Abstract
Despite evidence that exercise reduces the negative impacts of stressor exposure and promotes stress robustness, health and well-being, most people fail to achieve recommended levels of physical activity. One reason for this failure could be our fundamental lack of understanding the brain motivational and motor circuits underlying voluntary exercise behavior. Wheel running is an animal model used to reveal mechanisms of exercise-induced stress robustness. Here we detail the strengths and weakness of wheel running as a model; and propose that running begins as a purposeful, goal-directed behavior that becomes habitual with continued access. This fresh perspective could aid in the development of novel strategies to motivate and sustain exercise behavior and maximize the stress-robust phenotype.
Introduction
Physical activity impacts nearly every physiological system and tissue in the body including cardiorespiratory, nervous, reproductive, digestive, immune, endocrine, skeletal muscle, and bone. The deleterious effects of a lack of physical activity on health and disease are broad and indisputable. In fact, the World Health Organization lists physical inactivity as a leading cause of disease and disability; and Booth et al., (2017) presents compelling evidence that inactivity directly contributes to 35 chronic diseases and 10 leading causes of death in the US alone [1]. Importantly, increasing physical activity status can help ameliorate effects of a sedentary lifestyle [2]*. Recent studies using unbiased accelerometers, however, indicate that only 5 – 14% of adults achieve recommended levels of physical activity [3]*. This startling statistic reveals the failure of current strategies to encourage physical activity. One reason for this failure could be our lack of understanding of the mechanisms motivating voluntary exercise. Although progress is being made towards illuminating the means by which social, ethnic, economic, geographical and educational factors could motivate physical activity across the lifespan, how these and additional physiological factors capable of directly influencing physical activity interact with motivation and motor circuits in the brain remains relatively unknown.
Rodent models of exercise, such as voluntary wheel running, can be useful tools for revealing the beneficial physiological adaptions produced by physical activity, as well as the neural circuits motivating voluntary exercise behavior. Mice and rats, when housed with a running wheel, choose to voluntarily run on the wheel, find wheel running rewarding, and benefit from exercise in many of the same ways as humans; including a reduction in the negative impacts of stressor exposure on mind (e.g., mood, cognition) and body (e.g., immune dysregulation). While the benefits of wheel running on mind and body are robust and consistent, effects of other animal models of exercise, such as forced treadmill training or swimming, are more variable. The current review; therefore, focuses on voluntary wheel running as an animal model of physical activity. Limiting the translational potential of this model are long-standing debates over why captive rodents run on wheels and what this behavior could represent [4,5]. Given that rodents voluntarily choose to run if given access to wheel, it is reasonable to question if wheel running represents the experimental or control condition?
In the current review, we discuss issues associated with rodent voluntary wheel running as a model, present evidence that wheel running reduces the negative impacts of stressor exposure on mind and body, and propose the novel hypothesis that wheel running begins as a purposeful, goal-directed activity motivated by reward outcome and, with continued access, shifts to become a habit. We also introduce evidence of specific neural circuits that could control goal-directed and habitual exercise behavior. This fresh perspective on the impacts and neural control of wheel running could aid in the development of novel strategies to motivate exercise behavior and maximize the stress-robust phenotype.
Which is the experimental group?
One lingering question about wheel running as a model is, which is the experimental condition? This issue is predicated on the notion that human beings and rodents evolved to be physically active in their environments and only due to recent, rapid changes in the environment, such as advanced technology (humans) or laboratory caging (rodents), can these sedentary organisms survive without daily physical activity. If we focus specifically on voluntary wheel running as a rodent exercise model, one way to address this issue is by comparing measures of physical activity in laboratory rodents with and without running wheels, to those of their wild counterparts. This comparison is complicated by the fact that many generations of breeding of common laboratory strains may have altered normal activity levels when compared to wild rodents, and that wheel running behavior varies depending on age, sex, strain, and history of prior wheel running. Prior work in larger mammals indicate that animals in captivity tend to move around less than in their natural habitat [6]. If this observation applies to rodents, it would suggest that the sedentary group represents an experimental group, rather than a control. What is more difficult to understand is how wheel running compares to levels of physical activity in wild rodents. Neither the spontaneous activity [7], distance traveled in a naturalistic enclosure [8], nor speed or bout length of wheel running [9], seem to differ between wild rodents and common laboratory strains, thus comparisons between laboratory and wild rodents, if they existed, could be revealing.
If wild rodents and wheel running rodents have similar overall levels of activity, then the sedentary group would be the only experimental group since their levels of activity are diminished due to laboratory housing compared to levels attained in the wild. There is, however, evidence to suggest that this is not the whole story. An early study by Brant and Kavenau [8] reported dramatically greater distances traveled per day by captive mice running in a wheel placed in a large, naturalistic enclosure compared to the daily distance traveled in the enclosure when the wheel was locked. This observation is reasonable given evidence that wheel running is rewarding and rodents can prefer it over other enriching and rewarding stimuli. It seems feasible, in light of these data, that the sedentary and exercise conditions are actually both experimental groups each having opposing effects on physiological, immunological, and neurobiological outcomes. This idea is in agreement with the fact that effects of wheel running in rodents are often very robust; perhaps because studies capture both the negative effects of inactivity and positive impacts of physical activity. Supporting this view is the fact that a sedentary lifestyle increases susceptibility to the same maladaptive health outcomes that exercise is known to ameliorate. In the end, perhaps what wheel running studies reveal are differences in physiology and behavior along a continuum of physical activity status.
Benefits of physical activity are greatest in the face of challenge
Exercise-evoked positive effects are best revealed in the face of challenges such as disease, aging, and stressor exposure. There are recent reports, for example, that voluntary wheel running produces positive effects in studies testing animal models of multiple sclerosis [10], arterial inflammation [11], neural inflammation [12,13], Alzheimer’s disease [14], liver disease [15], immune aging [16], and adipose immunometabolism [17]. Results from these preclinical studies are significant because they identify novel adaptations in physiology produced by exercise that reduce pathologies associated with each disease state.
One challenge that readily reveals the benefits of regular physical activity is stressor exposure. Organisms have individual differences in stress vulnerability. Some individuals, for example, are better able to resist the negative impacts of chronic, repeated, or severe stressor exposure (i.e., stress resistance); and/or some individuals recover faster after suffering negative health consequences (i.e., stress resilient). We have described organisms that possess both stress resistance and stress resilience as stress robust [18]. Exercise is unique among stress-protective factors because it produces a stress robust phenotype.
To better understand the mechanisms of the stress robust phenotype using a preclinical model, we varied physical activity status by housing juvenile or adult rats (inbred and outbred strains) with access to either a mobile or locked running wheel in their home cages. After 3–6 weeks, rats housed with mobile running wheels display physical changes indicative of improved fitness, including increased endurance when tested on the treadmill, reduced abdominal adiposity when fed a high fat diet, increased lean body mass, and changes in muscle citrate synthase. Most importantly for our work, is that physically active compared to sedentary rats have reduced adipose inflammation [19], no antibody suppression [20], facilitated antibacterial innate immunity [21], reduced anxiety- and depressive-like behaviors [22], and faster diurnal rhythm and sleep disturbance recovery [23], after exposure to an acute, uncontrollable stressor (100, 1.5mA, 5-s tailshocks). Using this paradigm, we exploited the differences in stress robustness to reveal unique adaptations in stress-responsive neurocircuitry that were necessary and sufficient for specific outcomes, including adaptations in serotonergic dorsal raphe neuronal responses responsible for anxiety-like and depressive-like behaviors [22], and central sympathetic drive associated with immunomodulation [24–27]. Our most recent work extends our assessment of adaptations produced by exercise to include commensal intestinal microbes (gut microbiota). The gut microbiota contributes to many aspects of host physiology. Our recent evidence demonstrates that wheel running changes the gut microbial structure favoring a lean-promoting composition [28,29]; 2) increases the abundance of beneficial microbial species [28,29]; and 3) increases butyrate-producing bacteria and butyrate, a short chain fatty acid implicated in metabolism and epigenetic processes [30]. These effects are greater when running is initiated in adolescence compared to adulthood [29]. In light of the demonstrable mental and physical benefits of physical activity, it is important to develop novel strategies for motivating exercise behavior.
Factors influencing exercise behavior
Several factors act as barriers to reduce the chances of sustaining regular exercise behavior. In addition to factors that indirectly influence exercise behavior (e.g. social, ethnic, geographical, economic, educational factors), several physiological variables could directly act to decrease physical activity. Among these are obesity and psychologically stressful lifestyles, both of which are on the rise. Obesity is associated with a decline in physical activity [31], and both high-fat western diets [32] and stressor exposure [33,34] can reduce voluntary exercise. Stress, in particular, has been identified as a primary psychological determinant of low physical activity [35]*. Sterile inflammation, circadian disruption, and/or direct effects on motivation circuits could all at least partly mediate the effects of obesity and stress on physical activity. Non-exercise rewards also compete with exercise behavior to decrease physical activity, perhaps through direct interaction with hedonic circuits in the brain. Indeed, rodent studies indicate that non-exercise rewards such as cocaine compete with exercise and reduce wheel running behavior [36].
Interestingly, there is some evidence that these physiological factors have a stronger influence over exercise behavior when they occur during the early phase of exercise participation (acquisition phase) than after exercise has been maintained for long periods (maintenance phase). Wheel running initiated prior to psychological stress, for example, attenuates the ability of stress to reduce voluntary exercise [20]. These data could partly explain the difficulty in developing consistent exercise routines, as exercise programs could be most susceptible to interference during the early stages of their development. Indeed, individuals with a recent history of exercise are more likely to adhere to new exercise programs [37]. A better understanding of factors that modulate the initial acquisition and long-term maintenance of physical activity could help increase exercise participation and quality of life.
Goal-directed and habit circuits motivate voluntary exercise
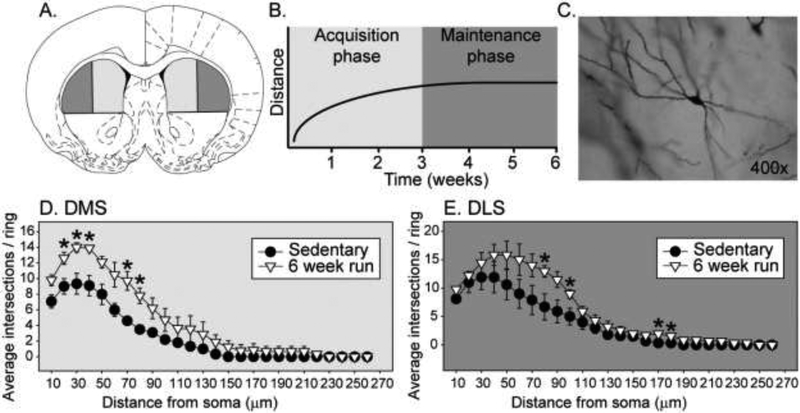
Although there are likely several explanations for why exercise is more susceptible to interference during the acquisition than the maintenance phase, one particularly intriguing idea is that distinct mechanisms that are differentially sensitive to interference by external factors might motivate exercise behavior depending on exercise history. This is a useful theory from a clinical perspective, as understanding these mechanisms could inform the development of unique interventions designed to maximize exercise adherence depending on exercise history. Two anatomically and functionally distinct strategies the brain uses to control behavior that differ in their sensitivity to disruption by external factors are goal-directed and habit-based strategies [38]*. Actions controlled by the goal-directed circuit are motivated by their anticipated outcome, remain flexible in the face of challenge, and involve the dorsomedial portion of the striatum (DMS). Habits are controlled by a more rigid, stimulus-driven circuit insensitive to reward outcome that involves the dorsolateral striatum (DLS). It is possible that during the acquisition of exercise behavior, when exercise is most vulnerable to disruption, goal-directed strategies involving the DMS predominate to motivate exercise. A shift in relative reliance on goal-directed to habit strategies involving the DLS, however, might be necessary for long-term maintenance of exercise behavior. In other words, to overcome factors that would interfere with exercise behavior and maintain a physically active lifestyle, exercise might need to become a habit. The different sub-regions of the dorsal striatum are shown in Figure 1A, and the hypothetical control exerted by the DMS and DLS in the acquisition and maintenance phases of voluntary exercise is depicted in Figure 1B.
Figure 1A-E. Different sub-regions of the dorsal striatum are proposed to control early vs late phases of voluntary wheel running behavior.
Figure 1A is schematic of a coronal section of the rat brain highlighting the dorsal medial striatum (DMS) and dorsal lateral striatum (DLS) sub-regions of the dorsal striatum. Figure 1B depicts our hypothetical distinct acquisition and maintenance phases of voluntary wheel running, differentially controlled by the DMS and DLS. To explore the effects of voluntary wheel running on dendritic complexity in the DMS and DLS, adult, male F344 rats (3 per group) were singly housed with either a locked or freely mobile running wheel. After 6 weeks of sedentary or voluntary exercise conditions, brain sections were prepared with FD Rapid Golgi Stain (FD NeuroTechnologies, Columbia, MD) following manufacturer’s instructions. Medium-spiny neurons were identified (Figure 1C), traced under 400X magnification using a drawing tube, and dendritic bifurcations were counted every 10μm from the soma using manual Sholl analyses [48]. Compared to sedentary rats, 6 weeks of wheel running increased dendritic bifurcations in both the DMS (Figure 1D) and the DLS (Figure 1E). Differences were greatest close to the soma (interaction between exercise and distance from soma: DMS (F (25,100) = 3.7; p < 0.0001); DLS, (F (25,100) = 2.3; p = 0.001)), resulting in higher peak bifurcations in exercised rats.
Switching from goal-directed to habit strategies commonly occurs during motor-skill and instrumental learning. In fact, there is evidence that this shift underlies the ability of rats to learn to balance in a running wheel during the acquisition phase of wheel running [39]. This shift in behavioral strategies is also thought to underlie the development of habitual drug-taking behavior. The DMS is involved in the acquisition phase of drug self-administration, when rodents learn to press a lever for a drug reinforcement, whereas drug self-administration relies more upon the DLS during the maintenance phase [40]. Interestingly, the long-term pattern of rodent voluntary exercise closely resembles that of free-access to drug self-administration: both escalate during the acquisition phase followed by a plateau at a high level during the maintenance phase (Figure 1B). This pattern of voluntary exercise suggests that goal-directed and habit circuits could differentially control voluntary exercise during these different phases, and makes rodents a useful translational tool for studying mechanisms underlying the acquisition and maintenance of voluntary exercise behavior.
It should be mentioned that although we are suggesting that habit circuits can control long-term maintenance of wheel running behavior, this does not imply that wheel running represents stereotypy previously suggested to underlie wheel running in captive rodents [4]. Meijer and Robbers [9] observed wild rodents running on wheels placed in their natural habitats, indicating that wheel running is not stereotypy. Wild rodents have even been observed to return to the wheel after leaving [9]; an observation suggesting that wheel running behavior begins as purposeful, goal-directed behavior that can develop into a habit. Lastly, although exercise habits are more likely to develop into overtraining syndrome or exercise dependence in humans, standard laboratory rodent strains display healthy amounts of wheel running [41] suggesting that wheel running behavior that develops into a habit is not analogous to human exercise dependence.
Exercise-induced plasticity in goal-directed and habit circuits
At present, there are no studies directly testing the idea that the acquisition and maintenance phases of voluntary exercise behavior differ in their relative reliance on goal-directed and habit circuits, respectively; however, several observations in the literature are consistent with this possibility. First, Cordony et al. [42]* recently demonstrated that during the acquisition phase of wheel running, when nightly wheel running was still escalating, motivation for wheel running (as assessed by breakpoints in operant responding for access to a running wheel) can be devalued by prior wheel running. Behaviors motivated by goal-directed processes remain sensitive to devaluation, whereas behaviors motivated by habit processes are resistant to devaluation. These data, therefore, indicate that goal-directed processes might motivate wheel running during the acquisition phase. Second, the maintenance of voluntary exercise does not seem to depend on functioning goal-directed circuits. The DMS supports goal-directed behavior through bi-directional communication with the prefrontal cortex (PFC), whereas performance strategies resort to the habit system in the absence of the PFC [43]. Although the effect of PFC damage prior to the acquisition of wheel running is unknown, rats quickly return to pre-surgical levels of voluntary exercise after PFC lesions if lesions occur after 3 weeks of wheel running [44], suggesting that functioning DMS-PFC circuits are not required for the maintenance of exercise after acquisition. Third, with a few notable exceptions, chronic wheel running produces neural adaptations in both the DMS and DLS (reviewed in [45]), suggesting that long-term wheel running recruits both goal-directed and habit-based circuits. If only one of these strategies motivates wheel running behavior, then wheel running might be expected to elicit neural adaptations in either the DMS or DLS, but not both. In both the DMS and DLS, 6 weeks of wheel running reduces mRNA for adenosine A1 and A2A receptors [46], increases mammalian target of rapamycin signaling [47], increases DA D2 receptor mRNA [46], potentiates stress-induced cfos mRNA in D1-expressing medium-spiny neurons and attenuates stress-induced cfos mRNA in D2-expressing medium-spiny neurons [46], and constrains stress-induced 5-HT efflux while potentiating stress-induced DA efflux [48].
Goal-directed and habit learning also alters structural plasticity, including dendritic complexity, within the DMS and DLS. There is evidence, for example, that high levels of habit behavior is associated with increased dendritic complexity in the DLS along with a reduction in dendritic complexity in the DMS [49]. If wheel running remains solely goal-directed or habitual, then one might expect wheel running to increase dendritic complexity only in the striatal region driving the behavior. We investigated the effects of voluntary exercise on dendritic complexity in the DMS and DLS of adult, male F344 rats (3 per group) singly housed with either a locked or freely mobile running wheel. All procedures were approved by the University of Colorado’s Institutional Animal Care and Use Committee and were compliant with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
After 6 weeks of sedentary or voluntary exercise conditions, brain sections were prepared with FD Rapid Golgi Stain (FD NeuroTechnologies, Columbia, MD) following manufacturer’s instructions. Medium-spiny neurons were identified (Figure 1C), traced under 400X magnification using a drawing tube, and dendritic bifurcations were counted every 10μm from the soma using manual Sholl analyses [50]. Compared to sedentary rats, 6 weeks of wheel running increased dendritic bifurcations in both the DMS (Figure 1D) and the DLS (Figure 1E). Differences were greatest close to the soma (interaction between exercise and distance from soma: DMS (F (25,100) = 3.7; p < 0.0001); DLS, (F (25,100) = 2.3; p = 0.001)), resulting in higher peak bifurcations in exercised rats. These data, together with prior findings mentioned above, suggest that voluntary wheel running recruits both DMS and DLS circuits. They do not; however, shed light on whether these circuits are differentially recruited during the acquisition vs. maintenance phase. More detailed time-course studies will be required to fully test this hypothesis.
Conclusions
A physical active lifestyle is associated with a plethora of positive health benefits and produces a stress robust phenotype. Unfortunately, few adults achieve recommended levels of physical activity, thus a better understanding of factors that modulate the initial acquisition and long-term maintenance of physical activity could help increase exercise participation and quality of life. Here we present evidence that rodent voluntary wheel running produces many of the same health benefits found in humans, making it a useful model for research designed to reveal physiological and neural adaptations contributing to these health benefits. In addition, this model is uniquely positioned to advance our understanding of the neural circuitry associated with acquisition and maintenance of exercise behavior. We hypothesize wheel running behavior initially begins as a goal-directed behavior and overtime becomes a habit, with an associated shift in neural control of exercise from goal-directed to habit circuits. This fresh perspective on the neural control and impacts of wheel running could aid in the development of novel strategies to motivate exercise behavior and maximize the stress-reducing and health-promoting impacts of regular physical activity.
Highlights.
Physical activity produces a stress robust phenotype
Rodent wheel running recapitulates benefits of a physically active lifestyle
Wheel running begins as a purposeful, goal-directed activity motivated by reward
Wheel running shifts to become a habit
Unique neural circuits control goal-directed and habitual exercise behavior
Acknowledgements
We thank Aggie Mika, Ph.D. for her technical contribution to the Golgi assay.
Funding: This work was supported in part by grant funding from the National Institutes of Health [R01-MH068283, mf; R15-MH114026, bg] and the Defense Science Office [DARPA, W911NF-10-1-0050, mf].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Special Interest (*)
- 1.Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN, Toedebusch RG: Role of inactivity in chronic diseases: Evolutionary insight and pathophysiological mechanisms. Physiol Rev (2017) 97(4):1351–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruegsegger GN, Booth FW: Health benefits of exercise. Cold Spring Harb Perspect Med (2018) 8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]; * An excellent review of how physical activity can both prevent and treat disease, including discussion of underlying mechanisms. Of particular interest is the argument that the benefits of exercise are too numerous and complex to replicate with pharmacological “exercise mimetics.”
- 3.Luzak A, Heier M, Thorand B, Laxy M, Nowak D, Peters A, Schulz H, Group K-S: Physical activity levels, duration pattern and adherence to who recommendations in german adults. PLoS One (2017) 12(2):e0172503. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Used unbiased accelorometers to demonstrate that levels of physical activity among a large population of german adults is far below world-heatlh organization guidelines. Includes analyses of associations between phsyical activity, age, sex and body mass index. Supports the need for new strategies to increase participation in physical activity.
- 4.Sherwin CM: Voluntary wheel running: A review and novel interpretation. Anim Behav (1998) 56(1):11–27. [DOI] [PubMed] [Google Scholar]
- 5.Novak CM, Burghardt PR, Levine JA: The use of a running wheel to measure activity in rodents: Relationship to energy balance, general activity, and reward. Neurosci Biobehav Rev (2012) 36(3):1001–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clubb R, Mason G: Animal welfare: Captivity effects on wide-ranging carnivores. Nature (2003) 425(6957):473–474. [DOI] [PubMed] [Google Scholar]
- 7.Avni R, Tzvaigrach Y, Eilam D: Exploration and navigation in the blind mole rat (spalax ehrenbergi): Global calibration as a primer of spatial representation. J Exp Biol (2008) 211(Pt 17):2817–2826. [DOI] [PubMed] [Google Scholar]
- 8.Brant DH, Kavanau JL: Exploration and movement patterns of the canyon mouse peromyscus crinitus in an extentive laboratory enclosure. Ecology (1965) 46(4):452–461. [Google Scholar]
- 9.Meijer JH, Robbers Y: Wheel running in the wild. Proc Biol Sci (2014) 281(1786). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mifflin KA, Frieser E, Benson C, Baker G, Kerr BJ: Voluntary wheel running differentially affects disease outcomes in male and female mice with experimental autoimmune encephalomyelitis. J Neuroimmunol (2017) 305(135–144. [DOI] [PubMed] [Google Scholar]
- 11.Trott DW, Henson GD, Ho MHT, Allison SA, Lesniewski LA, Donato AJ: Age-related arterial immune cell infiltration in mice is attenuated by caloric restriction or voluntary exercise. Exp Gerontol (2018) 109(99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadeghi M, Peeri M, Hosseini MJ: Adolescent voluntary exercise attenuated hippocampal innate immunity responses and depressive-like behaviors following maternal separation stress in male rats. Physiol Behav (2016) 163(177–183. [DOI] [PubMed] [Google Scholar]
- 13.Spielman LJ, Estaki M, Ghosh S, Gibson DL, Klegeris A: The effects of voluntary wheel running on neuroinflammatory status: Role of monocyte chemoattractant protein-1. Mol Cell Neurosci (2017) 79(93–102. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler-Waldkirch S, d’Errico P, Sauer JF, Erny D, Savanthrapadian S, Loreth D, Katzmarski N, Blank T, Bartos M, Prinz M, Meyer-Luehmann M: Seed-induced abeta deposition is modulated by microglia under environmental enrichment in a mouse model of alzheimer’s disease. EMBO J (2018) 37(2):167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bay ML, Gehl J, Pedersen BK, Hojman P: Voluntary wheel running reduces the acute inflammatory response to liver carcinogen in a sex-specific manner. Cancer Prev Res (Phila) (2017) 10(12):719–728. [DOI] [PubMed] [Google Scholar]
- 16.Lee JY, Paik IY, Kim JY: Voluntary exercise reverses immune aging induced by oxidative stress in aging mice. Exp Gerontol (2018). [DOI] [PubMed] [Google Scholar]
- 17.Zidon TM, Park YM, Welly RJ, Woodford ML, Scroggins RJ, Britton SL, Koch LG, Booth FW, Padilla J, Kanaley JA, Vieira-Potter VJ: Voluntary wheel running improves adipose tissue immunometabolism in ovariectomized low-fit rats. Adipocyte (2018) 7(1):20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleshner M, Greenwood BN, Yirmiya R: Neuronal-glial mechanisms of exercise-evoked stress robustness. Curr Top Behav Neurosci (2014) 18(1–12. [DOI] [PubMed] [Google Scholar]
- 19.Speaker KJ, Cox SS, Paton MM, Serebrakian A, Maslanik T, Greenwood BN, Fleshner M: Six weeks of voluntary wheel running modulates inflammatory protein (mcp-1, il-6, and il-10) and damp (hsp72) responses to acute stress in white adipose tissue of lean rats. Brain Behav Immun (2014) 39(87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moraska A, Fleshner M: Voluntary physical activity prevents stress-induced behavioral depression and anti-klh antibody suppression. Am J Physiol Regul Integr Comp Physiol (2001) 281(2):R484–489. [DOI] [PubMed] [Google Scholar]
- 21.Fleshner M, Campisi J, Deak T, Greenwood BN, Kintzel JA, Leem TH, Smith TP, Sorensen B: Acute stressor exposure facilitates innate immunity more in physically active than in sedentary rats. Am J Physiol Regul Integr Comp Physiol (2002) 282(6):R1680–1686. [DOI] [PubMed] [Google Scholar]
- 22.Greenwood BN, Fleshner M: Exercise, stress resistance, and central serotonergic systems. Exerc Sport Sci Rev (2011) 39(3):140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson RS, Roller R, Greenwood BN, Fleshner M: Wheel running improves rem sleep and attenuates stress-induced flattening of diurnal rhythms in f344 rats. Stress (2016) 19(3):312–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenwood BN, Kennedy S, Smith TP, Campeau S, Day HE, Fleshner M: Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience (2003) 120(1):269–281. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy SL, Nickerson M, Campisi J, Johnson JD, Smith TP, Sharkey C, Fleshner M: Splenic norepinephrine depletion following acute stress suppresses in vivo antibody response. J Neuroimmunol (2005) 165(1–2):150–160. [DOI] [PubMed] [Google Scholar]
- 26.Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Fleshner M: Adrenergic receptors mediate stress-induced elevations in extracellular hsp72. J Appl Physiol (2005) 99(5):1789–1795. [DOI] [PubMed] [Google Scholar]
- 27.Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M: Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience (2005) 135(4):1295–1307. [DOI] [PubMed] [Google Scholar]
- 28.Mika A, Rumian N, Loughridge AB, Fleshner M: Exercise and prebiotics produce stress resistance: Converging impacts on stress-protective and butyrate-producing gut bacteria. Int Rev Neurobiol (2016) 131(165–191. [DOI] [PubMed] [Google Scholar]
- 29.Mika A, Van Treuren W, Gonzalez A, Herrera JJ, Knight R, Fleshner M: Exercise is more effective at altering gut microbial composition and producing stable changes in lean mass in juvenile versus adult male f344 rats. PLoS One (2015) 10(5):e0125889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mika A, Fleshner M: Early-life exercise may promote lasting brain and metabolic health through gut bacterial metabolites. Immunology and cell biology (2016) 94(2):151–157. [DOI] [PubMed] [Google Scholar]
- 31.Shields M, Tremblay MS: Sedentary behaviour and obesity. Health Rep (2008) 19(2):19–30. [PubMed] [Google Scholar]
- 32.Ruegsegger GN, Toedebusch RG, Braselton JF, Roberts CK, Booth FW: Reduced metabolic disease risk profile by voluntary wheel running accompanying juvenile western diet in rats bred for high and low voluntary exercise. Physiol Behav (2015) 152(Pt A):47–55. [DOI] [PubMed] [Google Scholar]
- 33.DeVallance E, Riggs D, Jackson B, Parkulo T, Zaslau S, Chantler PD, Olfert IM, Bryner RW: Effect of chronic stress on running wheel activity in mice. PLoS One (2017) 12(9):e0184829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desan PH, Silbert LH, Maier SF: Long-term effects of inescapable stress on daily running activity and antagonism by desipramine. Pharmacol Biochem Behav (1988) 30(1):21–29. [DOI] [PubMed] [Google Scholar]
- 35.Cortis C, Puggina A, Pesce C, Aleksovska K, Buck C, Burns C, Cardon G, Carlin A, Simon C, Ciarapica D, Condello G et al. :Psychological determinants of physical activity across the life course: A “determinants of diet and physical activity” (dedipac) umbrella systematic literature review. PLoS One (2017) 12(8):e0182709. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Review of recent systematic literature reviews and meta-analyses on pyschological determinants of physical activity, inlcuding an assesment of the quality of the reviews. Analyses revealed that levels of stress were highly, and inversly, correlated to participation in physical activity. Highlights the bi-directional nature of the relationship between physical activity and stress.
- 36.Cosgrove KP, Hunter RG, Carroll ME: Wheel-running attenuates intravenous cocaine self-administration in rats: Sex differences. Pharmacol Biochem Behav (2002) 73(3):663–671. [DOI] [PubMed] [Google Scholar]
- 37.Rhodes RE, Martin AD, Taunton JE, Rhodes EC, Donnelly M, Elliot J: Factors associated with exercise adherence among older adults. An individual perspective. Sports Med (1999) 28(6):397–411. [DOI] [PubMed] [Google Scholar]
- 38.Knowlton BJ, Patterson TK: Habit formation and the striatum. Curr Top Behav Neurosci (2018) 37(275–295. [DOI] [PubMed] [Google Scholar]; Excellent review of the current state of knowledge on behavioral and neurobiological mechanisms underlying habit formation. Supports the notion that different biological substrates underly goal-directed and habit behaviors, with the dorsolateral striatum being most important for the latter.
- 39.Willuhn I, Steiner H: Skill-memory consolidation in the striatum: Critical for late but not early long-term memory and stabilized by cocaine. Behav Brain Res (2009) 199(1):103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith RJ, Laiks LS: Behavioral and neural mechanisms underlying habitual and compulsive drug seeking. Prog Neuropsychopharmacol Biol Psychiatry (2018) 87(Pt A):11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merritt JR, Rhodes JS: Mouse genetic differences in voluntary wheel running, adult hippocampal neurogenesis and learning on the multi-strain-adapted plus water maze. Behav Brain Res (2015) 280(62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]; *
- 42.Cordony MBL, Chow JYL, Boakes RA: Motivation to run measured by progressive ratio tests: Failure to support the addiction hypothesis for rats. Learn Behav (2018). [DOI] [PubMed] [Google Scholar]; Rats were trained to press a lever for wheel access using a progress-ratio schedule. All training and testing occurred when nightly running distances were still escalating, suggesting that rats were in the acqusition phase of wheel running behavior. Higher breakpoints for wheel access were found in rats without a recent running oppurtunity. These data suggest that wheel running can be devlaued during the acquisiton phase; data indicative of goal-directed circuits motivating exercise during the acquisition phase.
- 43.Ostlund SB, Balleine BW: Lesions of medial prefrontal cortex disrupt the acquisition but not the expression of goal-directed learning. J Neurosci (2005) 25(34):7763–7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenwood BN, Spence KG, Crevling DM, Clark PJ, Craig WC, Fleshner M: Exercise-induced stress resistance is independent of exercise controllability and the medial prefrontal cortex. The European journal of neuroscience (2013) 37(3):469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenwood BN: The role of dopamine in overcoming aversion with exercise. Brain Res (2018). [DOI] [PubMed] [Google Scholar]
- 46.Clark PJ, Ghasem PR, Mika A, Day HE, Herrera JJ, Greenwood BN, Fleshner M: Wheel running alters patterns of uncontrollable stress-induced cfos mrna expression in rat dorsal striatum direct and indirect pathways: A possible role for plasticity in adenosine receptors. Behav Brain Res (2014) 272(252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lloyd BA, Hake HS, Ishiwata T, Farmer CE, Loetz EC, Fleshner M, Bland ST, Greenwood BN: Exercise increases mtor signaling in brain regions involved in cognition and emotional behavior. Behav Brain Res (2017) 323(56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clark PJ, Amat J, McConnell SO, Ghasem PR, Greenwood BN, Maier SF, Fleshner M: Running reduces uncontrollable stress-evoked serotonin and potentiates stress-evoked dopamine concentrations in the rat dorsal striatum. PLoS One (2015) 10(11):e0141898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor SB, Anglin JM, Paode PR, Riggert AG, Olive MF, Conrad CD: Chronic stress may facilitate the recruitment of habit- and addiction-related neurocircuitries through neuronal restructuring of the striatum. Neuroscience (2014) 280(231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sholl DA: Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat (1953) 87(4):387–406. [PMC free article] [PubMed] [Google Scholar]