mRS) score were assigned by the stroke neurologist on call.
Final diagnosis and Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification was determined after full diagnostic work-up according to ESO guidelines unless impossible due to death. The diagnostic work-up included a 12-lead ECG on admission, parenchymal and vascular neuroimaging (computed tomography or magnetic resonance imaging), continuous ECG monitoring for at least 72 hours and transthoracic or transesophageal echocardiogram. We defined a stroke as cryptogenic when after full work-up there was no sufficient cause.
Recorded imaging variables included Alberta Stroke Program Early CT Score (ASPECTS), presence of a LVO (extracranial internal carotid artery, intracranial internal carotid artery, M1 and M2 segments of middle cerebral artery, basilar artery, A1 segment of anterior cerebral artery, P1 segment of posterior cerebral artery) and presence of an intracranial hemorrhage (ICH); these variables were determined by the local neuroradiologist. Workflow times including onset, arrival, imaging, and groin puncture were also recorded.
Patients received intravenous-tPA (tissue-type plasminogen activator) and endovascular treatment when indicated independently of COVID-19 diagnosis according to current guidelines. Endovascular procedures were performed by experienced interventionalists using commercially available stent retrievers and aspiration catheters. At the end of the procedure, recanalization was assessed according to modified Thrombolysis in Cerebral Infarction (TICI);13 successful recanalization was considered if the score was 2b or 3. We explored baseline and prognostic differences between patients who underwent EVT in the same period in function of SARS-CoV-2 status.
SARS-CoV-2 infection was considered when confirmed by real-time reverse transcription polymerase chain reaction (RT-PCR) assay on respiratory samples obtained in the two weeks prior to stroke onset or during hospital admission. COVID-19 symptoms, clinical signs, laboratory findings, chest imaging (radiography or CT scan) findings were collected by trained physicians. Severe COVID-19 was defined as1 patients requiring ICU admission,2 acute respiratory distress syndrome criteria defined as low oxygen saturation by pulse-oximetry correlated to the inspired fraction of oxygen (SpO2/FiO2 ratio < 315)3 or death due to acute respiratory failure.
Statistical analysis
We obtained descriptive and frequency statistical analyses using SPSS V.23.0 software. Shapiro-Wilk test was used to assure normality of continuous variables. Categorical variables were presented as absolute values and percentages and continuous variables as median (interquartile range (IQR)) or means (± standard deviation (SD)) as indicated. Statistical significance for intergroup differences was assessed by Pearson χ2 test or Fisher exact test for categorical variables and by Mann-Whitney U test or Student t test as indicated for continuous variables.
Multivariable logistic regression analyses were used to determine factors that could be considered as independent predictors of good functional outcomes. The analyses were adjusted using the variables that previously were shown statistical trends or differences between groups. The OR along with its 95% confidence interval based on logistic regression was reported. A p-value <0.05 was considered statistically significant.
Results
From March 2nd to April 30th, 2050 patients were admitted to our center with RT-PCR confirmed SARS-CoV-2 infection; of them 21 (1.02%) presented an acute ischemic stroke 21 and 4 (0.2%) suffered an ICH. Demographic and clinical data are shown in Table 1 .
Table 1.
Demographic and clinical data of patients with stroke possibly related and unrelated to COVID-19. COVID-19 indicates Coronavirus Disease 19; SD, standard deviation; IQR, interquartile range; ICU, intensive care unit; NIHSS, National Institutes of Health Stroke Scale; iv-tPA, intravenous tissue-type plasminogen activator; EVT, endovascular treatment.
| All stroke (N = 25) | Stroke unknown/infrequent etiology (N = 9) | Stroke known etiology (N = 16) | p value | |
|---|---|---|---|---|
| Age (mean, SD) | 66.5 (15.2) | 56.6 (17.3) | 72.1 (10.7) | 0.01 |
| Gender (male) | 14 (56.0%) | 4 (44.4%) | 10 (62.5%) | 0.38 |
| prestroke mRS (median, IQR) | 1 (0-2) | 0 (0-1) | 1 (0-2) | 0.22 |
| Vascular risk factors | ||||
|---|---|---|---|---|
| Hypertension | 14 (56.0%) | 5 (55.6%) | 9 (56.3%) | 0.97 |
| Diabetes mellitus | 9 (36.0%) | 3 (33.3%) | 6 (37.5%) | 0.83 |
| Hyperlipidemia | 11 (44.0%) | 3 (33.3%) | 8 (50.0%) | 0.42 |
| Current smoker | 8 (32.0%) | 3 (33.3%) | 5 (31.3%) | 0.92 |
| Atrial fibrillation | 4 (16.0%) | 0 | 4 (25.0%) | 0.1 |
| Obesity | 6 (24.0%) | 3 (33.3%) | 3 (18.8%) | 0.41 |
| COVID-19 infection | ||||
|---|---|---|---|---|
| Prestroke symptoms | 17 (68.0%) | 9 (100%) | 8 (50%) | 0.02 |
| Days of evolution (mean, SD) | 12 (7.5) | 14.9 (8.4) | 9.4 (5.9) | 0.14 |
| ICU admission | 8 (32.0%) | 6 (66.7%) | 2 (12.5%) | 0.02 |
| Severe infection | 14 (56.0%) | 9 (100%) | 6 (37.5%) | 0.01 |
| Stroke | ||||
|---|---|---|---|---|
| NIHSS (median, IQR) | 168, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 | 106, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 | 169, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 | 0.82 |
| Unknown onset | 14 (56.0%) | 6 (66.7%) | 8 (50.0%) | 0.52 |
| Ischemic stroke | 21 (84.0%) | 9 (100%) | 12 (75.0%) | 0.10 |
| Intracranial hemorrhage | 4 (16.0%) | 0 | 4 (25.0%) | 0.10 |
| iv-tPA | 5 (20.0%) | 2 (22.2%) | 3 (18.8%) | 0.84 |
| EVT | 10 (40.0%) | 4 (44.4%) | 6 (37.5%) | 0.61 |
Seventeen (68.0%) patients presented COVID-19 symptoms before stroke onset with a mean duration of 12 (SD 7.5) days; 6 patients without symptoms were diagnosed at admission by screening test, the 2 others presented symptoms in the first 48 hours after hospital admission. Severe COVID-19 infection was diagnosed in 14 (56.0%) patients, 8 of them were admitted to the ICU.
After the diagnostic work-up, in 12 (60.0%) ischemic stroke patients (4 large-artery atherosclerosis, 8 cardioembolism (4 atrial fibrillation, 1 atrial flutter, 3 severe depressed left ventricular ejection fraction) and all ICH patients (2 anticoagulated patients, 1 hypertensive ICH, 1 hemophilia) an etiology non-related with COVID-19 was identified. Among the remaining ischemic stroke patients, 3 died before completing etiologic work-up and in 6 (0.3% of all COVID-19 patients admitted in our hospital) the stroke cause was related to infrequent etiologies that could be related to or at less linked with SARS-CoV-2 infection. Three of them presented a posterior reversible encephalopathy syndrome (PRES);14 one presented a paradoxical embolism and in two no cause was identified after completing diagnostic work-up. Patients’ data are shown in Table 2 . Severe COVID-19 condition (100% vs. 37.5%, p = 0.01), ICU admission (66.7% vs. 12.5%, p = 0.02) and the presence of COVID-19 symptoms before stroke (100% vs. 50%, p = 0.02) were more frequent among patients in which stroke wasn't related to a usual stroke etiology. A logistic regression model adjusting for all these variables and age showed that severe COVID-19 condition was an independent predictor of stroke without a usual etiology (OR 13.3, 95% CI, 1.3-134.6; p 0.02).
Table 2.
Characteristics of six patients with stroke possibly related with SARS-CoV-2 infection. COVID-19 indicates Coronavirus Disease 19; ICU, intensive care unit; NIHSS, National Institutes of Health Stroke Scale; MCA, middle cerebral artery; CT, computed tomography; MRI, magnetic resonance imaging; PRES, posterior reversible encephalopathy syndrome. Reference range are as follows: D-dimer 0-243ng/ml; Ferritin 25-400ng/ml; Lymphocytes 1200-3500 per mm³.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Gender | Male | Female | Female | Female | Female | Female |
| Age (decade) | 60s | 40s | 40s | 50s | 30s | 40s |
| Risk factors for stroke | Hypertension | Puerperium | None | Hypertension, Diabetes mellitus, obesity, chronic kidney disease | None | None |
| COVID-19 features | ||||||
|---|---|---|---|---|---|---|
| Severe disease | Yes | Yes | Yes | Yes | Yes | Yes |
| Mechanical ventilation | Yes | Yes | Yes | Yes | Yes | Yes |
| ICU admission | Yes | Yes | Yes | Yes | Yes | Yes |
| Treatment | Hydroxychloroquine, Ritonavir/lopinavir, Glucocorticoids, Tocilizumab | Azitromicine, Hydroxychloroquine, Ritonavir/lopinavir, Tocilizumab | Azitromicine, Hydroxychloroquine, Ritonavir/lopinavir, Ceftriaxone | Azitromicine, Hydroxychloroquine, Ceftriaxone, Darunavir/cobicistat, | Azitromicine, Hydroxychloroquine, Ritonavir/lopinavir, Tocilizumab, Ceftriaxone | Azitromicine, Hydroxychloroquine, Ritonavir/lopinavir, Tocilizumab |
| D-dimer (ng/ml) | 6800 | 734 | 1833 | 1790 | 246 | 4430 |
| Ferritin (ng/ml) | 1054 | 164 | 236 | 2353 | 605 | 1020 |
| Lymphocytes per mm³ | 1224 | 1400 | 1900 | 1800 | 1600 | 1600 |
| Other relevant medical complications | Deep vein thrombosis | None | Catheter-associated subclavian vein thrombosis | Septic shock, pneumothorax | None | Staphylococcus aureus bacteriemia |
| Days from symptom onset to acute stroke | 35 days | 14 days | 30 days | 38 days | 19 days | 25 days |
| Stroke features | ||||||
|---|---|---|---|---|---|---|
| NIHSS | 3 | 1 | 18 | 22 | 8 | 10 |
| CT/MRI findings | Bilateral cortical infarcts | Tiny protuberance infarct | M1-segment of MCA occlusion | PRES | PRES | PRES, vasoconstriction |
| Probable cause | Unknown | Unknown | Paradoxical embolism | PRES | PRES | PRES |
Overall, in-hospital mortality rate was 36% (9/25): 5 (50%) LVO patients, 2 (18.2%) non-LVO ischemic stroke patients and 2 (50%) ICH patients died. Of them, 3 deaths were directly related with stroke severity (2 ICH and 1 LVO), 5 deaths were directly related with SARS-CoV-2 infection and 1 presented 2 possible causes (basilar artery occlusion successfully treated and severe hypoxemia).
Among patients with ischemic stroke, 5 (23.8%) received iv-tPA without any hemorrhagic transformation and 10 (47.6%) underwent EVT with a median time from last seen well to groin puncture of 222.5 (IQR 142.5-492.5) minutes. Among patients who underwent EVT, 8 presented intracranial LVO and two underwent acute extracranial stenting due to chronic carotid occlusion without intracranial LVO that became symptomatic in the context of severe hypoxemia. The median door-to-groin time was 118 minutes (IQR 45-134) and in 5 of the 8 patients (62.5%) with intracranial occlusion a successful recanalization was achieved. We did not find differences in baseline characteristics with patients who underwent EVT during the same period in our center (Table 3 ) except a higher rate of unknown onset stroke and a longer last-time-seen-well to groin time among patients without COVID-19.
Table 3.
Demographic and clinical data of patients who underwent EVT in function of COVID-19 status. COVID-19 indicates Coronavirus Disease 19; EVT, endovascular treatment; SD, standard deviation; IQR, interquartile range; ICU, intensive care unit; NIHSS, National Institutes of Health Stroke Scale; iv-tPA, intravenous tissue-type plasminogen activator; MCA, middle cerebral artery; TICA, terminal internal carotid artery; BA, basilar artery; TICI, Thrombolysis in Cerebral Infarction; ICH, intracranial hemorrhage; LTSW, last time seen well.
| COVID-EVT (N = 10) | NO COVID-EVT (N = 19) | p value | |
|---|---|---|---|
| Age (mean, SD) | 70.8 (14.8) | 71.0 (15.9) | 0.97 |
| Gender (male) | 6 (60.0%) | 11 (57.9%) | 0.91 |
| Vascular risk factors | |||
|---|---|---|---|
| Hypertension | 6 (60.0%) | 10 (52.6%) | 0.71 |
| Diabetes mellitus | 4 (40.0%) | 4 (21.0%) | 0.28 |
| Hyperlipidemia | 5 (50.0%) | 7 (36.8%) | 0.49 |
| Current smoker | 3 (30.0%) | 6 (31.6%) | 0.93 |
| Atrial fibrillation | 2 (20.0%) | 4 (21.0%) | 0.94 |
| COVID-19 infection | |||
|---|---|---|---|
| Prestroke symptoms | 9 (90.0%) | - | - |
| Days of evolution (mean, SD) | 11.7 (7.3%) | - | - |
| ICU admission | 2 (20.0%) | - | - |
| Severe infection | 7 (70.0%) | - | - |
| Stroke | |||
|---|---|---|---|
| NIHSS (median, IQR) | 1811, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 | 179, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 | 0.27 |
| Site of occlusion | 0.22 | ||
| MCA-M1 | 2 (20.0%) | 9 (47.4%) | |
| MCA-M2 | 4 (40.0%) | 5 (26.3%) | |
| TICA | 1 (10.0%) | 3 (15.8%) | |
| Extracranial ICA | 2 (20.0%) | 0 | |
| BA | 1 (10.0%) | 2 (10.5%) | |
| Unknown onset | 2 (20.0%) | 11 (57.9%) | 0.05 |
| iv-tPA | 1 (10.0%) | 5 (26.3%) | 0.27 |
| Endovascular procedure | |||
|---|---|---|---|
| TICI 2b-3 | 5 (62.5%)* | 16 (84.2%) | 0.30 |
| LTSW to groin time (minutes) (median, IQR) | 222.5 (142.5-492.5) | 570 (256-860) | 0.03 |
| Door to groin time (minutes) (median, IQR) | 118 (45.5-134.5) | 75 (46-93.5) | 0.15 |
In patients who underwent EVT during the study period, COVID-19 was an independent predictor of in-hospital mortality (50% versus 15%; Odds Ratio, 6.67; 95% CI, 1.1-40.4; p 0.04) and presented a trend to a higher median of NIHSS score at 24 hours (18 (IQR 7-42) vs. 15;5 – 21 p = 0.06).
Discussion
Our study shows that the frequency of acute stroke in patients with COVID-19 requiring hospital admission is low (1%) and in most cases a usual cause of stroke was identified. Moreover, all patients with an acute stroke without a usual etiology presented a severe infection requiring mechanical ventilation.
Severe respiratory disease has been previously linked to neurological symptoms and in particular to stroke.15 Neurological disorders were also described in patients affected by other coronavirus as Middle East Respiratory Syndrome or SARS-CoV.16 Cerebral ischemia associated with severe infections is not new to COVID-19; the association of sepsis with intravascular coagulopathy and platelet activation is well known and has been described as a potential cause of stroke.17 Recent bacterial and viral infections have been repeatedly reported as a risk factor for ischemic stroke.18 – 21 On the other hand, influenza vaccination, by reducing the infection rate, has been associated with a reduction in risk of stroke and myocardial infarction.22 , 23 In all cases, stroke is an uncommon complication of the infectious disease and according to our observations SARS-CoV2 seems to follow the same pattern. Therefore, among COVID-19 patients, and mainly in the absence of severe symptoms, the stroke cause should not be directly attributed to SARS-CoV-2, and an exhaustive diagnostic work-up must be completed.
In the majority of patients in which stroke etiology was possibly related to SARS-CoV-2 infection elevated D-Dimer and ferritin levels were found. High levels of D-Dimer and inflammatory markers have been previously related to COVID-19 severity.24 These laboratory findings, together with the fact that 3 patients presented PRES and 2 deep vein thrombosis, suggest an inflammatory and coagulopathy state possibly associated to the ischemic brain injury.25
The infrequent but possible causal relation of SARS-CoV-2 and ischemic stroke observed in our study could not be confirmed for ICH. During the study period we did not observe an increase in ICH admissions and in all cases a usual ICH cause was identified. Moreover none of the patients receiving reperfusion therapies experienced a symptomatic hemorrhagic transformation, suggesting that COVID-19 patients should not be excluded from these treatments for safety reasons.
It is not possible to determine if COVID-19 could act as a trigger in those patients with an identified usual stroke cause. Severe COVID-19 was less frequent among these stroke patients (37.5%), this rate is not higher than in previously published series of COVID patients admitted to a hospital.26 , 27 In two of our cases, hypoxemia was considered the triggering stroke factor in patients with chronic extracranial carotid occlusion. Therefore COVID-19 patients with pre-existing vascular risk factors should undergo regular neurological exams.
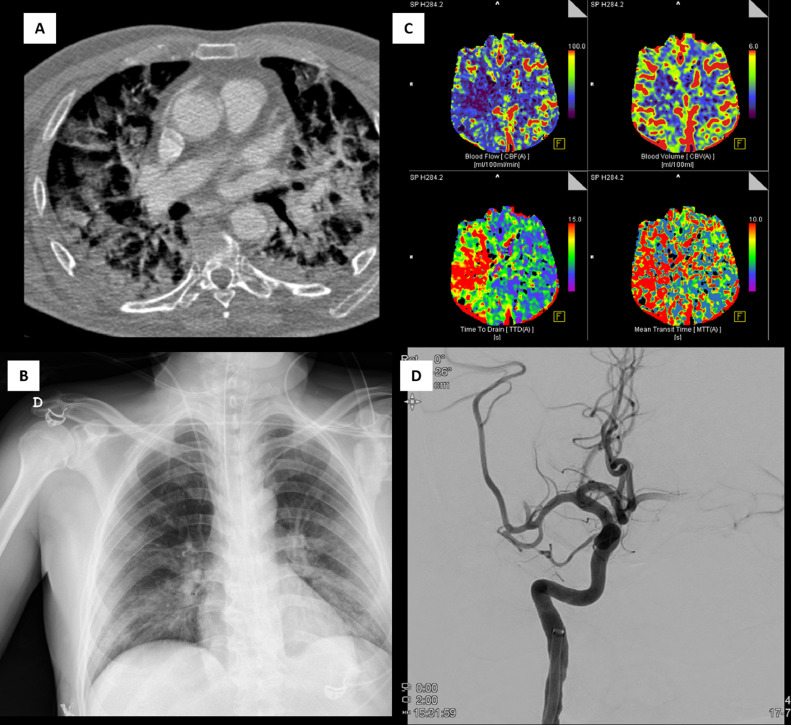
In our study, the concomitant presence of LVO and SARS-CoV-2 infection was associated with a high rate of in-hospital mortality (50%) despite of mechanical thrombectomy. Of the 5 patients who died, 4 (80%) presented a severe infection (Figure 1 ) and the remaining was an elder patient in whom mechanical thrombectomy was not successful (TICI 0 at the end of EVT). A logistic regression model applied all patients undergoing EVT during the study period confirmed SARS-CoV2 infection as an independent predictor of mortality.
Fig. 1.
Acute ischemic stroke due to a large vessel occlusion in a patient with a severe COVID-19. A and B, severe pulmonary injury showed on CT angio and radiography. C, CT perfusion maps. D, M2-middle cerebral artery occlusion.
This study has some limitations and strengths. The main limitations are:1 it is a single center study;2 even despite stroke neurologists were involved in COVID-19 teams in different settings (Emergency department, hospitalization and ICU) cases of minor stroke have not been detected, mainly in critically ill patients in which neurological exam may be challenging;3 some patients did not have a complete diagnostic work-up and4 most asymptomatic patients were not tested for COVID-19 by RT-PCR. On the other hand, all COVID-19 patients admitted during the study period were accounted, the etiological diagnostic work-up was always guided by a stroke neurologist and all data were obtained from a mandatory prospective registry.
In conclusion, less than 2% of COVID-19 patients admitted to our hospital presented an associated stroke. In most cases a usual stroke cause was identified. In patients without any other potential cause, stroke was an uncommon complication only seen in patients with a severe pulmonary injury. In stroke patients with LVO, the presence of COVID-19 was a strong predictor of in-hospital mortality.
Disclosures
Marc Ribó receives payment from Philips as Co-Principal Investigator of the WE TRUST study and he has a consulting agreement with Medtronic, Stryker, Cerenovus, CVAid, Methinks, Anaconda Biomed and Apta Targets.
The others authors reports no conflict.
References
- 1.Lodigiani C, Iapichino G, Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. PubMed PMID: 32353746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yaghi S, Ishida K, Torres J. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51(7):2002–2011. doi: 10.1161/STROKEAHA.120.030335. PubMed PMID: 32432996. Pubmed Central PMCID: 7258764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothstein A, Oldridge O, Schwennesen H. Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke. 2020 doi: 10.1161/STROKEAHA.120.030995. Online published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oxley TJ, Mocco J, Majidi S. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020 doi: 10.1056/NEJMc2009787. Online published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Xiao M, Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. PubMed PMID: 32268022. Pubmed Central PMCID: 7161262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez-Fernandez F, Valencia HS, Barbella-Aponte RA. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain: a J Neurol. 2020 doi: 10.1093/brain/awaa239. Online published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poissy J, Goutay J, Caplan M. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. PubMed PMID: 32330083. [DOI] [PubMed] [Google Scholar]
- 8.Grillet F, Behr J, Calame P. Acute Pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020 doi: 10.1148/radiol.2020201544. 201544. PubMed PMID: 32324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost JTH. 2020;18(4):844–847. doi: 10.1111/jth.14768. PubMed PMID: 32073213. Pubmed Central PMCID: 7166509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barton LM, Duval EJ, Stroberg E. COVID-19 Autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153(6):725–733. doi: 10.1093/ajcp/aqaa062. PubMed PMID: 32275742. Pubmed Central PMCID: 7184436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escalard S, Maier B, Redjem H. Treatment of acute ischemic stroke due to large vessel occlusion with COVID-19: experience from Paris. Stroke. 2020;51(8):2540–2543. doi: 10.1161/STROKEAHA.120.030574. PubMed PMID: 32466736. Pubmed Central PMCID: 7282400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin C, Zhou L, Hu Z. Clinical characteristics and outcomes of COVID-19 patients with a history of stroke in Wuhan, China. Stroke. 2020;51(7):2219–2223. doi: 10.1161/STROKEAHA.120.030365. PubMed PMID: 32466735. Pubmed Central PMCID: 7282412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaidat OO, Yoo AJ, Khatri P. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650–2663. doi: 10.1161/STROKEAHA.113.001972. PubMed PMID: 23920012. Pubmed Central PMCID: 4160883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fugate JE, Claassen DO, Cloft HJ. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proceed. 2010;85(5):427–432. doi: 10.4065/mcp.2009.0590. PubMed PMID: 20435835. Pubmed Central PMCID: 2861971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao L, Jin H, Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. PubMed PMID: 32275288. Pubmed Central PMCID: 7149362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umapathi T, Kor AC, Venketasubramanian N. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS) J Neurol. 2004;251(10):1227–1231. doi: 10.1007/s00415-004-0519-8. PubMed PMID: 15503102. Pubmed Central PMCID: 7088071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeerleder S, Hack CE, Wuillemin WA. Disseminated intravascular coagulation in sepsis. Chest. 2005;128(4):2864–2875. doi: 10.1378/chest.128.4.2864. PubMed PMID: 16236964. [DOI] [PubMed] [Google Scholar]
- 18.Syrjanen J, Valtonen VV, Iivanainen M. Preceding infection as an important risk factor for ischaemic brain infarction in young and middle aged patients. Br Med J. 1988;296(6630):1156–1160. doi: 10.1136/bmj.296.6630.1156. PubMed PMID: 3132245. Pubmed Central PMCID: 2545622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grau AJ, Buggle F, Heindl S. Recent infection as a risk factor for cerebrovascular ischemia. Stroke. 1995;26(3):373–379. doi: 10.1161/01.str.26.3.373. PubMed PMID: 7886709. [DOI] [PubMed] [Google Scholar]
- 20.Grau AJ, Buggle F, Becher H. Recent bacterial and viral infection is a risk factor for cerebrovascular ischemia: clinical and biochemical studies. Neurology. 1998;50(1):196–203. doi: 10.1212/wnl.50.1.196. PubMed PMID: 9443480. [DOI] [PubMed] [Google Scholar]
- 21.Bova IY, Bornstein NM, Korczyn AD. Acute infection as a risk factor for ischemic stroke. Stroke. 1996;27(12):2204–2206. doi: 10.1161/01.str.27.12.2204. PubMed PMID: 8969781. [DOI] [PubMed] [Google Scholar]
- 22.Smeeth L, Thomas SL, Hall AJ, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611–2618. doi: 10.1056/NEJMoa041747. PubMed PMID: 15602021. [DOI] [PubMed] [Google Scholar]
- 23.Grau AJ, Fischer B, Barth C. Influenza vaccination is associated with a reduced risk of stroke. Stroke. 2005;36(7):1501–1506. doi: 10.1161/01.STR.0000170674.45136.80. PubMed PMID: 15947266. [DOI] [PubMed] [Google Scholar]
- 24.Wu C, Chen X, Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. PubMed PMID: 32167524. Pubmed Central PMCID: 7070509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17(7):796–808. doi: 10.1038/nm.2399. PubMed PMID: 21738161. Pubmed Central PMCID: 3137275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. PubMed PMID: 32171076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan WJ, Ni ZY, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. PubMed PMID: 32109013. Pubmed Central PMCID: 7092819. [DOI] [PMC free article] [PubMed] [Google Scholar]