Abstract
N-linked glycosylation is the most common form of protein glycosylation and is required for the proper folding, trafficking, and/or receptor binding of some host and viral proteins. As viruses lack their own glycosylation machinery, they are dependent on the host's machinery for these processes. Certain iminosugars are known to interfere with the N-linked glycosylation pathway by targeting and inhibiting α-glucosidases I and II in the endoplasmic reticulum (ER). Perturbing ER α-glucosidase function can prevent these enzymes from removing terminal glucose residues on N-linked glycans, interrupting the interaction between viral glycoproteins and host chaperone proteins that is necessary for proper folding of the viral protein. Iminosugars have demonstrated broad-spectrum antiviral activity in vitro and in vivo against multiple viruses. This review discusses the broad activity of iminosugars against Flaviviridae. Iminosugars have shown favorable activity against multiple members of the Flaviviridae family in vitro and in murine models of disease, although the activity and mechanism of inhibition can be virus-specfic. While iminosugars are not currently approved for the treatment of viral infections, their potential use as future host-targeted antiviral (HTAV) therapies continues to be investigated.
Keywords: Iminosugars, Glycosylation, Flavivirus, Flaviviridae, Antiviral therapy, ER α-glucosidases
1. Introduction
Host factors required for the replication of multiple viruses provide for a good target for the development of broad-spectrum antiviral drugs. Recent advances in the development of novel antiviral therapies have taken advantage of similarities in viral replication strategies by identifying and targeting host-cell pathways required for viral replication that are common to Flaviviridae or other viruses. One such pathway is the host N-linked glycosylation pathway, which has been shown to be required for the replication of multiple enveloped viruses (Watanabe et al., 2019). The aim of this review is to highlight the anti-Flaviviridae activity of iminosugars, a promising class of host-targeted antivirals (HTAVs) that can interfere with the host's N-linked glycosylation pathway by inhibiting α-glucosidases in the endoplasmic reticulum (ER).
2. N-linked glycosylation
Protein glycosylation is an essential post-translational modification required for the proper folding and function of glycoproteins and plays a key role in many cellular processes, including protein-protein and cell-cell interactions. N-linked glycosylation, the most common form of protein glycosylation, is catalyzed at the luminal face of the ER. The oligosaccharyltransferase complex (OST) co-translationally attaches the preformed N-linked glycan core, Glc3Man9GlcNAc2, to an asparagine residue within the sequence asparagine-X-serine/threonine (N-X-S/T) of the target peptide, where “X” represents any amino acid residue except proline. Several factors can contribute to the glycosylation efficiency of N-X-S/T motifs, including the ability of the OST complex to access the sequon as well as the sequence content (Kasturi et al., 1997; Martinez-Duncker et al., 2014). Glycosylation efficiency is typically higher when the target sequence contains a threonine rather than a serine (Kasturi et al., 1997).
Processing of the attached N-linked oligosaccharide begins with sequential cleavage of terminal glucose residues by the ER α-glucosidases I and II. The distal glucose residue (α1,2-glucose) is cleaved by α-glucosidase I to generate Glc2Man9GlcNAc2 (Kornfeld and Kornfeld, 1985), which can then associate with the ER membrane-bound lectin, malectin, or be further processed by α-glucosidase II (Schallus et al., 2008). The ER α-glucosidase II enzyme is responsible for consecutively cleaving the second and third glucose residues to generate Man9GlcNAc2 (Kornfeld and Kornfeld, 1985). However, prior to cleavage of the third glucose residue, the chaperone proteins, calnexin and calreticulin, can bind to the monoglucosidated glycan and assist with folding and proper disulfide bond formation through interaction with a protein disulfide isomerase (Hebert et al., 1995; Molinari and Helenius, 1999). Once the third glucose residue is cleaved, proteins that are not properly folded can be transiently reglycosylated by UDP glucotransferase 1 to prevent further processing and return to the calnexin/calreticulin cycle. Proteins may progress through a series of reglycosylation, refolding, and processing steps until properly folded and sent to the Golgi apparatus for further processing. If proper folding is not achieved, the ER enzyme mannosidase I may remove the mannose residues from the oligosaccharide to prevent reglycosylation and reprocessing through the calnexin cycle, and the protein may be targeted for degradation by the ER-associated degradation pathway (Avezov et al., 2008).
The ER glycosylation machinery is not only required for host protein glycosylation, it is also critical for the lifecycle of diverse viruses. N-linked glycosylation is a necessary component for the replication of many viruses as it is required for the proper folding, trafficking, and/or receptor binding of some viral proteins (Watanabe et al., 2019; Yap et al., 2017). Because viruses do not possess their own glycosylation machinery, they rely on host-cell machinery for these processes. Viral dependency on this pathway was demonstrated in a case study of two patients with MOGS-CDG, a rare congenital disorder caused by absent expression of α-glucosidase I and a lack of N-linked glycan processing in the ER (Sadat et al., 2014). Although these patients had hypogammaglobulinemia and other complications, they also had no documented history of viral illness. These patients had no immune recognition against childhood vaccinations consisting of live virus vaccines directed against measles, mumps, rubella, and varicella viruses, which contain glycosylated envelopes, but had normal antibody responses when vaccinated with nonreplicating infectious agents presenting proteins, polysaccharides, and other immunogens, including the diphtheria-tetanus-acellular pertussis, hepatitis B, 23-valent pneumococcal polysaccharide, and conjugated Haemophilus influenzae type B vaccines. Additionally, cells isolated from these patients had increased resistance to infection by the glycosylation-dependent human immunodeficiency virus (HIV) and influenza A virus in vitro, but not to the nonenveloped viruses, adenovirus or poliovirus 1, confirming this pathway has an essential role in the replication of certain viruses.
3. Flaviviridae replication and the role of the host glycosylation pathway
3.1. Genomic organization and replication strategies employed by Flaviviridae
Although there are distinct differences in the mode of transmission, tissue tropism, and course of infection between different members of the Flaviviridae family (Supplemental Table 1), all viruses in this family have similar replication cycles and genomic organization. The Flaviviridae RNA genome ranges from 9,000 to 13,000 bases containing one open reading frame that encodes for a single polyprotein (Simmonds et al., 2017). After the virus is internalized through receptor-mediated endocytosis, the viral RNA genome is released into the cytoplasm where it is translated into the polyprotein, which is processed by viral and host-cell proteases to produce ~3–4 structural and ~6–8 non-structural (NS) proteins, depending on the virus. RNA replication occurs in membrane-associated viral replication organelles that are derived from the ER (Neufeldt et al., 2018; Paul and Bartenschlager, 2015). Immature viral particles are assembled on the surface of the ER and are transported through the trans-Golgi network where they are further processed to produce mature virus particles that are subsequently released from the cell by exocytosis.
Structural proteins are derived from the amino terminus end of the polyprotein to allow the virus to maximize production of these proteins, while the NS proteins are derived from the carboxyl-terminal end. NS proteins are involved with proteolysis, viral assembly, regulation of host immunity, and RNA replication, and include sequences that are characteristic of an RNA-dependent RNA polymerase, serine protease, and RNA helicase (Brinton, 2002; Fernandez-Garcia et al., 2009; Simmonds et al., 2017; Zhang et al., 2017). Pestiviruses, pegiviruses, and hepaciviruses also contain an additional P7/P13 protein that is believed to be important for viral maturation that is not expressed by members of the flavivirus genus. Structural proteins, which are required to form progeny virus particles, include two or three membrane-associated glycoproteins that differ slightly between genera. Hepaciviruses, flaviviruses, and pestiviruses also contain a structural basic capsid protein (C) which assembles to form the nucleocapsid that encases the viral genetic material.
The membrane-associated glycoproteins contain a variable number of potential N-linked glycosylation sites depending on the virus and the protein. During viral replication, N-linked glycans are added to the target N-X-S/T motifs in viral glycoproteins and processed by the host ER glycan processing and protein folding machinery.
3.2. Flavivirus replication and protein glycosylation
Flavivirus structural proteins include the C protein, precursor membrane glycoprotein (prM), and envelope (E) protein, although only membrane-associated E proteins are exposed on the surface of mature particles. The E protein is glycosylated and co-translationally processed in the ER lumen where it heterodimerizes with prM, which can function as a chaperone to stabilize E proteins (Idris et al., 2016; Zhang et al., 2003) and ensure proper protein folding (Li et al., 2008; Lorenz et al., 2002; Yap et al., 2017; Yu et al., 2009). The prM-E heterodimers further oligomerize (Konishi and Mason, 1993; Zhang et al., 2003) and assemble into immature, noninfectious virions containing nucleocapsid protein complexed with viral RNA (Lorenz et al., 2002, 2003; Mackenzie and Westaway, 2001). The immature virions are translocated to the Golgi complex where the low pH environment promotes reorganization of the prM-E heterodimers, allowing for the host protease, furin, to cleave prM to the mature membrane protein (M) (Fernandez-Garcia et al., 2009; Li et al., 2008; Stadler et al., 1997; Yu et al., 2008a). This results in the formation of head-to-tail E homodimers and the generation of mature infectious particles that are eventually released from the cell by exocytosis (Kuhn et al., 2002; Mukhopadhyay et al., 2003; Stiasny and Heinz, 2006). The dengue virus (DENV) prM/M protein contains an N-linked glycosylation site at N69 and may contain other sites at additional locations (N7, N31, N52) (Courageot et al., 2000). The ability of prM to act as a chaperone for E protein is thought to be dependent on glycosylation of prM (Courageot et al., 2000; Idris et al., 2016). Removal of the N-linked glycosylation site from the prM protein of Japanese encephalitis virus (JEV) and certain West Nile virus (WNV) strains can significantly decrease the amount of newly formed virus particles secreted from mammalian cells (Hanna et al., 2005; Kim et al., 2008; Zai et al., 2013).
Several potential N-linked glycosylation motifs have also been identified in the flavivirus E glycoprotein (Dai et al., 2016; Gavel and von Heijne, 1990; Hahn et al., 1987; Ishak et al., 2001; Johnson et al., 1994; Mukhopadhyay et al., 2003; Zhang et al., 2003). The DENV E protein is glycosylated at two asparagines, N67 and N153 (Chambers et al., 1990; Hacker et al., 2009; Johnson et al., 1994). N153 is a conserved glycosylation site in most Flaviviridae (present as N154 in some viruses), whereas N67 is unique to DENV. Glycosylation of N67 is thought to mediate the interaction between the virus and the host-cell receptor DC-SIGN and is required for assembly and egress of DENV (Bryant et al., 2007; Mondotte et al., 2007; Pokidysheva et al., 2006).
The E protein of most flaviviruses, aside from DENV, contains only the single, conserved N153/154 glycosylation site. N-linked glycosylation of the flavivirus E protein has been associated with pH sensitivity, neurovirulence, infectivity, and virus secretion (Beasley et al., 2005; Bryant et al., 2007; Guirakhoo et al., 1993; Hanna et al., 2005; Kawano et al., 1993; Lee et al., 1997, 2010; Mondotte et al., 2007; Mossenta et al., 2017; Vorndam et al., 1993). Deletion of the N153/154 glycosylation site results in decreased secretion of DENV (Lee et al., 2010), tick-borne encephalitis virus (TBEV) (Goto et al., 2005; Yoshii et al., 2013), ZIKV (Mossenta et al., 2017), and WNV (Hanna et al., 2005) from mammalian cells and attenuation of Zika virus (ZIKV) in mouse models of disease (Fontes-Garfias et al., 2017). Despite the importance of this glycosylation site for many flaviviruses, not all flavivirus E proteins are N-glycosylated. For WNV, there is a correlation between viruses that contain the E protein glycosylation site and those that are responsible for many of the major outbreaks of human disease (Hanna et al., 2005).
The flavivirus genome also encodes seven NS proteins: NS1, NS2A/B, NS3, NS4A, 2K, NS4B, and NS5 (Apte-Sengupta et al., 2014; Lopez-Denman and Mackenzie, 2017; Yu et al., 2008b; Zhang et al., 2017). NS1 contains two potential N-glycosylation sites (N130 and N207) that are conserved in flaviviruses (Flamand et al., 1999; Putnak et al., 1988; Winkler et al., 1989), in addition to less conserved sites (e.g. N175 in WNV) (Akey et al., 2014). NS1 glycosylation is necessary for proper replication and virulence of DENV, although the mechanism of how NS1 glycosylation is involved in these processes is not well understood (Muylaert et al., 1996; Somnuke et al., 2011).
3.3. HCV replication and protein glycosylation
Hepatitis C virus (HCV) structural proteins include the C protein and two envelope proteins, E1 and E2, which are dependent on each other for proper protein folding (Brazzoli et al., 2005; Cocquerel et al., 2001). E1 and E2 contain 9–11 and 4–5 highly conserved glycosylation sites, respectively, depending on the viral genotype (Goffard and Dubuisson, 2003). Following glycosylation, E1 and E2 can form noncovalently-bound heterodimers that interact with the ER chaperone protein, calnexin, to assist with productive folding of the proteins (Beljelarskaya et al., 2016; Chapel et al., 2006; Choukhi et al., 1998; Op De Beeck et al., 2001). Alternatively, the glycoproteins can interact with calreticulin and form disulfide bridges, resulting in the production of misfolded protein aggregates (Beljelarskaya et al., 2016; Chapel et al., 2006; Choukhi et al., 1998; Dubuisson, 2000; Op De Beeck et al., 2001) Formation of E1-E2 heterodimers is important for viral fusion, entry, and secretion (Lindenbach and Rice, 2013). Mutation studies demonstrated that specific glycosylation sites in the HCV envelope proteins are important for the correct folding and heterodimerization of E1 and E2, including N1 and N4 in E1, and N1, N8 and N10 in E2 (Freedman et al., 2016; Goffard et al., 2005; Helle et al., 2010). Glycosylation of several sites in E2 is also believed to contribute to immune evasion (Helle et al., 2010).
3.4. Evidence of Flaviviridae dependency on host ER α-glucosidases
Considering the important role N-linked glycosylation can have in the folding and function of Flaviviridae glycoproteins, it is likely that impeding this pathway could lead to detrimental effects on viral replication. Consistent with this concept, the ER α-glucosidases have been confirmed as antiviral targets for multiple viruses in the Flaviviridae family, including HCV, DENV, yellow fever virus (YFV), and (ZIKV). Downregulation of either α-glucosidase enzyme in Huh7.5 cells by shRNA resulted in a 5-fold reduction in the production of HCV particles without impacting cell growth or viability (Qu et al., 2011). In a separate study, CRISPR/Cas9 technology was used to generate Huh7.5 cell lines lacking expression of α-glucosidase I or α-glucosidase II, and the impact on viral replication and particle secretion was measured following infection with DENV, YFV, or ZIKV (Ma et al., 2018a). A significant reduction in intracellular viral RNA and virus secretion (~10–1,000-fold) was observed for DENV and YFV in α-glucosidase I and II knockout Huh7.5 cells compared to control parental cells. Expression of α-glucosidase II was not required for the replication of ZIKV; however, loss of α-glucosidase 1 expression reduced ZIKV secretion by greater than 10-fold. These studies provide evidence that the ER α-glucosidases have a functional role in the replication of several virulent Flaviviridae pathogens.
4. Iminosugars
The required involvement of the host's ER glycosylation machinery for replication of diverse viruses makes it a key target for broad-spectrum HTAV therapies. Iminosugars are sugar mimetics that can function as competitive inhibitors of a variety of glycosidase and glycosyltransferase enzymes that interact with sugar substrates. Certain iminosugars are known to inhibit the ER glycosylation pathway by targeting and inhibiting α-glucosidase I and α-glucosidase II and comprise the largest class of α-glucosidase inhibitors currently in development (Simone et al., 2018). Perturbing ER α-glucosidase function can prevent the enzymes from removing terminal glucose residues on N-linked glycans, thereby inhibiting the interaction with the calnexin/calreticulin chaperone proteins and proper folding of some viral glycoproteins. This can ultimately lead to the activation of the cellular unfolded protein response, which can detect the misfolded viral proteins and send them for degradation, resulting in decreased production of virions. Alternatively, it is possible that some misfolded viral glycoproteins may escape detection and incorporate into viral particles, greatly reducing their infectivity (Fischer et al., 1996).
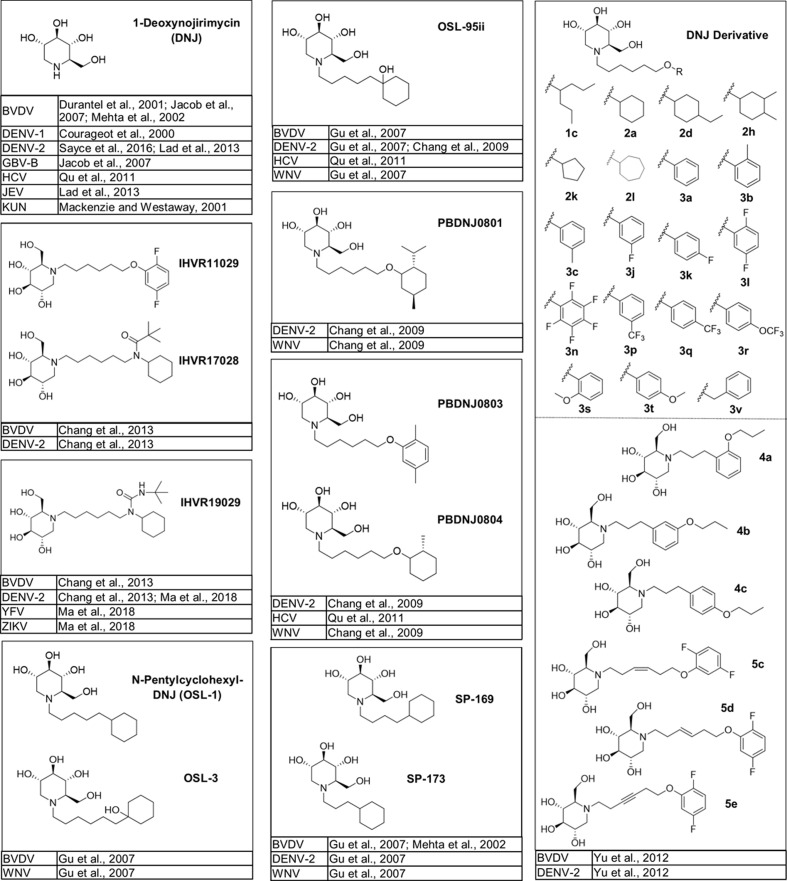
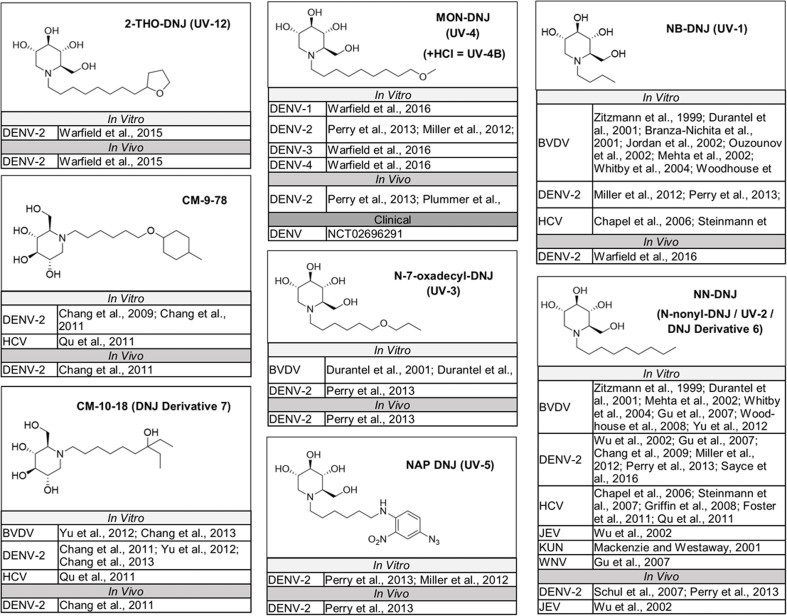
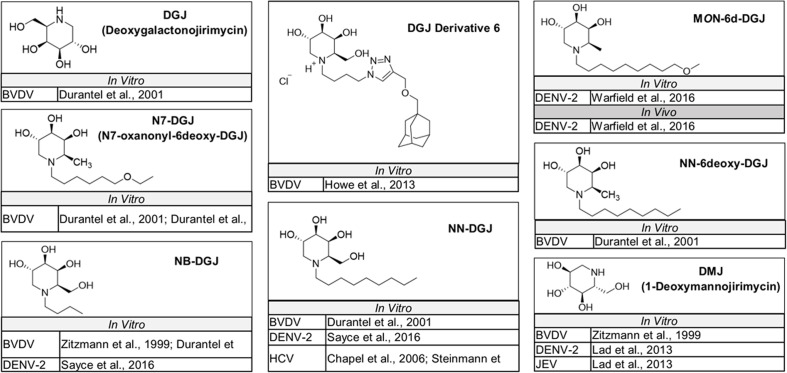
The naturally occurring compounds 1-deoxynojirimycin (DNJ) and castanospermine (CAST) were some of the first iminosugars identified to have antiviral properties. Medicinal chemistry strategies to design derivatives of these iminosugars have led to the development of compounds with increased activity and decreased associated cytotoxicity. Numerous analogs and derivatives of DNJ and other iminosugars have been evaluated for antiviral activity against a diverse range of enveloped viruses, including members of the Flaviviridae family (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5 ) and Ebola, Marburg, influenza A and B, herpes simplex, hepatitis B, Sindbis, Semliki Forest, chikungunya, measles, Newcastle disease, severe acute respiratory syndrome, Lassa, Junín, Rift Valley fever, vesicular stomatitis, and human immunodeficiency viruses (Chang et al., 2013a; Miller et al., 2018).
Fig. 1.
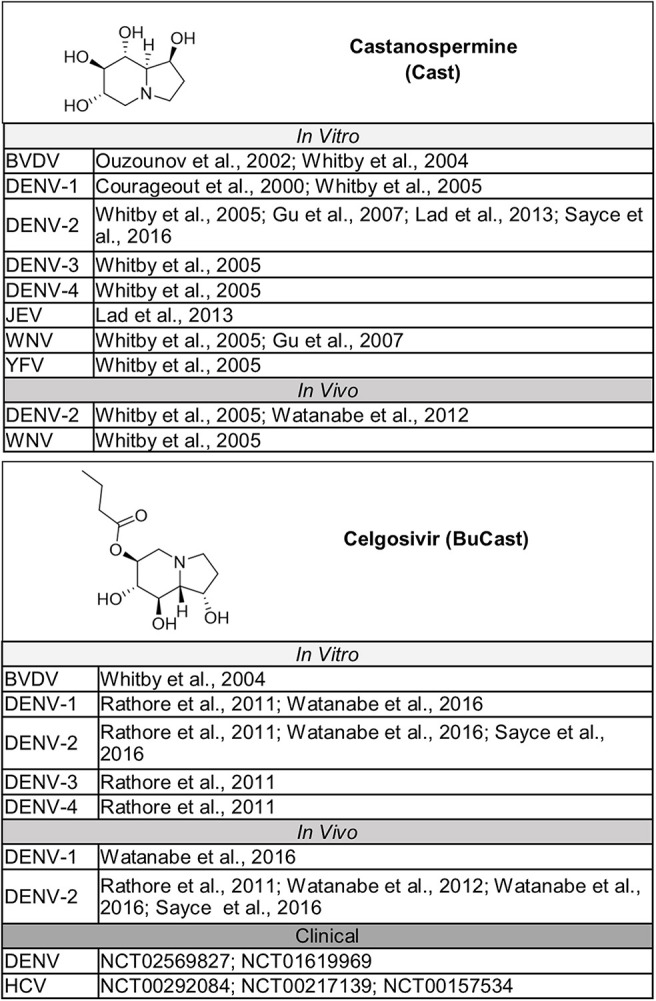
Bicyclic iminosugars evaluated for activity against members of the Flaviviridae family. Structures for bicyclic iminosugars that have been tested for activity in vitro and in vivo against members of the Flaviviridae family, both of which have advanced to in clinical trials as potential HTAVs to combat Flaviviridae infections (Watanabe et al., 2012).
Fig. 2.
DNJ derivatives evaluated in vitro for activity against a single member of the Flaviviridae family. Structures for monocyclic glucose-mimicking iminosugars that have been tested for activity in vitro against a single virus in the Flaviviridae family (Kiappes et al., 2018).
Fig. 3.
DNJ derivatives evaluated in vitro for activity against multiple members of the Flaviviridae family. Structures for monocyclic glucose-mimicking iminosugars that have been tested for activity in vitro against two or more members of the Flaviviridae family (Schul et al., 2007).
Fig. 4.
DNJ derivatives evaluated in vitro and in vivo for activity against members of the Flaviviridae family. Structures for monocyclic glucose-mimicking iminosugars that have been tested for activity against Flaviviridae in vitro and in animal models of disease or that have advanced to clinical development for Flaviviridae infections.
Fig. 5.
DGJ and other non-glucose-mimicking iminosugars evaluated in vitro or in vivo for activity against members of the Flaviviridae family. Structures for iminosugars containing headgroups that mimic galactose (DGJ and DGJ-derivatives) or mannose (DMJ) that have been tested for activity against Flaviviridae in vitro or in animal models of disease.
Although iminosugars are not currently approved for use as antiviral therapies, they have a good safety profile and a long history of use in humans. Several iminosugars are approved by the Food and Drug Administration (FDA) for other indications which require long-term treatment. The N-alkylated DNJ derivative, N-butyl-deoxynojirimycin (NB-DNJ/Miglustat/Zavesca®), is a glucosylceramide synthase enzyme inhibitor approved for use to treat adult patients with type 1 Gaucher's disease, a lysosomal storage disorder that results from impaired break down of glucocerebroside due to decreased production of the enzyme, glucocerebrosidase. Migalastat (Galafold™) is approved for the treatment of another type of genetic lysosomal storage disorder, Fabry disease, which is caused by increased levels of globotriaosylceramide due to a deficiency in the production of the enzyme, alpha-galactosidase A. An FDA-approved DNJ derivative, N-hydroxyethyl-1-deoxynojirimycin (BAY m 1099/Miglitol/Glyset®), is used to help control type 2 diabetes by targeting an α-glucosidase in the small intestines to prevent the breakdown of large carbohydrates into glucose. Acarbose (PRECOSE®) is similarly approved in the United States and other countries for the treatment of diabetes, as is voglibose (Voglib) in Japan. Several of these medications have been used in human patients for over 25 years and are considered safe treatment options for the intended indications. The clinical long-term use of iminosugars to treat these chronic diseases demonstrates the safety of this class of compounds and supports the further development of iminosugars for other indications, such as the treatment or prevention of certain viral infections. Although treatment of viral infections would require systemic delivery, for many viruses it would consist of a much shorter duration of treatment than for chronic indications, which is expected to limit the impact on host physiology. One of the practical challenges that needs to be considered when developing novel iminosugars for antiviral indications is the interaction between iminosugars and intestinal glucosidases, which is associated with undesired gastrointestinal side-effects. This can potentially be overcome by designing selective ER α-glucosidase inhibitors with reduced activity against the gastrointestinal enzymes and ensuring good bioavailability to minimize inhibition of intestinal glucosidases. It should be pointed out that gastrointestinal distress caused by long-term NB-DNJ (Zavesca®) use can be mitigated by changing dietary habits, such as reducing carbohydrate load with oral administration of the drug.
5. In vitro efficacy and mechanism of anti-Flaviviridae activity
A substantial amount of research has been performed in the past 20 years evaluating the activity of iminosugars against members of the Flaviviridae family (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5). Data from published studies assessing the antiviral effect of iminosugars against Flaviviridae in cell culture are summarized in Table 1 (bicyclic iminosugars), Table 2 (monocyclic glucose-mimicking iminosugars), and Table 3 (galactose-mimicking and other iminosugars). The details for each study are provided in Supplemental Table 2.
Table 1.
In vitro activity of bicyclic iminosugars against members of the Flaviviridae family. References are provided in Fig. 1 and details for each study are included in Supplemental Table 2.
| Bicyclic Iminosugars | ||||||
|---|---|---|---|---|---|---|
| Compound(s) |
Virus |
Summary of Results | ||||
| BVDV | DENV | JEV | WNV | YFV | ||
| Castanospermine (CAST) | X | X | X | X | X |
DENV (1–4) in multiple cell types: Reduced production of infective particles. IC50 = 1–85.7 μm. Resulted in altered prM glycosylation (DENV-2 in BHK-21 cells) and impaired formation of prME heterodimers (DENV-1 in Neuro 2a cells). WNV in BHK-21 cells: minimal to no inhibitory effect at up to 500 μM YFV in BHK-21 cells: 57% reduction at 50 μM JEV in PS cells: ≤10-fold reduction in virus titer BVDV in MDBK cells: IC50 = 5–367 μM; additive activity when used in combination with NB-DNJ |
| Celgosivir (BuCast) | X | X |
DENV-1 and -2 in multiple cell types: EC50 = 0.06–51 μM. Blocked transport of NS1 and E proteins from ER to Golgi and resulted in defect in NS1 glycosylation in BHK-21 cells DENV-3 and -4 in BHK-21 cells: EC50 = 0.31–0.68 μM. BVDV: Additive or synergistic activity in MDBK cells when used in combination with ribavirin or IFN-α. |
|||
Abbreviations: BVDV, bovine viral diarrhea virus; DENV, dengue virus; EC50, half maximal effective concentration; ER, endoplasmic reticulum; IC50, half maximal inhibitory concentration; IFN-α, interferon alpha; JEV, Japanese encephalitis virus; MDBK, Madin-Darby bovine kidney; NS1, non-structural protein 1; PS, porcine stable; prM: precursor membrane protein; prME: precursor membrane envelope protein complex; WNV, West Nile virus; YFV, yellow fever virus.
Table 2.
In vitro activity of DNJ and DNJ derivatives against members of the Flaviviridae family. References are provided in Fig. 2, Fig. 3, Fig. 4 and details for each study are included in Supplemental Table 2.
| Monocyclic Glucose-mimicking Iminosugars | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound(s) |
Virus |
Summary of Results | ||||||||
| BVDV | DENV | HCV | GBV-B | JEV | KUN | WNV | YFV | ZIKV | ||
| 2-THO-DNJ (UV-12) | X | DENV-2 in Vero cells: IC50 = 27.71 μM, CC50 > 500 μM | ||||||||
| CM-9-78 | X | X |
DENV-2 in BHK-21 or A549 cells: EC50 = 1.5–6.75 μM, CC50 > 40 μM HCV chimera in Huh7.5 cells: EC50 = 52.5 μM, CC50 > 500 μM; shift in electrophoresis mobility for E2 and E2-p7 proteins |
|||||||
| CM-10-18 (DNJ Derivative 7) | X | X | X | All viruses, multiple cell lines: EC50 = 1.1–27.2 μM, CC50 > 100 μM. Synergistic antiviral activity against DENV-2 in A549 cells when combined with ribavirin. | ||||||
| 1-Deoxynojirimycin (DNJ) | X | X | X | X | X | X | Low activity against most viruses with reported EC50 and EC90 values typically >100 μM. Low cytotoxicity (CC50s reported as >500 to >10,000 μM, depending on cell type and highest concentration tested). DENV-1 in Neuro 2a cells: E protein misfolding and impaired formation of prME heterodimers Greater effect on DENV-2 secretion (6 log pfu/mL reduction) at 100 μm/mL compared to JEV (~0.6–1 log pfu/mL reduction) in PS cells No inhibitory effect observed against KUN in Vero cells at 100 μM |
|||
| IHVR11029; IHVR17028 | X | X | BVDV in MDBK cells and DENV-2 in BHK-21 cells: EC50 = 0.3–1.3 μM, CC50 > 500 μM | |||||||
| IHVR19029 | X | X | X | X | Various cell types: EC50 = 0.25–1.7 μM (BVDV, DENV-2) and 21.5–30 μM (YFV, ZIKV), CC50 > 100–500 μM. Synergistic antiviral effect against YFV when used in combination with T-705. | |||||
| MON-DNJ (UV-4/UV-4B) | X |
DENV 1–4 in Vero cells: IC50 = 2.1–86.49 μM, CC50 > 500-1,000 μM DENV-2 in MDMs: IC50 = 3.09 μM, CC50 = 3,150 μM |
||||||||
| N-5-Oxaheptyl-DNJ (5–07); N-5-Oxahexyl-DNJ (5–06); N-5-Oxaoctyl-DNJ (5–08); N-6-Oxadecyl-DNJ (6–10); N-6-Oxanonyl-DNJ (6–09); N-6-Oxaocytyl-DNJ (6–08) |
X | MDBK cells: 5–07 and 5–06, no antiviral activity (up to 100 μM); 5–08 and 6–10, minimal antiviral activity (up to 100 μM); 6–09 and 6–08, moderate antiviral activity (up to 100 μM) but not improved compared to NN-DNJ. Series not advanced. CC50 > 300 μM for all compounds. | ||||||||
| N-7-Oxadecyl-DNJ (UV-3) | X | X |
BVDV in MDBK cells: IC50 = 17–80 μM, CC50 > 5,000 μM, reduced secretion of viral RNA in MDBK cells. DENV-2 in Vero cells: IC50 = 41 μM, CC50 = >500 μM |
|||||||
| NAP DNJ (UV-5) | X | Vero cells: IC50 = 2 μM, CC50 = 350 μM; MDMs: IC50 = 0.04 μM, CC50 = 300 μM | ||||||||
| NB-DNJ (UV-1) | X | X | X |
BVDV in MDBK cells: Large range in IC50s (2.5 to >200 μm), depending on MOI and assay readout. Infecting with lower MOI resulted in lower IC50 across assays; Dose dependent reduction in secretion of infectious virus particles that correlated with misfolding of viral glycoprotein E2; Synergistic when used in combination with IFN-α2b; Treatment of persistently infected MDBK cells in combination with IFN and ribavirin (containing 10 μM NB-DNJ) was sufficient to permanently eliminate virus after 9 passages following drug withdrawal. HCV chimera/replicon system: Dose dependent decrease in production of infectious particles in Huh7-Lunet and Huh7.5 cells; Reduced electrophoretic mobility of E1 and E2 glycoproteins. In Sf9 cells, E1 and E1 glycoproteins accumulated in ER, had reduced mobility shift, and interaction with calnexin was disrupted. DENV-2: IC50 = 6–10.6 μM, CC50 > 100 μM (MDMs); IC50 = 162 μM, CC50 > 500 μM (Vero cells) |
||||||
| NN-DNJ (N-nonyl-DNJ/UV-2/DNJ Derivative 6) | X | X | X | X | X | X |
BVDV in MBDK cells: Range in IC50s depending on assay and publication, with most ranging between 3 and 115 μM, CC50s mostly > 100 μM DENV-2 in multiple cell lines: IC50 = 1–9 μM, CC50 = 20–317 μM; interfered with prM, E, and NS1 protein interaction with calnexin in BHK-21 cells HCV in Huh7/Huh7.5/Huh7-Lunet cells: Multiple genotypes evaluated. Dose dependent decrease in production of infectious particles. Reduced electrophoretic mobility shift of E1 and E2 glycoproteins (JFH1 replicon system); inhibition of p7 function for most genotypes tested except for JFH-1/452 (genotype 3a) in which a lower inhibition of infectious particle secretion (only ~40% reduction at 50 μm) and minimal reduction in p7 function were observed. In Sf9 cells using HCV VLPs, E1 and E2 glycoproteins accumulated in ER and had reduced mobility shifts; VLPs produced had reduced binding to huh7 cells. HCV JFH-1 replicon: Additive effect on reduction of secreted infectious virus when used in combination with Rimantadine. JEV in BHK-21 cells: Dose dependent inhibition (2–3 log reduction at 100 μM), CC50 = 150 μM; interfered with transient interaction between viral proteins (prM, E, and NS1) and calnexin. KUN in Vero cells: secreted infectious virus titer reduced by 3.5 log at 100 μM WNV in BHK-21 cells: IC50 < 4 μM, CC50 = 20–100 μM |
|||
| OSL-1 (N-Pentylcyclohexyl-DNJ); OSL-3 | X | X |
BVDV in MDBK cells: IC50 < 20 μM, CC50 = ~200 μM WNV in BHK-21 cells: IC50 = 4–10 μM, dose dependent inhibition. |
|||||||
| OSL-2 | X | MDBK cells: IC50 > 70 μM, CC50 > 300 μM | ||||||||
| OSL-12-31; OSL-12-51 | X | MDBK cells: IC50 = ~70 μM (OSL-12-31) or >70 μM (OSL-1-51), CC50 > 300 μM | ||||||||
| OSL-95ii | X | X | X | X | All viruses, various cell lines: IC50 = 2–28 μM (WNV <4 μM), CC50 > 40–500 μM HCV JC1 chimera in Huh7.5 cells: shift in electrophoresis mobility for E2 and E2-p7 proteins |
|||||
| PBDNJ0801 | X | X | BHK-21 cells: EC50 = 0.1 μM (DENV-2) or 4.75 μM (WNV), CC50 = 70–80 μM | |||||||
| PBDNJ0802 | X | Huh7.5 cells: EC50 = 3.5 μM, CC50 > 500 μM | ||||||||
| PBDNJ0803; PBDNJ0804 |
X | X | X | BHK-21 cells: EC50 = 0.07–0.1 μM (DENV-2) and 1.5–3.5 μM (WNV), CC50 = 65–85 μM HCV chimera in Huh7.5 cells: EC50 = 1.7–5.4 μM, CC50 > 200 μM, electrophoretic mobility shift and degradation of E2 and E2-p7 proteins (PBDNJ0804) |
||||||
| SP-169; SP-173 | X | X | X | BVDV in MDBK cells, DENV-2/WNV in BHK-21 cells: IC50 = 3–5 μM, CC50 > 100 μM | ||||||
| ToP-DNJ | X | DENV-2: No inhibition observed in Huh7.5 cells (up to 50 μM), IC50 = 12.7 μM in MDMs | ||||||||
| DNJ Derivatives 7 - 13 | X | Reduction in viral RNA and infectious virus secretion measured in MDBK cells. Derivative 7: no antiviral activity at up to 50 μM Derivatives 8 and 9: No reduction at 10 μM, 25–75% reduction at 50 μM, CC50 > 250 μM Derivatives 10 and 11: ~50% reduction at 10 μM, CC50 ≥ 200 μM Derivatives 12 and 13: ≥75% reduction at 10 μM, CC50 = 29–51 μM |
||||||||
| DNJ Derivatives: 1a, 1b; 2b, 2c, 2e, 2f, 2g, 2i, 2j; 3d, 3e, 3f, 3g, 3h, 3i, 3m, 30, 3u; 4d, 4e, 4f; 5a, 5b | X | Single cycle virus yield reduction plaque assay using MDBK cells: Derivative 1 compounds: EC50 = 3–7.5 μM, CC50 ≥ 450 μM Derivative 2 compounds: EC50 = 0.3–1.8 μM, CC50 ≥ 150–500 μM Derivative 3 compounds: EC50 = 0.4–5.0 μM, CC50 ≥ 62–500 μM Derivative 4 compounds: EC50 = 3.5–24 μM, CC50 = 295–420 μM Derivative 5 compounds: EC50 = 11 μM (5a) and >100 μM (5b), CC50 > 500 μM |
||||||||
| DNJ Derivatives: 1c, 2a, 2d, 2h, 2k, 2l, 3a, 3b, 3c, 3j, 3k, 3l, 3n, 3p, 3q, 3r, 3s, 3t, 3v, 4a, 4b, 4c, 5d | X | X |
BVDV in MDBK cells and DENV-2 in BHK-21 cells: Activity of individual compounds comparable against DENV and BVDV. Derivative 1 compounds: EC50 = 0.3–0.6 μM, CC50 ≥ 480 μM Derivative 2, 3, and 4 compounds: EC50 = 0.3–4 μM, CC50 ≥ 400 μM Derivative 5 compounds: EC50 = 0.9–47 μM, CC50 > 500 μM |
|||||||
| SP-116; SP-150; SP-156; SP-159; SP-164; SP-165; SP-166; SP-168; SP-187 | X | Virus yield reduction using MDBK cells: SP-165, SP-166, SP-187: IC50 = 3–12 μM, CC50 ≥ 400 μM SP-116, SP-150, SP-156, SP-159, SP-168: IC50 = 24–40 μM, CC50 ≥ 500 μM SP-164: IC50 = 150 μM, CC50 > 1,000 μM |
||||||||
Abbreviations: BVDV, bovine viral diarrhea virus; CC50, 50% cytotoxic concentration; DENV, dengue virus; EC50, half maximal effective concentration; ER, endoplasmic reticulum; GBV-B, GB virus B; HCV, hepatitis C virus; IC50, half maximal inhibitory concentration; IFN, interferon; JEV, Japanese encephalitis virus; KUN, Kunjin virus; MDBK, Madin-Darby bovine kidney; MDM, monocyte-derived macrophages; MOI, multiplicity of infection; PS, porcine stable; pfu, plaque-forming unit; prM: precursor membrane protein; prME: precursor membrane-envelope protein complex; VLP, virus-like particle; WNV, West Nile virus; YFV, yellow fever virus; ZIKV, zika virus.
Table 3.
In vitro activity of iminosugars containing headgroups that mimic galactose (DGJ and DGJ-derivatives) or mannose (DMJ) tested for activity against members of the Flaviviridae family. References are provided in Fig. 5 and details for each study are included in Supplemental Table 2.
| Galactose-mimicking and other iminosugars | |||||
|---|---|---|---|---|---|
| Compound(s) | Virus |
Summary of Results | |||
| BVDV | DENV | HCV | JEV | ||
| DGJ (Deoxygalactonojirimycin) | X | No antiviral activity, CC50 > 10,000 μM | |||
| DGJ Derivative 6 | X | Minimal to no antiviral activity, CC50 > 250 μM | |||
| MON-6d-DGJ | X | DENV-2 in MDMs: no inhibitory effect up to 100 μM. | |||
| N7-DGJ (N7-oxanonyl-6deoxy-DGJ) | X | MDBK cells: IC50 = 2.5–125 μM depending on MOI (MOI 0.1 = 2.5 μM; MOI 1.0 = 125 μm), CC50 > 4,000 μM No viral rebound after 22 passages in persistently infected MDBK cells when used in combination with IFN and ribavirin |
|||
| NB-DGJ | X | X | No antiviral activity observed against BVDV in MDBK cells or DENV-2 in MDMs | ||
| NN-6deoxy-DGJ | X | MDBK cells: IC50 = 2–25 μM, CC50 = 187.5 μM | |||
| NN-DGJ | X | X | X |
BVDV in MDBK cells: IC50 = 12.5–25 μM, CC50 = 237.5 μM DENV-2 in MDMs, no antiviral activity (up to 31.6 μM) HCV in multiple genotypes and cell types: results varied, mostly ~moderate inhibition of secreted infectious virus. No change in the electrophoretic mobility of E1 or E2 in Huh7 or Sf9 cells |
|
| DMJ (1-Deoxymannojirimycin) | X | X | X | Virus secretion: BVDV in MDBK cells, minimal to no inhibition (up to 15,000 μM); DENV-2 in PS cells, reduced by ~6 log pfu/mL (at 200 μM/mL); JEV in PS cells, reduced by 0.6–1.0 log pfu/mL (at 200 μM/mL) | |
Abbreviations: BVDV, bovine viral diarrhea virus; CC50, 50% cytotoxic concentration; DENV, dengue virus; EC50, half maximal effective concentration; HCV, hepatitis C virus; IC50, half maximal inhibitory concentration; IFN, interferon; JEV, Japanese encephalitis virus; MDBK, Madin-Darby bovine kidney; MDM, monocyte-derived macrophages; MOI, multiplicity of infection; PS, porcine stable; pfu, plaque-forming unit.
5.1. Flaviviruses
The antiviral activity of iminosugars has been evaluated in vitro against multiple flaviviruses that pose a risk to human health, including all four DENV serotypes, with a strong focus on DENV-2 (Miller et al., 2018), as well as JEV (Wu et al., 2002), YFV (Ma et al., 2018a; Whitby et al., 2005), ZIKV (Ma et al., 2018a), WNV (Chang et al., 2009; Gu et al., 2007; Whitby et al., 2005), and the WNV variant strain, Kunjin virus (KUNV) (Mackenzie and Westaway, 2001). A large number of iminosugars tested exhibited moderate to strong activity against flaviviruses in vitro, with IC50s ranging from 40 nM to 20 μM, although the level of inhibition was dependent on the molecule, cell type, and virus. The variable response of flaviviruses was demonstrated when the antiviral activity of celgosivir, the butylated pro-drug of the bicyclic iminosugar, CAST, was measured against all four DENV serotypes in multiple cell lines. Celgosivir inhibited DENV 1–4 in BHK-12 cells, with half maximal inhibitory concentrations (IC50s) in the nanomolar range (Rathore et al., 2011; Watanabe et al., 2016). However, when efficacy was evaluated against DENV-1 and DENV-2 in Vero, Huh-7, and THP-1 cells, the IC50 varied, ranging from 0.75 to 51 μM depending on the serotype, MOI, and cell type (Watanabe et al., 2016).
The variable response of flaviviruses to iminosugar treatment was also recognized when the antiviral activity of CAST was evaluated against multiple viruses in parallel, including DENV, WNV, and YFV, in BHK-21 cells (Whitby et al., 2005). CAST treatment demonstrated inhibition against all four DENV serotypes, with >95% reduction in the number of infected cells at 50 μM, as measured by immunostaining and flow cytometry, but resulted in minimal to no inhibitory effect against WNV at concentrations up to 500 μM. Decreased inhibition of YFV was also observed compared to DENV, although there was still a 93% reduction at 500 μM relative to untreated cells. Plaque assays of cell supernatants confirmed this differential inhibitory response to treatment. Cells exposed to 50 μM of CAST had a 910-fold reduction in the production of infectious DENV particles, but only a 1.7- and 6.8-fold reduction was observed for WNV and YFV, respectively (Whitby et al., 2005). Despite the lack of antiviral activity of the bicyclic iminosugar CAST against WNV, several monocyclic iminosugars demonstrated strong anti-WNV activity in BHK-21 cells with dose-dependent inhibition and IC50s ranging between 1 and 10 μM (Chang et al., 2009; Gu et al., 2007), although most were still less efficacious against WNV than DENV (Chang et al., 2009).
The difference in sensitivity to iminosugar treatment between DENV and other flaviviruses was further demonstrated in HEK293 cells treated with IHVR-19029. The IC50 was 12.5 times higher for YFV than DENV, and 17.6 times higher for ZIKV than DENV (Ma et al., 2018a). Iminosugar efficacy has also been assessed against JEV in two published studies, both demonstrating a similar trend in antiviral activity when compared to DENV (Wu et al., 2002; Lad et al., 2013). The DNJ derivative, NN-DNJ, which is known to have more potent antiviral activity than DNJ, inhibited the production of infectious JEV and DENV-2 in BHK-12 cells, however, the reduction was much greater at lower concentrations for DENV-2 compared to JEV. Other iminosugars (CAST, DNJ, and DMJ) had minimal to no impact on JEV replication, reducing viral RNA or infectious particle secretion by only ≤10-fold, but inhibited DENV secretion by up to 106 plaque forming units (pfu). Similarly, NN-DNJ (100 μM) also reduced the secretion of infectious KUNV particles by more than 103 pfu in Vero cells despite DNJ having no inhibitory effect when tested at the same concentration (Mackenzie and Westaway, 2001). It is possible that differences in the number and/or location of glycosylation sites within viral proteins may contribute to the range in antiviral activity of iminosugars observed against different flaviviruses (Miller et al., 2018).
Depending on the structure, iminosugars are capable of differentially inhibiting glycolipid or glycoprotein processing enzymes. The ability of some iminosugars to inhibit glycolipid processing was originally thought to contribute to the anti-DENV activity of this class of compounds (Chatel-Chaix and Bartenschlager, 2014; Sayce et al., 2016; Welsch et al., 2009; Wichit et al., 2011). To better understand the mechanism iminosugars use to inhibit DENV, Sayce et al., evaluated the enzymatic targets of diverse bicyclic glucose- (CAST and celgosivir), monocyclic glucose- (DNJ, NB-DNJ, and NN-DNJ) and galactose-mimicking (deoxygalactonojirimycin (DGJ), NB-DGJ, and NN-DGJ) iminosugars, and correlated the target with the anti-DENV activity in primary human monocyte derived macrophages (MDMs) (Sayce et al., 2016). To identify the enzymatic target, they performed both in vitro glucosidase and glycosidase enzyme inhibition assays as well as measured the generation of glycosphingolipids (GSLs) or free oligosaccharides (FOS). Generation of triglucosylated or monoglucosylated FOS is used as a marker for ER α-glucosidase I and II inhibition, respectively, whereas the generation of the glycosphingolipid monosialodihexosylganglioside (GM3) is used as an indicator to measure glycolipid processing (Alonzi et al., 2008; Neville et al., 2004; Platt et al., 1994a, 1994b; Warfield et al., 2016b). Sayce et al., demonstrated that monocyclic and bicyclic glucose-mimicking iminosugars inhibit glycoprotein processing, but only monocyclic compounds also inhibit glycolipid processing enzymes. Galactose-mimicking iminosugars inhibited glycolipid processing but not glycoprotein processing. Interestingly, the level of glucosyltransferase enzymatic inhibition correlated with the length of the alkyl tail, despite whether the iminosugar mimicked glucose or galactose, with longer tails having stronger inhibition (Butters et al., 2000; Caputo et al., 2016; Mellor et al., 2002, 2003; Sayce et al., 2016). Consistent with the enzymatic data, the glucose-mimicking iminosugars celgosivir and NB-DNJ generated FOS (Sayce et al., 2016; Warfield et al., 2016b). In contrast, DGJ derivatives reduced GM3 levels but did not generate triglucosylated or monoglucosylated FOS. Only the glucose-mimicking iminosugars that inhibited enzymes required for either glycoprotein processing, or both glycoprotein and glycolipid processing, were efficacious against DENV-2, whereas iminosugars with a galactose-like configuration had no measurable effect on the virus.
In a similar study, the glucose-mimicking iminosugar, UV-4B (MON-DNJ), inhibited the enzymatic activity of both α-glucosidase I and II, but when an isomer of UV-4B designed to have a galactose-like configuration was tested, α-glucosidase I and II inhibition was lost (Warfield et al., 2016b). MDMs treated with UV-4B containing either a glucose- or galactose-like configuration had reduced levels of GM3; however, only UV-4B with a glucose-like configuration generated FOS and was active against DENV, while the galactose-mimicking isomer lacking α -glucosidase inhibition was not efficacious against the virus.
Because iminosugars have the potential to alter the levels and/or folding of viral glycoproteins, treatment can ultimately result in decreased viral production or reduced infectivity of progeny viruses. In vitro studies have been performed to evaluate the effects of DNJ, NN-DNJ, CAST, and celgosivir on DENV or JEV glycoproteins, and to determine whether misfolded viral proteins are degraded or incorporated into newly released virus particles with decreased infectivity (Courageot et al., 2000; Rathore et al., 2011; Sayce et al., 2016; Warfield et al., 2016b; Wu et al., 2002). These studies demonstrated that treatment with iminosugars can alter glycan processing of the virus prM, E, and NS1 glycoproteins (Courageot et al., 2000; Rathore et al., 2011), prevent their interaction with calnexin (Wu et al., 2002), reduce proper folding of prM and E (Courageot et al., 2000), inhibit the formation and stability of prM-E heterodimer complexes (Courageot et al., 2000), prevent the transport of E and NS1 from the ER to the Golgi (Rathore et al., 2011), and reduce secretion of both E and NS1 from cells (Rathore et al., 2011). The fact that iminosugars can block the transport and secretion of some flavivirus glycoproteins suggests that inhibiting ER α-glucosidase function likely results in decreased formation and secretion of new viruses rather than the formation of less infectious particles. Two separate studies have confirmed that this is the main mechanism employed by UV-4B, NB-DNJ, and celgosivir to inhibit DENV (Sayce et al., 2016; Warfield et al., 2016b). In these studies, the authors performed plaque assays to measure the amount of infectious virus secreted from iminosugar treated MDMs and compared that to quantitative polymerase chain reaction results measuring the total amount of viral RNA secreted from the cells. Data from both studies were consistent and found that there was a direct correlation between the decrease in secreted infectious virus titer and total virus production, providing evidence that the viral glycoproteins are likely misfolded and targeted for degradation rather than partially misfolded and incorporated into newly released particles with decreased infectivity.
5.2. HCV and BVDV
Prior to the development of in vitro culture systems for HCV, the closely related virus, bovine viral diarrhea virus (BVDV), was commonly used as a surrogate model. Cytopathic and noncytopathic BVDV culture systems have been used to evaluate the antiviral activity and mechanism of action of iminosugars in Madin-Darby bovine kidney (MDBK) cells (Branza-Nichita et al., 2001; Chang et al., 2013b; Chapman et al., 2005; Durantel et al., 2001, 2004; Gu et al., 2007; Howe et al., 2013; Jacob et al., 2007; Jordan et al., 2002; Mehta et al., 2002; Ouzounov et al., 2002; Whitby et al., 2004; Woodhouse et al., 2008; Yu et al., 2012; Zitzmann et al., 1999). Several DNJ derivatives were shown to inhibit BVDV by preventing the secretion of viral particles and viral RNA (Durantel et al., 2001; Jordan et al., 2002; Zitzmann et al., 1999). Similar to subsequent observations for flaviviruses, the antiviral effect elicited by the long-alkyl-chain DNJ derivative, NN-DNJ, was stronger than that of the short-alkyl-chain derivative, NB-DNJ. Treatment with DNJ derivatives was also shown to result in the assembly and release of low levels of viral particles with decreased infectivity (Durantel et al., 2001). The production of noninfectious particles was less pronounced when cells were treated with NB-DNJ compared to NN-DNJ, which may account for the stronger anti-BVDV activity associated with the long-alkyl-chain derivatives (Durantel et al., 2001).
To further evaluate the impact of the alkyl-chain length, as well as other potential inhibitory mechanisms of iminosugars, Durantel, et al., evaluated and compared the anti-BVDV activity of long- and short-alkyl-chain glucose-derived DNJ derivatives to that of long-alkyl-chain galactose-derived iminosugar derivatives of DGJ, which do not inhibit ER α-glucosidases (Durantel et al., 2001). Both DGJ and DNJ derivatives inhibited BDBV in cell culture, unlike what was later observed for flaviviruses, in which only DNJ derivatives demonstrated antiviral activity. However, only the long-alkyl-chain DGJ derivative and not the short-alkyl-chain derivative was efficacious against BVDV. This effect was solely the consequence of the production of noninfectious virions, rather than a reduction in viral secretion. Deletion of the viral membrane-spanning protein p7 has a similar impact on BVDV replication in vitro, also resulting in the production of viral particles with decreased infectivity without inhibiting viral secretion in media (Harada et al., 2000). P7 was also shown to be essential for the production of infectious HCV virus (Jones et al., 2007; Sakai et al., 2003; Steinmann et al., 2007). The long-alkyl-chain iminosugar derivatives, NN-DNJ, N7-DGJ, and NN-DGJ, were found to inhibit HCV p7 ion channel activity in a dose-dependent manner (Pavlovic et al., 2003). Interestingly, DENV-2 and JEV, which do not encode for the p7/p13 protein, are not inhibited by DGJ derivatives (Wu et al., 2002).
While many of the initial studies used BVDV as a surrogate for HCV, the use of HCV virus-like particles (VLPs) and the later developed in vitro HCV infectivity systems helped to confirm the anti-HCV activity of iminosugars against multiple genotypes (Fig. 1 and Table 2). Exposure to NN-DNJ inhibited the release of HCV particles in a dose-dependent manner in Huh7 cells transfected with JFH-1 or chimeric derivatives containing structural proteins of other genotypes, including 1a (H77), 1b (CON-1), 2a (JS, CJ-1), and 3a (452), but was less active against the 3a (452) chimera (Griffin et al., 2008). To evaluate the potential cause for decreased activity against the 3a (452) chimera, the impact of iminosugar treatment on p7 function was evaluated by measuring the ability of p7 to release a fluorescent dye from liposomes. NN-DNJ had minimal to no impact on 3a (452) p7 function; however, NN-DNJ reduced the activity of p7 from all other HCV isolates tested, correlating with the in vitro viral inhibition data (Griffin et al., 2008). Alignment of the p7 sequences from different HCV isolates identified a polymorphism (F25A) in the 3a HCV genotype (Foster et al., 2011). When a mutant chimeric JFH-1 virus containing the p7 F25A polymorphism was exposed to NN-DNJ or NN-DGJ treatment, minimal to no effect was observed on virus secretion, whereas secretion of wild-type JFH-1 virus was largely inhibited following exposure to the iminosugars.
Data from mechanistic studies show that iminosugars have similar effects on HCV and the surrogate BVDV culture systems. Iminosugars prevent the release of BVDV and HCV viral RNA and infectious virus with minimal to no impact on viral RNA synthesis (Durantel et al., 2001; Jordan et al., 2002; Qu et al., 2011; Zitzmann et al., 1999). Treatment with iminosugars also alters the interaction between BVDV or HCV glycoproteins and ER chaperone proteins (Branza-Nichita et al., 2001; Chapel et al., 2006; Choukhi et al., 1998; Durantel et al., 2007), and results in an electrophoretic mobility shift caused by deficient glycan processing, which is accompanied by the production of misfolded glycoproteins and/or decreased formation of E1-E2 heterodimers (Branza-Nichita et al., 2001; Chapel et al., 2006; Qu et al., 2011).
The antiviral effect of NB-DNJ was attributed to inefficient glucose trimming of BVDV envelope glycoproteins by the ER α-glucosidases, inhibiting the association of the envelope proteins with calnexin and resulting in the production of misfolded viral envelope glycoproteins and decreased E1-E2 heterodimer formation (Branza-Nichita et al., 2001). The HCV glycoproteins E1 and the E2 precursor, E2-NS2, were found to interact with multiple ER chaperones, although only calnexin interacted primarily with monomers or noncovalent E1-E2 complexes, which are formed prior to the final folding stage (Choukhi et al., 1998). By using HCV VLPs, it was shown that iminosugars inhibit N-linked glycan processing to interrupt this interaction, resulting in the production of VLPs containing unprocessed N-linked glycans and misfolded proteins that have a decreased binding affinity to hepatoma cells (Chapel et al., 2006).
In addition to having a direct effect on viral glycoproteins, it is possible iminosugars may also regulate infectivity by altering the expression or perturbing the function of host glycoproteins that are required for viral entry and replication. Although changes to host glycoproteins have been implicated as a contributor to the antiviral activity of iminosugars against the severe acute respiratory syndrome coronavirus (SARS-CoV), the impact of potential alterations to host-protein glycosylation on Flaviviridae entry or replication has not been evaluated (Zhao et al., 2015). Miller et al., 2012 recently reported that iminosugars may alter the function and expression of some host glycoproteins involved in DENV replication, although the possible effect these changes may have on DENV infection is not yet understood (Miller et al., 2019).
6. In vivo efficacy of iminosugars against Flaviviridae
Despite the extensive in vitro efficacy studies that have been performed evaluating the antiviral activity of iminosugars against multiple members of the Flaviviridae family, in vivo studies have been primarily limited to murine models of DENV infection, particularly DENV-2 (for a more in-depth review, refer to Miller et al., 2018). This has been due, in part, to a lack of suitable small animal models available for HCV and BVDV (Billerbeck et al., 2013), and because of the stronger in vitro antiviral activity observed against DENV compared to other flaviviruses. Animal models of JEV and WNV disease are the only Flaviviridae infection models, other than DENV, that have been used in published studies to evaluate the antiviral activity of iminosugars (Whitby et al., 2005; Wu et al., 2002). In fact, the first in vivo model system used to evaluate the efficacy of an iminosugar against a Flaviviridae family member was the murine model of JEV disease (Wu et al., 2002). A survival benefit was observed in comparison to control treated animals when female ICR mice were administered NN-DNJ (200 mg/kg, oral administration, once daily) beginning one day before simultaneous intraperitoneal and intracranial challenge with JEV. Considering that NN-DNJ was notably more efficacious against DENV-2 than JEV (RP9 strain) in vitro, the observation that NN-DNJ protected mice against a lethal JEV infection in vivo supported the continued examination of iminosugars against other flaviviruses.
The potential in vivo anti-flavivirus activity of iminosugars was next examined against WNV and DENV-2 (Whitby et al., 2005). C57BL/6 mice were infected with 102 pfu of WNV (3000.0259 strain) by footpad injection and A/J mice were infected intracranially with 105 pfu of mouse-neuroadapted DENV-2 (NGC strain). Mice were treated intraperitoneally with up to 250 mg/kg CAST or vehicle once daily beginning on the day of infection (Whitby et al., 2005). While CAST treatment resulted in a significant survival benefit against DENV-2 compared to control treated mice, it had no measurable effect on mortality when mice were infected with WNV. This in vivo response correlated with observations in vitro, where CAST had minimal to no inhibitory effect against WNV at concentrations of up to 500 μM. However, unlike CAST, which is a bicyclic iminosugar, several monocyclic iminosugars, such as NB-DNJ (Table 1), have proven to be efficacious in vitro against WNV but have not been tested in vivo (Chang et al., 2009; Gu et al., 2007). Despite the lack of activity of CAST against WNV in the murine model, it is possible treatment with monocyclic iminosugars would result in a survival benefit based on the results of in vitro studies. These monocyclic compounds may elicit their anti-WNV activity through a separate or additional mechanism, such as through their ability to also inhibit the glycolipid biosynthesis pathway, although alteration of glycosphingolipid activity was shown to not be the mechanism of anti-DENV activity for the iminosugar, UV-4 (Sayce et al., 2016; Warfield et al., 2016b).
Two iminosugars, UV-4B and celgosivir, successfully protected mice against a lethal challenge with DENV-2 when treatment was delayed and initiated up to 48 h post-challenge. (Perry et al., 2013; Rathore et al., 2011; Warfield et al., 2016b). Both of these molecules have advanced to clinical trials, with UV-4B being evaluated in early phase safety and pharmacokinetic studies, and celgosivir in adults with confirmed dengue fever (discussed below) (Low et al., 2014; Spurgers et al., unpublished data). Several other iminosugars have demonstrated efficacy in murine models of DENV-2 disease, including antibody dependent enhancement (ADE) models. ADE is an increase in the infectivity of a virus due to preexisting, poorly neutralizing antibodies that can facilitate entry of the virus into host cells and exacerbate disease severity. The highest known risk of severe dengue disease is in patients previously infected with a different DENV serotype who have intermediate levels of pre-existing anti-DENV antibodies (Katzelnick et al., 2017). Murine models of ADE have been used to better represent severe DENV infections in humans. Passive administration of anti-DENV antibodies has been shown to be sufficient to enhance DENV disease in mice using both mouse-adapted and clinical DENV isolates (Balsitis et al., 2010). Clinical features of lethal DENV-ADE disease models include thrombocytopenia, vascular leakage, elevated serum cytokine levels, and increased systemic viral burden in serum and tissue phagocytes, all hallmarks of severe dengue disease in humans (Balsitis et al., 2010).
Celgosivir is the only iminosugar for which data has been published evaluating the in vivo efficacy against a DENV serotype other than DENV-2 (Watanabe et al., 2016). AG129 mice were infected intravenously with 7 × 107 pfu DENV-1 (2402 strain) after being exposed to the 4G2 anti-E antibody to induce ADE, and mice were treated orally with vehicle or celgosivir (10 mg/kg or 50 mg/kg) twice daily, starting at the time of infection. Although a survival benefit was not observed for animals in the low-dose group (10 mg/kg), all mice in the high-dose group (50 mg/kg) survived infection.
7. Anti-Flaviviridae activity of iminosugar drug combinations
Drug combination therapies are often considered for the treatment of viral infections. Combination treatment may minimize the potential for resistant strains to develop against an individual drug, as well as maximize compound efficacy if a synergistic benefit is observed with the combination treatment. Iminosugars were first evaluated in clinical trials for an antiviral indication as part of a combination therapy with zidovudine (AZT) for the treatment of HIV infections, although these studies did not proceed further than Phase 2 (Fischl et al., 1994). Prior to the recent development and approval of highly efficacious direct-acting antivirals against HCV (discussed in more detail below), the standard therapy for patients consisted of a less effective combination of interferon (IFN) and ribavirin, which could be associated with multiple, and sometimes serious, side effects (Das and Pandya, 2018; Elloumi et al., 2007; Ferenci et al., 2005; Manns et al., 2006). In pursuit of improving the treatment regimen, the potential benefit of incorporating iminosugars into this therapeutic modality was evaluated. In vitro drug synergy studies were performed using BVDV culture systems to evaluate the antiviral efficacy of iminosugars when used in combination with IFN, ribavirin, or a second iminosugar (Jacob et al., 2007; Ouzounov et al., 2002; Whitby et al., 2004; Woodhouse et al., 2008). When BVDV-infected MDBK cells were treated with a combination of two iminosugars, CAST and NB-DNJ, the observed antiviral activity was additive with no statistically significant synergistic or antagonistic antiviral effect, which would be expected considering CAST and NB-DNJ have the same cellular target (Ouzounov et al., 2002). However, when NB-DNJ was combined with IFN-α2b under the same conditions, treatment resulted in synergistic antiviral activity against BVDV. In a separate study, synergistic antiviral activity was also observed when BVDV-infected MDBK cells were treated with celgosivir plus INF-α or celgosivir plus ribavirin in two or three out of four experiments, respectively (Whitby et al., 2004).
To extend observations beyond dual-drug treatment combinations, the antiviral activity of triple-drug combinations, consisting of IFN, ribavirin, and an iminosugar, was evaluated in MDBK cells persistently infected with a noncytopathic strain of BVDV (Woodhouse et al., 2008). Cells were passaged in the presence of IFN and ribavirin, with or without the addition of one of three iminosugars (N7-DGJ, NB-DNJ, or NN-DNJ). Treatment was sustained over several passages and the impact on long-term viral clearance was measured following removal of one or all drugs. Cells treated with the dual-drug combination of IFN and ribavirin maintained viral clearance only if treatment was continued; removal of the drugs resulted in a loss of viral clearance over time. However, with the addition of an iminosugar to the treatment regimen, viral clearance was sustained, even after removal of the drugs. NB-DNJ had the greatest impact on maintaining viral clearance. Treatment of cells for nine weeks with the three-drug cocktail containing 10 μM NB-DNJ was sufficient to permanently eliminate the virus.
The antiviral activity of iminosugar drug combinations was also evaluated against members of the Flavivirus genera. Synergistic antiviral activity was observed when HEK293 cells were infected with the vaccine strain of YFV (17D) and treated with the iminosugar, IHVR-19029, and the viral RNA polymerase inhibitor, favipiravir (T-705). While the antiviral efficacy of this drug combination was not evaluated in an in vivo flavivirus disease model, T-705 was found to potentiate the antiviral activity of IHVR-19029 in a murine model of Ebola virus disease (Ma et al., 2018a). Ribavirin was also found to have a synergistic antiviral effect in vitro against DENV-2 when combined with the iminosugar CM-10-18 (Chang et al., 2011). In this study, the enhanced activity of ribavirin by CM-10-18 was confirmed using a murine model of DENV-2 infection. These studies support the potential use of iminosugars in drug combination therapies for the treatment of flavivirus infections.
8. Broad-spectrum antiviral activity of iminosugars
Considering that many viruses exploit the host's N-linked glycosylation machinery for the morphogenesis of progeny virus particles, it is not surprising that the antiviral activity of some iminosugars expands far beyond members of the Flaviviridae family. Several iminosugars have proven to be efficacious in small animal models of disease for influenza A, influenza B, herpes simplex, Ebola, and Marburg viruses (Bridges et al., 1995; Chang et al., 2013b; Stavale et al., 2015; Warfield et al., 2015, 2016a; Zhang et al., 2013), as well as against many diverse DNA and RNA viruses using cell culture systems (refer to table 20.3 from Miller et al., 2018 and Table 2 from Chang et al., 2013a, Chang et al., 2013b). Included in this group of viruses are the coronaviruses, SARS-CoV and the recently identified SARS-CoV-2, responsible for the ongoing global pandemic (Wu et al., 2004; Zhao et al., 2015; Rajasekharan et al., 2020). Iminosugars have been shown to decrease the production of infectious virions in vitro as well as alter the N-linked glycan structure of the SARS-CoV and SARS-CoV-2 cellular receptor, angiotensin I-converting enzyme 2 (ACE2), impairing entry of SARS-CoV and human coronavirus NL63 (HCoV-NL63) spike glycoprotein-pseudotyped lentiviral particles through a post-receptor binding mechanism (Zhao et al., 2015).
Similar to what has been observed for Flaviviridae, the antiviral activity of iminosugars against other viruses varies with the compound, virus, and cell type. The mechanism of antiviral activity can also vary depending on the compound and the virus. Influenza virus and HIV are both good examples of the differential effects that can be observed following treatment with iminosugars. Extensive research has been performed evaluating the effect of iminosugars against influenza virus, which is discussed further in a separate review (Plummer et al., 2015). Interestingly, iminosugars tend to have low potency against influenza virus in vitro, yet treatment provides partial or full protection against lethal infections in murine models of disease (Warfield et al., 2015). Studies evaluating the mechanism of anti-HIV activity of iminosugars found that treating infected cells with iminosugars can alter the glycosylation of the viral gp120 glycoprotein (Fischer et al., 1995, 1996). This can impair the ability of new HIV particles to enter cells following binding to CD4 rather than reduce the production of secreted progeny viruses, as is observed for flaviviruses. Despite the differences in the level and mechanism of antiviral activity of iminosugars, the broad antiviral potential of this class of compounds supports further research into the potential use of these molecules for the treatment of diverse viral infections.
9. High barrier to development of in vivo drug resistance to iminosugars
One of the many challenges in the antiviral drug development field is understanding how to design compounds that will remain efficacious despite the high genetic variability of most viruses, which leads to rapid generation of antiviral drug resistance. For example, influenza virus resistance to oseltamivir (Tamiflu®) has been noted after a single mutation, and recent publications discussed resistance to baloxavir marboxil (XOFLUXA™), which was approved in the U.S. in October, 2018 (Bloom et al., 2010; Hauge et al., 2009; Meijer et al., 2009; Omoto et al., 2018; Takashita et al., 2019a, 2019b). Current strategies proposed for overcoming this challenge include the development of drug combination therapies that contain drugs targeting multiple viral proteins and HTAVs, both of which are less likely to generate resistant strains compared to direct-acting antivirals targeting a single viral protein. For HTAVs, this is due to the detrimental effect resistant mutations would likely have on viral replication if the virus was critically dependent on the host factor targeted by the drug.
In order to determine whether DENV has the potential to mutate and develop resistance to iminosugars, Plummer et al., performed an in vivo passaging study in mice (Plummer et al., 2015). DENV (DENV-2 strain S221) was passaged in STAT1−/−/2−/− mice treated orally with either UV-4B or vehicle (water) for 4 serial passages; samples were pooled by group after each passage and evaluated to determine whether the virus could mutate and develop resistant strains. Only 13 nonsynonymous mutations were identified in pooled samples from UV-4B treated mice, 12 of which were also identified in virus isolated from vehicle-treated control animals. Viruses isolated after each passage were still susceptible to treatment with UV-4B. Considering that DENV is transmitted from invertebrate (mosquito) to vertebrate hosts rather than from vertebrate to vertebrate (e.g., mouse to mouse), and to address the possible impact pooling the samples may have had on diluting mouse-specific responses, the authors also identified mutations in viruses isolated from individual mice after a single passage in the presence or absence of UV-4B. After only a single passage in vivo, 19 nonsynonymous mutations were identified in DENV glycosylated proteins (M, E, and NS1) that were specific to UV-4B treatment. Despite the increased number of nonsynonymous mutations identified, these viruses were still susceptible to treatment with UV-4B providing no evidence that viral escape mutants were generated after single or multiple cycles of proliferation in the presence of the iminosugar in vivo. A high barrier to the development of drug resistant DENV viral strains was also observed in vitro when DENV was passaged up to 30 times in cells in the presence of UV-4B (Warfield et al., unpublished data).
The potential for UV-4B to induce the development of drug resistant viral strains was also evaluated in a murine model of influenza A virus (Warfield et al., 2019). Similar to what was observed in the DENV model, when mouse-adapted influenza A virus was passaged in the presence or absence of UV-4B for five successive passages, the virus maintained sensitivity to UV-4B treatment in mice. Mice infected with recombinant viruses containing individual or combinations of nonsynonymous mutations identified in the passaged virus were also still sensitive to UV-4B treatment, further confirming the high genetic barrier to the generation of drug resistant strains following exposure to an iminosugar antiviral.
10. Clinical trials: iminosugars as antivirals
Three candidate iminosugars have advanced to clinical trials to evaluate safety and efficacy against viral infections: celgosivir (HCV, DENV, and HIV), NB-DNJ (HIV), and UV-4B (DENV). HCV was the first of the two Flaviviridae family members to be included in a clinical trial assessing the therapeutic potential of an iminosugar (Table 4 ). Prior to the approval of current direct-acting antiviral therapies for HCV infections, several Phase 2 clinical trials were completed to evaluate the safety and efficacy of celgosivir against chronic HCV genotype 1 infections when administered as a monotherapy (NCT00157534) or in combination with PEGylated IFN-α2b or PEGylated IFN-α2b plus ribavirin (NCT00332176 and NCT00217139). In the initial Phase 2a randomized, dose-ranging trial, 43 patients with chronic HCV infections who were either IFN-intolerant or had never been treated with IFN were enrolled to receive celgosivir treatment (200 or 400 mg once daily or 200 mg twice daily) for 12 weeks. Viral load reduction was only observed for 2 of the 35 patients who completed the full treatment course (Durantel, 2009; Yoshida et al., 2006). Although well tolerated, celgosivir treatment only provided a measurable therapeutic benefit when used as part of a drug combination in other trials. Clinical trials were conducted to evaluate the safety and efficacy of celgosivir plus either PEGylated IFN-α2b or PEGylated IFN-α2b and ribavirin in patients not previously treated with IFN (NCT00332176) or patients who did not respond or only partially responded to prior treatment with PEGylated IFN-α2b (NCT00217139). Non-responders treated with the triple drug combination had an increased average early viral response (42%) when compared to patients that received the standard treatment of PEGylated IFN-α2b and ribavirin (10%), and a greater reduction in viral load (−1.63 log10) compared to the control group receiving the standard treatment (−0.92 log10) (NCT00217139) (Durantel, 2009). A follow-up trial was conducted (NCT00292084) as an extension of the original clinical trial (NCT00217139) to allow patients from the original trial an opportunity to switch treatments and receive therapy for an additional 36 weeks, although results from this study are not yet reported.
Table 4.
Clinical stage development of iminosugars for the treatment of Flaviviridae infections.
| Compound | Indication/Virus | Study Details | Study Endpoint/Readouts | Results | References |
|---|---|---|---|---|---|
| Celgosivir | HCV (genotype 1) | Phase 2a randomized, dose-ranging trial; 43 adult participants, ages 18–65 with chronic HCV infections who were IFN-intolerant or never treated with IFN; 200 or 400 mg once daily or 200 mg twice daily for 12 weeks | Safety and efficacy (viral load reduction) | Viral load reduction observed for 2 of the 35 patients who completed the full treatment course. Well tolerated but no measurable therapeutic benefit. | NCT00157534; Durantel 2009; Yoshida et al. (2006) |
| DENV | Phase 1 randomized, double-blind, placebo-controlled study; 50 adult participants, ages 21–60 with uncomplicated DENV fever (fever ≥38 °C for less than 48 h); 24 participants received celgosivir (400 mg loading dose, 200 mg maintenance dose every 12 h for a total of 9 doses) and 26 participants received placebo control | Safety, pharmacokinetics, and efficacy (primary: viral load reduction and fever reduction; secondary: NS1 and NS1 clearance) | Celgosivir was well tolerated by patients; no measurable reduction in viral load or fever burden. |
NCT01619969; Low et al. (2014) |
|
| Phase 2 double-blind, placebo-controlled study; Adult participants ages 21–65 with acute febrile illness with fever >37.5 °C for less than 48 h); celgosivir arm: 150 mg every 6 h for a total of 20 doses | Safety and efficacy (primary: viral load reduction, AUC; secondary: fever clearance time, duration of illness, time to NSI clearance) | NA/not yet recruiting | NCT02569827 | ||
| Celgosivir ± standard care (PEGylated IFN-α2b + Ribavirin) | HCV (genotype 1) | Phase 2 randomized, active controlled study; 50 adult participants, ages 18–65 with chronic HCV infections who were not previously treated with IFN; 400 mg or 600 mg celgosivir qd plus standard care, or standard care alone, for 12 weeks | Safety, tolerability, pharmacokinetics, efficacy (viral load reduction) | NA | NCT00332176 |
| Celgosivir + PEGylated IFN-α2b +/− Ribavirin |
HCV (genotype 1) | Phase 2 randomized, active controlled study; 60 participants ages 18–65 with chronic HCV infections who did not respond or only partially responded to prior treatment with PEGylated IFN-α2b; Study arms not indicated, 12-week treatment | Safety and efficacy (viral load reduction) | Non-responders treated with the triple drug combination had an increased average early viral response (42%) when compared to patients that received the standard treatment of PEGylated IFN-α2b and ribavirin (10%), and a greater reduction in viral load (−1.6310) compared to the control group receiving the standard treatment (−0.92 log10). | NCT00217139 |
| HCV (genotype 1) | Phase 2 (extension of clinical trial NCT00217139); allow patients to switch and/or continue celgosivir (plus IFN or IFN and ribavirin) treatment for an additional 36 weeks | Safety and efficacy (viral load reduction) | N/A | NCT00292084 | |
| UV-4B | DENV | Phase 1 randomized, double-blind, placebo-controlled, single-ascending dose study; 64 participants, healthy subjects received placebo control or up to 1,000 mg UV-4B (single dose) | Safety, tolerability, and pharmacokinetics | No serious adverse events. | NCT02061358; Spurgers et al., unpublished data |
| Phase 1 randomized, double-blind, placebo-controlled, multiple ascending dose study; healthy adult subjects, ages 18–45; placebo or UV-4B TID treatment (ascending doses) for 7 days | Safety, tolerability, and pharmacokinetics | Terminated early before DENV-infected patients were dosed; product development ceased for business reasons. | NCT02696291 | ||
Abbreviations: BID, twice daily; DENV, dengue virus; h, hours; HCV, hepatitis C virus; IFN, interferon; NA, not available; NS1, non-structural protein 1; qd, once daily; RT-PCR, reverse transcription polymerase chain reaction; TID, three times daily; WNV, West Nile virus.
More recently, celgosivir entered a proof of concept, Phase 1b double-blind, randomized clinical trial (NCT01619969) for the treatment of DENV infections in adult patients with uncomplicated dengue fever (as defined by patients having a fever of at least 38 °C for less than 48 h) (Low et al., 2014). Fifty patients were enrolled in the study, with 24 assigned to the celgosivir group and 26 to the placebo group. Patients receiving celgosivir treatment were administered an initial loading dose of 400 mg, followed by a maintenance dose of 200 mg every 12 h. Treatment was continued until patients received a total of nine doses. While celgosivir was well tolerated by patients in the test group, there was no measurable reduction in viral load or fever burden. It is possible that use of a subtherapeutic dose may have contributed to these results. A follow-up Phase 2 clinical trial (NCT02569827) was recently approved to evaluate a higher-dose regimen of 150 mg, administered every 6 h for a total of 20 doses, but is not yet recruiting. A potent derivative of NB-DNJ, UV-4B, also advanced to Phase 1 clinical trials as a prelude to trials in DENV infections. No serious adverse events were reported in a Phase 1 single-ascending dose study at doses up to 1,000 mg (NCT02569827). However, the subsequent Phase 1 multiple-ascending dose trial (NCT02696291) was terminated early before DENV-infected patients were dosed.
The iminosugars NB-DNJ and celgosivir also entered clinical trials as a combination therapy with zidovudine for HIV infections (celgosivir: NCT00002151, NCT00002150; NB-DNJ: NCT00001993, NCT00002079, NCT00000791), but neither advanced past Phase 2 (Fischl et al., 1994). These studies demonstrated that both NB-DNJ and celgosivir are well tolerated by patients, although minor but reversible osmotic diarrhea was observed due to inhibition of α-glucosidases in the gut.
11. Other Flaviviridae ER-associated host dependency factors
Several gain- or loss-of-function genetic screens have been performed to identify host factors required for flavivirus or HCV replication in various cell lines (Lin et al., 2017; Ma et al., 2015; Marceau et al., 2016; Petrova et al., 2019; Savidis et al., 2016; Zhang et al., 2016). While considerable overlap was observed for flavivirus host dependency factors between screens, there was substantial diversity between genes required for DENV and HCV replication, with DENV being largely affected by the loss of protein expressing genes that comprise different ER-associated protein complexes, while viral receptors, metabolic enzymes, and RNA binding proteins, were among the genes that had the most significant impact on HCV replication.
Several screens identified members of the OST complex, which is an essential component in the N-linked glycosylation pathway, as being essential for flavivirus propagation. The catalytic subunit of the OST complex consists of one of two paralogous forms of STT3, STT3A and STT3B, which have partial functional redundancy (Shrimal et al., 2015). STT3A/B associates with at least six other subunits, including Ribophorin I (RPN1), ribophorin II (RPN2), DDOST (OST48), DAD1, OST4, and MAGT1 (IAP) or Tusc3 (N33), to make up different forms of the complex (Shrimal et al., 2015). Viral dependency on OST was confirmed for multiple flaviviruses, including DENV 1–4, WNV, ZIKV, JEV and YFV, although dependency varied between virus, cell-type, and subunit (Lin et al., 2017; Marceau et al., 2016; Savidis et al., 2016; Zhang et al., 2016). STT3A and STT3B expression were shown to be required for DENV propagation, while STT3A, but not STT3B, is required for ZIKV, JEV, WNV, and YFV replication in 293T and/or HAP1 cells. Despite the involvement of the OST complex in the N-linked glycosylation pathway, the N-glycosylation catalytic activity of STT3A/B was not required for DENV replication (Lin et al., 2017; Marceau et al., 2016). Expression of a STT3A or STT3B mutant lacking catalytic activity was able to overcome DENV inhibition observed in STT3A or STT3B knockout cell lines, respectively, suggesting the mechanism is at least partially independent of viral protein glycosylation. Instead, the OST complex was found to interact with viral NS proteins of the RNA replication complex, demonstrating that OST plays a structural role though a physical interaction with a viral protein that is required for DENV RNA replication. Although, because STT3A and STT3B may have partially redundant functions, a possible impact on N-glycosylation of viral glycoproteins cannot be eliminated as any substantial change on viral protein glycosylation would be observed at a later stage in the viral replication cycle than the primary mechanism observed.
The anti-flavivirus potential of perturbing OST function was confirmed using the OST inhibitor, NG-1, which was shown to have broad anti-flavivirus activity in vitro, reducing viral RNA production of DENV, ZIKV, WNV and YFV (Lopez-Sambrooks et al., 2016; Puschnik et al., 2017). The mechanism of anti-flavivirus activity of NG-1 was also shown to be independent of the catalytic N-glycosylation function of the OST complex; however, it is possible that higher concentrations could alter viral protein glycosylation (Puschnik et al., 2017).
In addition to the OST complex, these genome-wide screens identified multiple other host protein candidates important for flavivirus replication that could be targeted in the future development of novel HTAVs. Specifically, several other ER-associated proteins were identified in loss-of-function screens as flavivirus host dependency factors, including members of the translocon-associated protein (TRAP) complex, ERAD pathway, ER-associated signal peptidase complex (SPCS1), and the ER membrane complex (EMC) (Le Sommer et al., 2012; Marceau et al., 2016; Savidis et al., 2016; Zhang et al., 2016). The TRAP complex, along with Sec61 and OST complexes, form the translocon where the cotranslational insertion of proteins occurs in the ER, and is required for proper expression of ER-targeted glycoproteins (Braunger et al., 2018; Pfeffer et al., 2016). The SPCS complex cleaves signal peptides on various membrane-associated and secreted proteins. SPC1 was demonstrated to affect posttranslational processing of the JEV NS2B protein rather than viral entry, RNA replication, or translation (Ma et al., 2018b). The EMC complex may associate with the OST complex and is involved in the processing and maturation of transmembrane proteins (Volkmar et al., 2019; Zakaria et al., 2018). EMC proteins were found to have a role in regulating the levels of host AXL or heparin sulfate protein expression on the cell surface, which is important for the early phase of replication for DENV and ZIKV (Jemielity et al., 2013; Meertens et al., 2012; Richard et al., 2015; Savidis et al., 2016). Infectivity assays using Huh-7 cells lacking expression of the EMC component, EMC4, confirmed the requirement of this host protein for DENV-2, DENV-4, YFV, and ZIKV replication, although WNV replication was unchanged in EMC4 knockout cells (Barrows et al., 2019). Similarly, the EMC2 subunit was also found to be dispensable for WNV propagation, although replication appeared to be slightly delayed (Ma et al., 2015).
12. Other clinical therapeutics for the treatment of flavivirus infections
While numerous vaccines and other biologics have advanced to clinical development for the prevention or treatment of flavivirus infections, only a few small molecules have reached this stage of development, with most targeting DENV (Table 5 ). As of August 2019, all drugs that have been evaluated in the clinic for DENV (safety and/or efficacy studies) were previously approved for other indications, except for three experimental drugs: the iminosugars UV-4B and celgosivir (discussed above), and balapiravir. Balapiravir is a prodrug of a cytidine analog that functions by inhibiting the viral polymerase protein and was originally developed for the treatment of chronic HCV infections. Balapiravir demonstrated promising results against HCV in a Phase 1b study (Roberts et al., 2008); however, clinical development was terminated following dose-related adverse events in patients in a Phase 2 clinical trial (NCT00517439) investigating extended combination therapy with pegylated IFN and ribavirin (Nelson et al., 2012; Roberts et al., 2008). The positive safety profile observed during the short course treatment in the Phase 1b trial led to balapiravir being investigated for the treatment of DENV infections, which would require only acute treatment unlike chronic HCV infections (Roberts et al., 2008). Although well-tolerated, balapiravir did not measurably reduce viremia, NS1 antigenemia, or fever clearance time. Failure to meet the efficacy endpoint is thought to be due, in part, to a decrease in the conversion of the prodrug to the active triphosphate form in infected patients (Chen et al., 2014).
Table 5.
Clinical stage development of small molecules for the treatment of flavivirus infections or symptoms associated with disease caused by flavivirus infections.
| Compound | Indication/Virus | Study Details | Study Endpoint/Readouts | Results | References |
|---|---|---|---|---|---|
| Approved Drugs | |||||
| Chloroquine | DENV | Randomized, double-blind, placebo-controlled study; Participants included patients over 14 years of age hospitalized in Ho Chi Minh City, Vietnam, with suspected uncomplicated DENV infections, including 257 confirmed cases; Participants received 600 mg chloroquine (day 1–2) and 200 mg on day 3, or placebo control | Safety and efficacy (negative RT-PCR detection of viral RNA in plasma and clearance of NS1 from blood). | Trend of lower incidence of hemorrhagic fever with chloroquine treatment; no reduction in duration of NS1 antigenaemia or DENV viremia; treatment was associated with more adverse events (e.g. vomiting) compared to the placebo-treated control group, with one case of hematemesis, with no reduction in the duration of NS1 antigenaemia or DENV viremia. | ISRCTN38002730; Tricou et al. (2010) |
| Ivermectin | DENV | Phase 2/3, randomized, double-blind, placebo-controlled study; 360 participants, children and adult patients ages 15 and older with DENV infection (fever >38 °C for ≤ 72 h); 200–400 μg/kg ivermectin or placebo once daily for 2 or 3 days | Safety and efficacy (primary: time to resolution of viremia; secondary: time to clearance of NS1 antigen, time until fever resolved) | Preliminary data suggests that there is no difference in viremia with ivermectin treatment. Treatment may result in a reduction in body temperature and NS1 serum levels with administration of a high dose. | NCT02045069; Low et al. (2017) |
| DENV | Phase 2 non-randomized, open label study; Pediatric participants, ages 1–15, with DENV infections (acute fever within last 72 h); 400 μg or 600 μg ivermectin per 1 kg body weight once daily for three days | Primary: pharmacokinetics, Secondary: pharmacodynamics, safety (occurrences of adverse events), viremia and NS1 antigenemia clearance | NA; recruiting, current status unknown | NCT03432442 | |
| Ketotifen | Vascular leakage during DENV fever | Phase 4 randomized, double-blind, placebo-controlled study; Adult participants ages 21–60 with DENV fever (≥ 37.5 °C for ≤ 72 h), excluding patients with severe dengue; 2 mg Ketotifen or placebo BID for a total of 10 doses | Efficacy (primary: fluid accumulation in the pleural cavity; secondary: multiple secondary outcome measures) | NA; recruitment status unknown | NCT02673840 |
| Lovastatin | DENV | Phase 2, randomized, double-blind, placebo-controlled study; Adult Vietnamese participants with acute DENV infections; 40 mg or 80 mg lovastatin or placebo control once daily for up to 5 days | Safety and tolerability of lovastatin, including rate of adverse events | Well-tolerated; No benefit observed, although not powered to statistically measure efficacy and selected dose may have been subtherapeutic. | ISRCTN03147572; Whitehorn et al. (2016) |
| Oseltamivir | Thrombocyt-openia and plasma leakage in DENV infections | Phase 2 randomized, placebo-controlled, double-blinded study; Participants ages 16 and above with DENV infections (fever ≤ 6 days, admitted to hospital); 75 mg oseltamivir phosphate BID or placebo for a maximum of 5 days | Safety and effects of treatment on plasma leakage and thrombocytopenia (time to platelet recovery) | NA; ongoing, no longer recruiting | ISRCTN35227717 |
| Prednisolone | Prevention of DENV associated complications without prolonging viral clearance | Randomized, placebo-controlled, partially blinded study; Participants included Vietnamese children and young adults, ages 6–20, with suspected DENV infection (fever ≤ 72 h); Low or high dose prednisolone (doses based on body weight ranges) or placebo daily for 3 days | Safety (clinical and virological parameters) | Adverse effects, including prolongation of viremia, were not observed in patients following prednisolone treatment. No benefit to disease progression observed with prednisolone treatment compared to the placebo treated control group, although the study was not powered to statistically assess such effects. | ISRCTN39575233; Tam et al. (2012) |
| Novel Inhibitors | |||||
| AVI-4020 | WNV neuroinvasive disease | Phase 1 exploratory study; Adult participants ages 18 to 75 with presumptive acute neuroinvasive WNV disease | Safety, tolerability, pharmacokinetics, and potential effectiveness on neurological status (measured by NIH Stroke Scale and Galsgow Coma Score) | Study terminated due to limited number of patients meeting eligibility criteria. | NCT00091845 |
| Balapiravir | DENV | Phase 1, randomized, double-blind, placebo-controlled study; 64 adult male participants, ages 18–65, with confirmed DENV infections and symptom onset of < 48 h; 1,500 mg or 3,000 mg balapiravir, or placebo, BID for 5 days | Safety. tolerability, pharmacokinetics, and efficacy (viral load reduction, body temperature, quality of life) | Well-tolerated; no measurable reduction in viremia, NS1 antigenemia, or fever clearance time. | NCT01096576; Nguyen et al. (2013) |
| Galidesivir | YFV | Phase 1, randomized, double-blind, placebo-controlled study; 2 part study with 66 adult participants, ages 18 and older, hospitalized in Brazil with first onset of symptoms occurring within previous 7 days; part 1 is a dose-ranging study (3 doses), IV treatment every 12 h for 7 days, optimized dose level will be selected for part 2 | Safety, tolerability, pharmacokinetics, and efficacy (viral load reduction, improvement in signs and symptoms and clinical manifestations) | NA; recruiting | NCT03891420 |
Abbreviations: BID, twice daily; DENV, dengue virus; h, hours; HCV, hepatitis C virus; IFN, interferon; NA, not available; NS1, non-structural protein 1; RT-PCR, reverse transcription polymerase chain reaction; TID, three times daily; WNV, West Nile virus; YFV, yellow fever virus.
The FDA-approved compounds chloroquine, ivermectin, prednisolone, and lovastatin have also been evaluated in clinical trials for the treatment of DENV infections or for their ability to relieve associated symptoms without exacerbating infectivity. These compounds were considered to be promising candidates with the potential to be fast-tracked for approval because of their known safety profiles. Unfortunately, in a majority of these clinical studies, treatment did not provide a measurable benefit or was associated with adverse events.
Unlike for flaviviruses, significant advancement has been made for HCV antiviral drug development in the last five to ten years, with recent approval of multiple direct-acting antivirals. These combination therapies, which are discussed more thoroughly in recent reviews (Asselah et al., 2018; Das and Pandya, 2018; Vermehren et al., 2018), have increased safety, tolerability, and efficacy, and require shorter treatment regimens compared to the previous standard of care consisting of IFN and ribavirin.
13. Considerations for future research
It is nearly impossible to halt trends that promote the geographical expansion of some flaviviruses, such as climate change, global travel, and rapidly growing urbanization (Boldescu et al., 2017). Considering the paramount international health problems that are associated with some flavivirus infections, the lack of available therapeutics to treat most diseases caused by these infections, and the continued risk for the development of drug resistant strains, further development of novel antivirals for the treatment of flavivirus infections will continue to remain a priority. The dependency of Flaviviridae and other viruses on the host for N-linked glycosylation and glycoprotein production provides an opportunity for a host-targeted approach to broad-spectrum antiviral drug development.
While significant advances have been made in HCV antiviral drug development, most available therapies are not suitable for use in children, and there are a small percentage of adults (<5%) that do not respond to treatment. Drug development for HCV infections is still advancing with multiple other direct-acting antivirals being developed and tested in preclinical and clinical studies. The possible emergence of drug resistant strains will continue to have to be monitored, particularly because nearly all of the approved therapies and investigational agents directly target viral proteins. While the use of drug combinations can minimize the risk of resistant strains from developing, the inclusion of a host-targeted antiviral, such as an iminosugar, in a combination cocktail could further reduce the potential for viruses to mutate and no longer respond to the approved treatment.
One of the practical challenges that needs to be considered when developing novel iminosugars as HTAVs is that, because both the virus and the host depend on ER glucosidases for glycan processing, the development of potent compounds could potentially have a negative impact on the host. Regulatory agency concerns around this issue have likely slowed development of HTAV therapies compared to direct-acting antivirals. However, good therapeutic window and high tolerability have been observed for some iminosugars in animal infection models, suggesting that viral proteins may be more susceptible to enzyme inhibition than host proteins. It is likely viral proteins are being synthesized at an exponentially higher rate compared to host proteins during the proliferative phase of an acute viral infection, which may provide a window between drug levels required to impact the virus and levels that might result in significant impact on the host. Experimentally, the unique sensitivity of M-protein to glucosidase inhibition in hepatitis B virus infected cells, demonstrated by Block et al., supports the idea that a therapeutic window can be found (Block and Jordan, 2001; Simsek et al., 2005). However, replication rates and viral burden can vary both in infected individuals and for different viruses, which may require some ‘tailoring’ of drug dosage or schedule. Demonstrating an acceptable therapeutic window for HTAVs remains the next hurdle for approval of HTAV drug candidates by international regulatory agencies.
Further research investigating virus lifecycles and viral/host interactions could eventually lead to the identification of novel host targets for the development of HTAV to combat flavivirus infections. As with many other viruses, a combination of antivirals may be necessary to maximize the response and, as drugs become available, research providing insight into their potential synergy when used in combination with other antivirals is warranted. With continued efforts, the future provides hope for available treatment options to combat flavivirus infections caused by both known viruses and those yet to be identified.
Acknowledgements
We sincerely thank Christine Butler for reviewing and editing early drafts of the manuscript and Kezia Suwintono and Hemin Chon for assisting with preparing figures and tables.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.antiviral.2020.104881.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Akey D.L., Brown W.C., Dutta S., Konwerski J., Jose J., Jurkiw T.J., DelProposto J., Ogata C.M., Skiniotis G., Kuhn R.J., Smith J.L. Flavivirus NS1 structures reveal surfaces for associations with membranes and the immune system. Science. 2014;343:881–885. doi: 10.1126/science.1247749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzi D.S., Neville D.C., Lachmann R.H., Dwek R.A., Butters T.D. Glucosylated free oligosaccharides are biomarkers of endoplasmic- reticulum alpha-glucosidase inhibition. Biochem. J. 2008;409:571–580. doi: 10.1042/BJ20070748. [DOI] [PubMed] [Google Scholar]
- Apte-Sengupta S., Sirohi D., Kuhn R.J. Coupling of replication and assembly in flaviviruses. Curr. Opin. Virol. 2014;9:134–142. doi: 10.1016/j.coviro.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselah T., Marcellin P., Schinazi R.F. Treatment of hepatitis C virus infection with direct-acting antiviral agents: 100% cure? Liver Int. 2018;38(Suppl. 1):7–13. doi: 10.1111/liv.13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avezov E., Frenkel Z., Ehrlich M., Herscovics A., Lederkremer G.Z. Endoplasmic reticulum (ER) mannosidase I is compartmentalized and required for N-glycan trimming to Man5-6GlcNAc2 in glycoprotein ER-associated degradation. Mol. Biol. Cell. 2008;19:216–225. doi: 10.1091/mbc.E07-05-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsitis S.J., Williams K.L., Lachica R., Flores D., Kyle J.L., Mehlhop E., Johnson S., Diamond M.S., Beatty P.R., Harris E. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrows N.J., Anglero-Rodriguez Y., Kim B., Jamison S.F., Le Sommer C., McGee C.E., Pearson J.L., Dimopoulos G., Ascano M., Bradrick S.S., Garcia-Blanco M.A. Dual roles for the ER membrane protein complex in flavivirus infection: viral entry and protein biogenesis. Sci. Rep. 2019;9:9711. doi: 10.1038/s41598-019-45910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley D.W., Whiteman M.C., Zhang S., Huang C.Y., Schneider B.S., Smith D.R., Gromowski G.D., Higgs S., Kinney R.M., Barrett A.D. Envelope protein glycosylation status influences mouse neuroinvasion phenotype of genetic lineage 1 West Nile virus strains. J. Virol. 2005;79:8339–8347. doi: 10.1128/JVI.79.13.8339-8347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beljelarskaya S.N., Orlova O.V., Drutsa V.L., Orlov V.A., Timohova A.V., Koroleva N.N., Popenko V.I., Ivanov A.V., Spirin P.V., Prassolov V.S., Rubtsov P.M., Kochetkov S.N. Hepatitis C virus: the role of N-glycosylation sites of viral genotype 1b proteins for formation of viral particles in insect and mammalian cells. Biochem. Biophys. Rep. 2016;7:98–105. doi: 10.1016/j.bbrep.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billerbeck E., de Jong Y., Dorner M., de la Fuente C., Ploss A. Animal models for hepatitis C. Curr. Top. Microbiol. Immunol. 2013;369:49–86. doi: 10.1007/978-3-642-27340-7_3. [DOI] [PubMed] [Google Scholar]
- Block T.M., Jordan R. Iminosugars as possible broad spectrum anti hepatitis virus agents: the glucovirs and alkovirs. Antivir. Chem. Chemother. 2001;12:317–325. doi: 10.1177/095632020101200601. [DOI] [PubMed] [Google Scholar]
- Bloom J.D., Gong L.I., Baltimore D. Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science. 2010;328:1272–1275. doi: 10.1126/science.1187816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldescu V., Behnam M.A.M., Vasilakis N., Klein C.D. Broad-spectrum agents for flaviviral infections: dengue, Zika and beyond. Nat. Rev. Drug Discov. 2017;16:565–586. doi: 10.1038/nrd.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branza-Nichita N., Durantel D., Carrouee-Durantel S., Dwek R.A., Zitzmann N. Antiviral effect of N-butyldeoxynojirimycin against bovine viral diarrhea virus correlates with misfolding of E2 envelope proteins and impairment of their association into E1-E2 heterodimers. J. Virol. 2001;75:3527–3536. doi: 10.1128/JVI.75.8.3527-3536.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunger K., Pfeffer S., Shrimal S., Gilmore R., Berninghausen O., Mandon E.C., Becker T., Forster F., Beckmann R. Structural basis for coupling protein transport and N-glycosylation at the mammalian endoplasmic reticulum. Science. 2018;360:215–219. doi: 10.1126/science.aar7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazzoli M., Helenius A., Foung S.K., Houghton M., Abrignani S., Merola M. Folding and dimerization of hepatitis C virus E1 and E2 glycoproteins in stably transfected CHO cells. Virology. 2005;332:438–453. doi: 10.1016/j.virol.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Bridges C.G., Ahmed S.P., Kang M.S., Nash R.J., Porter E.A., Tyms A.S. The effect of oral treatment with 6-O-butanoyl castanospermine (MDL 28,574) in the murine zosteriform model of HSV-1 infection. Glycobiology. 1995;5:249–253. doi: 10.1093/glycob/5.2.249. [DOI] [PubMed] [Google Scholar]
- Brinton M.A. The molecular biology of West Nile Virus: a new invader of the western hemisphere. Annu. Rev. Microbiol. 2002;56:371–402. doi: 10.1146/annurev.micro.56.012302.160654. [DOI] [PubMed] [Google Scholar]
- Bryant J.E., Calvert A.E., Mesesan K., Crabtree M.B., Volpe K.E., Silengo S., Kinney R.M., Huang C.Y., Miller B.R., Roehrig J.T. Glycosylation of the dengue 2 virus E protein at N67 is critical for virus growth in vitro but not for growth in intrathoracically inoculated Aedes aegypti mosquitoes. Virology. 2007;366:415–423. doi: 10.1016/j.virol.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Butters T.D, van den Broek L.A.G.M, Fleet G.W.J, Wormald M.R., Dwek R.A., Platt F.M. Molecular requirements of imino sugars for the selective control of N-linked glycosylation and glycosphingolipid biosynthesis. Tetrahedron: Asymmetry. 2000;11:113–124. [Google Scholar]
- Caputo A.T., Alonzi D.S., Marti L., Reca I.B., Kiappes J.L., Struwe W.B., Cross A., Basu S., Lowe E.D., Darlot B., Santino A., Roversi P., Zitzmann N. Structures of mammalian ER alpha-glucosidase II capture the binding modes of broad-spectrum iminosugar antivirals. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E4630–E4638. doi: 10.1073/pnas.1604463113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers T.J., Hahn C.S., Galler R., Rice C.M. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- Chang J., Wang L., Ma D., Qu X., Guo H., Xu X., Mason P.M., Bourne N., Moriarty R., Gu B., Guo J.T., Block T.M. Novel imino sugar derivatives demonstrate potent antiviral activity against flaviviruses. Antimicrob. Agents Chemother. 2009;53:1501–1508. doi: 10.1128/AAC.01457-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Schul W., Butters T.D., Yip A., Liu B., Goh A., Lakshminarayana S.B., Alonzi D., Reinkensmeier G., Pan X., Qu X., Weidner J.M., Wang L., Yu W., Borune N., Kinch M.A., Rayahin J.E., Moriarty R., Xu X., Shi P.Y., Guo J.T., Block T.M. Combination of alpha-glucosidase inhibitor and ribavirin for the treatment of dengue virus infection in vitro and in vivo. Antivir. Res. 2011;89:26–34. doi: 10.1016/j.antiviral.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Guo J.T., Du Y., Block T. Imino sugar glucosidase inhibitors as broadly active anti-filovirus agents. Emerg. Microb. Infect. 2013;2:e77. doi: 10.1038/emi.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Warren T.K., Zhao X., Gill T., Guo F., Wang L., Comunale M.A., Du Y., Alonzi D.S., Yu W., Ye H., Liu F., Guo J.T., Mehta A., Cuconati A., Butters T.D., Bavari S., Xu X., Block T.M. Small molecule inhibitors of ER alpha-glucosidases are active against multiple hemorrhagic fever viruses. Antivir. Res. 2013;98:432–440. doi: 10.1016/j.antiviral.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapel C., Garcia C., Roingeard P., Zitzmann N., Dubuisson J., Dwek R.A., Trepo C., Zoulim F., Durantel D. Antiviral effect of alpha-glucosidase inhibitors on viral morphogenesis and binding properties of hepatitis C virus-like particles. J. Gen. Virol. 2006;87:861–871. doi: 10.1099/vir.0.81503-0. [DOI] [PubMed] [Google Scholar]
- Chapman T.M., Davies I.G., Gu B., Block T.M., Scopes D.I., Hay P.A., Courtney S.M., McNeill L.A., Schofield C.J., Davis B.G. Glyco- and peptidomimetics from three-component Joullie-Ugi coupling show selective antiviral activity. J. Am. Chem. Soc. 2005;127:506–507. doi: 10.1021/ja043924l. [DOI] [PubMed] [Google Scholar]
- Chatel-Chaix L., Bartenschlager R. Dengue virus- and hepatitis C virus-induced replication and assembly compartments: the enemy inside--caught in the web. J. Virol. 2014;88:5907–5911. doi: 10.1128/JVI.03404-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.L., Abdul Ghafar N., Karuna R., Fu Y., Lim S.P., Schul W., Gu F., Herve M., Yokohama F., Wang G., Cerny D., Fink K., Blasco F., Shi P.Y. Activation of peripheral blood mononuclear cells by dengue virus infection depotentiates balapiravir. J. Virol. 2014;88:1740–1747. doi: 10.1128/JVI.02841-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choukhi A., Ung S., Wychowski C., Dubuisson J. Involvement of endoplasmic reticulum chaperones in the folding of hepatitis C virus glycoproteins. J. Virol. 1998;72:3851–3858. doi: 10.1128/jvi.72.5.3851-3858.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocquerel L., Meunier J.C., Op de Beeck A., Bonte D., Wychowski C., Dubuisson J. Coexpression of hepatitis C virus envelope proteins E1 and E2 in cis improves the stability of membrane insertion of E2. J. Gen. Virol. 2001;82:1629–1635. doi: 10.1099/0022-1317-82-7-1629. [DOI] [PubMed] [Google Scholar]
- Courageot M.P., Frenkiel M.P., Dos Santos C.D., Deubel V., Despres P. Alpha-glucosidase inhibitors reduce dengue virus production by affecting the initial steps of virion morphogenesis in the endoplasmic reticulum. J. Virol. 2000;74:564–572. doi: 10.1128/jvi.74.1.564-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L., Song J., Lu X., Deng Y.Q., Musyoki A.M., Cheng H., Zhang Y., Yuan Y., Song H., Haywood J., Xiao H., Yan J., Shi Y., Qin C.F., Qi J., Gao G.F. Structures of the zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe. 2016;19:696–704. doi: 10.1016/j.chom.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Das D., Pandya M. Recent advancement of direct-acting antiviral agents (DAAs) in hepatitis C therapy. Mini Rev. Med. Chem. 2018;18:584–596. doi: 10.2174/1389557517666170913111930. [DOI] [PubMed] [Google Scholar]
- Dubuisson J. Folding, assembly and subcellular localization of hepatitis C virus glycoproteins. Curr. Top. Microbiol. Immunol. 2000;242:135–148. doi: 10.1007/978-3-642-59605-6_7. [DOI] [PubMed] [Google Scholar]
- Durantel D. Celgosivir, an alpha-glucosidase I inhibitor for the potential treatment of HCV infection. Curr. Opin. Invest. Drugs. 2009;10:860–870. [PubMed] [Google Scholar]
- Durantel D., Branza-Nichita N., Carrouee-Durantel S., Butters T.D., Dwek R.A., Zitzmann N. Study of the mechanism of antiviral action of iminosugar derivatives against bovine viral diarrhea virus. J. Virol. 2001;75:8987–8998. doi: 10.1128/JVI.75.19.8987-8998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durantel D., Carrouee-Durantel S., Branza-Nichita N., Dwek R.A., Zitzmann N. Effects of interferon, ribavirin, and iminosugar derivatives on cells persistently infected with noncytopathic bovine viral diarrhea virus. Antimicrob. Agents Chemother. 2004;48:497–504. doi: 10.1128/AAC.48.2.497-504.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durantel D., Alotte C., Zoulim F. Glucosidase inhibitors as antiviral agents for hepatitis B and C. Curr. Opin. Invest. Drugs. 2007;8:125–129. [PubMed] [Google Scholar]
- Elloumi H., Houissa F., Hadj N.B., Gargouri D., Romani M., Kharrat J., Ghorbel A. Sudden hearing loss associated with peginterferon and ribavirin combination therapy during hepatitis C treatment. World J. Gastroenterol. 2007;13:5411–5412. doi: 10.3748/wjg.v13.i40.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci P., Fried M.W., Shiffman M.L., Smith C.I., Marinos G., Goncales F.L., Jr., Haussinger D., Diago M., Carosi G., Dhumeaux D., Craxi A., Chaneac M., Reddy K.R. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. J. Hepatol. 2005;43:425–433. doi: 10.1016/j.jhep.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Fernandez-Garcia M.D., Mazzon M., Jacobs M., Amara A. Pathogenesis of flavivirus infections: using and abusing the host cell. Cell Host Microbe. 2009;5:318–328. doi: 10.1016/j.chom.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Fischer P.B., Collin M., Karlsson G.B., James W., Butters T.D., Davis S.J., Gordon S., Dwek R.A., Platt F.M. The alpha-glucosidase inhibitor N-butyldeoxynojirimycin inhibits human immunodeficiency virus entry at the level of post-CD4 binding. J. Virol. 1995;69:5791–5797. doi: 10.1128/jvi.69.9.5791-5797.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer P.B., Karlsson G.B., Dwek R.A., Platt F.M. N-butyldeoxynojirimycin-mediated inhibition of human immunodeficiency virus entry correlates with impaired gp120 shedding and gp41 exposure. J. Virol. 1996;70:7153–7160. doi: 10.1128/jvi.70.10.7153-7160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl M.A., Resnick L., Coombs R., Kremer A.B., Pottage J.C., Jr., Fass R.J., Fife K.H., Powderly W.G., Collier A.C., Aspinall R.L., et al. The safety and efficacy of combination N-butyl-deoxynojirimycin (SC-48334) and zidovudine in patients with HIV-1 infection and 200-500 CD4 cells/mm3. J. Acquir. Immune Defic. Syndr. 1994;7:139–147. [PubMed] [Google Scholar]
- Flamand M., Megret F., Mathieu M., Lepault J., Rey F.A., Deubel V. Dengue virus type 1 nonstructural glycoprotein NS1 is secreted from mammalian cells as a soluble hexamer in a glycosylation-dependent fashion. J. Virol. 1999;73:6104–6110. doi: 10.1128/jvi.73.7.6104-6110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes-Garfias C.R., Shan C., Luo H., Muruato A.E., Medeiros D.B.A., Mays E., Xie X., Zou J., Roundy C.M., Wakamiya M., Rossi S.L., Wang T., Weaver S.C., Shi P.Y. Functional analysis of glycosylation of zika virus envelope protein. Cell Rep. 2017;21:1180–1190. doi: 10.1016/j.celrep.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T.L., Verow M., Wozniak A.L., Bentham M.J., Thompson J., Atkins E., Weinman S.A., Fishwick C., Foster R., Harris M., Griffin S. Resistance mutations define specific antiviral effects for inhibitors of the hepatitis C virus p7 ion channel. Hepatology. 2011;54:79–90. doi: 10.1002/hep.24371. [DOI] [PubMed] [Google Scholar]
- Freedman H., Logan M.R., Law J.L., Houghton M. Structure and function of the hepatitis C virus envelope glycoproteins E1 and E2: antiviral and vaccine targets. ACS Infect. Dis. 2016;2:749–762. doi: 10.1021/acsinfecdis.6b00110. [DOI] [PubMed] [Google Scholar]
- Gavel Y., von Heijne G. Sequence differences between glycosylated and non-glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein Eng. 1990;3:433–442. doi: 10.1093/protein/3.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffard A., Dubuisson J. Glycosylation of hepatitis C virus envelope proteins. Biochimie. 2003;85:295–301. doi: 10.1016/s0300-9084(03)00004-x. [DOI] [PubMed] [Google Scholar]
- Goffard A., Callens N., Bartosch B., Wychowski C., Cosset F.L., Montpellier C., Dubuisson J. Role of N-linked glycans in the functions of hepatitis C virus envelope glycoproteins. J. Virol. 2005;79:8400–8409. doi: 10.1128/JVI.79.13.8400-8409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto A., Yoshii K., Obara M., Ueki T., Mizutani T., Kariwa H., Takashima I. Role of the N-linked glycans of the prM and E envelope proteins in tick-borne encephalitis virus particle secretion. Vaccine. 2005;23:3043–3052. doi: 10.1016/j.vaccine.2004.11.068. [DOI] [PubMed] [Google Scholar]
- Griffin S., Stgelais C., Owsianka A.M., Patel A.H., Rowlands D., Harris M. Genotype-dependent sensitivity of hepatitis C virus to inhibitors of the p7 ion channel. Hepatology. 2008;48:1779–1790. doi: 10.1002/hep.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B., Mason P., Wang L., Norton P., Bourne N., Moriarty R., Mehta A., Despande M., Shah R., Block T. Antiviral profiles of novel iminocyclitol compounds against bovine viral diarrhea virus, West Nile virus, dengue virus and hepatitis B virus. Antivir. Chem. Chemother. 2007;18:49–59. doi: 10.1177/095632020701800105. [DOI] [PubMed] [Google Scholar]
- Guirakhoo F., Hunt A.R., Lewis J.G., Roehrig J.T. Selection and partial characterization of dengue 2 virus mutants that induce fusion at elevated pH. Virology. 1993;194:219–223. doi: 10.1006/viro.1993.1252. [DOI] [PubMed] [Google Scholar]
- Hacker K., White L., de Silva A.M. N-linked glycans on dengue viruses grown in mammalian and insect cells. J. Gen. Virol. 2009;90:2097–2106. doi: 10.1099/vir.0.012120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C.S., Dalrymple J.M., Strauss J.H., Rice C.M. Comparison of the virulent Asibi strain of yellow fever virus with the 17D vaccine strain derived from it. Proc. Natl. Acad. Sci. U. S. A. 1987;84:2019–2023. doi: 10.1073/pnas.84.7.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna S.L., Pierson T.C., Sanchez M.D., Ahmed A.A., Murtadha M.M., Doms R.W. N-linked glycosylation of west nile virus envelope proteins influences particle assembly and infectivity. J. Virol. 2005;79:13262–13274. doi: 10.1128/JVI.79.21.13262-13274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T., Tautz N., Thiel H.J. E2-p7 region of the bovine viral diarrhea virus polyprotein: processing and functional studies. J. Virol. 2000;74:9498–9506. doi: 10.1128/jvi.74.20.9498-9506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauge S.H., Dudman S., Borgen K., Lackenby A., Hungnes O. Oseltamivir-resistant influenza viruses A (H1N1), Norway, 2007-08. Emerg. Infect. Dis. 2009;15:155–162. doi: 10.3201/eid1502.081031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert D.N., Foellmer B., Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 1995;81:425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- Helle F., Vieyres G., Elkrief L., Popescu C.I., Wychowski C., Descamps V., Castelain S., Roingeard P., Duverlie G., Dubuisson J. Role of N-linked glycans in the functions of hepatitis C virus envelope proteins incorporated into infectious virions. J. Virol. 2010;84:11905–11915. doi: 10.1128/JVI.01548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J.D., Smith N., Lee M.J., Ardes-Guisot N., Vauzeilles B., Desire J., Baron A., Bleriot Y., Sollogoub M., Alonzi D.S., Butters T.D. Novel imino sugar alpha-glucosidase inhibitors as antiviral compounds. Bioorg. Med. Chem. 2013;21:4831–4838. doi: 10.1016/j.bmc.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Idris F., Muharram S.H., Diah S. Glycosylation of dengue virus glycoproteins and their interactions with carbohydrate receptors: possible targets for antiviral therapy. Arch. Virol. 2016;161:1751–1760. doi: 10.1007/s00705-016-2855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishak H., Takegami T., Kamimura K., Funada H. Comparative sequences of two type 1 dengue virus strains possessing different growth characteristics in vitro. Microbiol. Immunol. 2001;45:327–331. doi: 10.1111/j.1348-0421.2001.tb02627.x. [DOI] [PubMed] [Google Scholar]
- Jacob J.R., Mansfield K., You J.E., Tennant B.C., Kim Y.H. Natural iminosugar derivatives of 1-deoxynojirimycin inhibit glycosylation of hepatitis viral envelope proteins. J. Microbiol. 2007;45:431–440. [PubMed] [Google Scholar]
- Jemielity S., Wang J.J., Chan Y.K., Ahmed A.A., Li W., Monahan S., Bu X., Farzan M., Freeman G.J., Umetsu D.T., Dekruyff R.H., Choe H. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.J., Guirakhoo F., Roehrig J.T. The envelope glycoproteins of dengue 1 and dengue 2 viruses grown in mosquito cells differ in their utilization of potential glycosylation sites. Virology. 1994;203:241–249. doi: 10.1006/viro.1994.1481. [DOI] [PubMed] [Google Scholar]
- Jones C.T., Murray C.L., Eastman D.K., Tassello J., Rice C.M. Hepatitis C virus p7 and NS2 proteins are essential for production of infectious virus. J. Virol. 2007;81:8374–8383. doi: 10.1128/JVI.00690-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R., Nikolaeva O.V., Wang L., Conyers B., Mehta A., Dwek R.A., Block T.M. Inhibition of host ER glucosidase activity prevents Golgi processing of virion-associated bovine viral diarrhea virus E2 glycoproteins and reduces infectivity of secreted virions. Virology. 2002;295:10–19. doi: 10.1006/viro.2002.1370. [DOI] [PubMed] [Google Scholar]
- Kasturi L., Chen H., Shakin-Eshleman S.H. Regulation of N-linked core glycosylation: use of a site-directed mutagenesis approach to identify Asn-Xaa-Ser/Thr sequons that are poor oligosaccharide acceptors. Biochem. J. 1997;323(Pt 2):415–419. doi: 10.1042/bj3230415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzelnick L.C., Gresh L., Halloran M.E., Mercado J.C., Kuan G., Gordon A., Balmaseda A., Harris E. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358:929–932. doi: 10.1126/science.aan6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano H., Rostapshov V., Rosen L., Lai C.J. Genetic determinants of dengue type 4 virus neurovirulence for mice. J. Virol. 1993;67:6567–6575. doi: 10.1128/jvi.67.11.6567-6575.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiappes J.L., Hill M.L., Alonzi D.S., Miller J.L., Iwaki R., Sayce A.C., Caputo A.T., Kato A., Zitzmann N. ToP-DNJ, a selective inhibitor of endoplasmic reticulum alpha-glucosidase II exhibiting antiflaviviral activity. ACS Chem. Biol. 2018;13:60–65. doi: 10.1021/acschembio.7b00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.M., Yun S.I., Song B.H., Hahn Y.S., Lee C.H., Oh H.W., Lee Y.M. A single N-linked glycosylation site in the Japanese encephalitis virus prM protein is critical for cell type-specific prM protein biogenesis, virus particle release, and pathogenicity in mice. J. Virol. 2008;82:7846–7862. doi: 10.1128/JVI.00789-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi E., Mason P.W. Proper maturation of the Japanese encephalitis virus envelope glycoprotein requires cosynthesis with the premembrane protein. J. Virol. 1993;67:1672–1675. doi: 10.1128/jvi.67.3.1672-1675.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kuhn R.J., Zhang W., Rossmann M.G., Pletnev S.V., Corver J., Lenches E., Jones C.T., Mukhopadhyay S., Chipman P.R., Strauss E.G., Baker T.S., Strauss J.H. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108:717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad V.J., Shende V.R., Gupta A.K. Effect of catanospermine, 1-deoxynojirimycin or 1-deoxymannojirimycin on biological and functional activities of Japanese encephalitis virus in porcine stable kidney cells. Microbiol. Res. 2013;4(e3):12–15. [Google Scholar]
- Le Sommer C., Barrows N.J., Bradrick S.S., Pearson J.L., Garcia-Blanco M.A. G protein-coupled receptor kinase 2 promotes flaviviridae entry and replication. PLoS Neglected Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E., Weir R.C., Dalgarno L. Changes in the dengue virus major envelope protein on passaging and their localization on the three-dimensional structure of the protein. Virology. 1997;232:281–290. doi: 10.1006/viro.1997.8570. [DOI] [PubMed] [Google Scholar]
- Lee E., Leang S.K., Davidson A., Lobigs M. Both E protein glycans adversely affect dengue virus infectivity but are beneficial for virion release. J. Virol. 2010;84:5171–5180. doi: 10.1128/JVI.01900-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Lok S.M., Yu I.M., Zhang Y., Kuhn R.J., Chen J., Rossmann M.G. The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science. 2008;319:1830–1834. doi: 10.1126/science.1153263. [DOI] [PubMed] [Google Scholar]
- Lin D.L., Cherepanova N.A., Bozzacco L., MacDonald M.R., Gilmore R., Tai A.W. Dengue virus hijacks a noncanonical oxidoreductase function of a cellular oligosaccharyltransferase complex. mBio. 2017;8 doi: 10.1128/mBio.00939-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach B.D., Rice C.M. The ins and outs of hepatitis C virus entry and assembly. Nat. Rev. Microbiol. 2013;11:688–700. doi: 10.1038/nrmicro3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Denman A.J., Mackenzie J.M. The IMPORTance of the nucleus during flavivirus replication. Viruses. 2017;9 doi: 10.3390/v9010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Sambrooks C., Shrimal S., Khodier C., Flaherty D.P., Rinis N., Charest J.C., Gao N., Zhao P., Wells L., Lewis T.A., Lehrman M.A., Gilmore R., Golden J.E., Contessa J.N. Oligosaccharyltransferase inhibition induces senescence in RTK-driven tumor cells. Nat. Chem. Biol. 2016;12:1023–1030. doi: 10.1038/nchembio.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz I.C., Allison S.L., Heinz F.X., Helenius A. Folding and dimerization of tick-borne encephalitis virus envelope proteins prM and E in the endoplasmic reticulum. J. Virol. 2002;76:5480–5491. doi: 10.1128/JVI.76.11.5480-5491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz I.C., Kartenbeck J., Mezzacasa A., Allison S.L., Heinz F.X., Helenius A. Intracellular assembly and secretion of recombinant subviral particles from tick-borne encephalitis virus. J. Virol. 2003;77:4370–4382. doi: 10.1128/JVI.77.7.4370-4382.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low J.G., Sung C., Wijaya L., Wei Y., Rathore A.P.S., Watanabe S., Tan B.H., Toh L., Chua L.T., Hou Y., Chow A., Howe S., Chan W.K., Tan K.H., Chung J.S., Cherng B.P., Lye D.C., Tambayah P.A., Ng L.C., Connolly J., Hibberd M.L., Leo Y.S., Cheung Y.B., Ooi E.E., Vasudevan S.G. Efficacy and safety of celgosivir in patients with dengue fever (CELADEN): a phase 1b, randomised, double-blind, placebo-controlled, proof-of-concept trial. Lancet Infect. Dis. 2014;14:706–715. doi: 10.1016/S1473-3099(14)70730-3. [DOI] [PubMed] [Google Scholar]
- Low J.G., Ooi E.E., Vasudevan S.G. Current status of dengue therapeutics research and development. J. Infect. Dis. 2017;215:S96–S102. doi: 10.1093/infdis/jiw423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Dang Y., Wu Y., Jia G., Anaya E., Zhang J., Abraham S., Choi J.G., Shi G., Qi L., Manjunath N., Wu H. A CRISPR-based screen identifies genes essential for west-nile-virus-induced cell death. Cell Rep. 2015;12:673–683. doi: 10.1016/j.celrep.2015.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Zhang X., Soloveva V., Warren T., Guo F., Wu S., Lu H., Guo J., Su Q., Shen H., Solon E., Comunale M.A., Mehta A., Guo J.T., Bavari S., Du Y., Block T.M., Chang J. Enhancing the antiviral potency of ER alpha-glucosidase inhibitor IHVR-19029 against hemorrhagic fever viruses in vitro and in vivo. Antivir. Res. 2018;150:112–122. doi: 10.1016/j.antiviral.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Li F., Zhang J.W., Li W., Zhao D.M., Wang H., Hua R.H., Bu Z.G. Host factor SPCS1 regulates the replication of Japanese encephalitis virus through interactions with transmembrane domains of NS2B. J. Virol. 2018;92(12) doi: 10.1128/JVI.00197-18. e00197-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie J.M., Westaway E.G. Assembly and maturation of the flavivirus Kunjin virus appear to occur in the rough endoplasmic reticulum and along the secretory pathway, respectively. J. Virol. 2001;75:10787–10799. doi: 10.1128/JVI.75.22.10787-10799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns M.P., Wedemeyer H., Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350–1359. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau C.D., Puschnik A.S., Majzoub K., Ooi Y.S., Brewer S.M., Fuchs G., Swaminathan K., Mata M.A., Elias J.E., Sarnow P., Carette J.E. Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nature. 2016;535:159. doi: 10.1038/nature18631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Duncker I., Diaz-Jimenez D.F., Mora-Montes H.M. Comparative analysis of protein glycosylation pathways in humans and the fungal pathogen Candida albicans. Internet J. Microbiol. 2014;2014:267497. doi: 10.1155/2014/267497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meertens L., Carnec X., Lecoin M.P., Ramdasi R., Guivel-Benhassine F., Lew E., Lemke G., Schwartz O., Amara A. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe. 2012;12:544–557. doi: 10.1016/j.chom.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A., Ouzounov S., Jordan R., Simsek E., Lu X., Moriarty R.M., Jacob G., Dwek R.A., Block T.M. Imino sugars that are less toxic but more potent as antivirals, in vitro, compared with N-n-nonyl DNJ. Antivir. Chem. Chemother. 2002;13:299–304. doi: 10.1177/095632020201300505. [DOI] [PubMed] [Google Scholar]
- Meijer A., Lackenby A., Hungnes O., Lina B., van-der-Werf S., Schweiger B., Opp M., Paget J., van-de-Kassteele J., Hay A., Zambon M. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007-08 season. Emerg. Infect. Dis. 2009;15:552–560. doi: 10.3201/eid1504.081280. European Influenza Surveillance, S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor H.R., Nolan J., Pickering L., Wormald M.R., Platt F.M., Dwek R.A., Fleet G.W., Butters T.D. Preparation, biochemical characterization and biological properties of radiolabelled N-alkylated deoxynojirimycins. Biochem. J. 2002;366:225–233. doi: 10.1042/BJ20020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor H.R., Platt F.M., Dwek R.A., Butters T.D. Membrane disruption and cytotoxicity of hydrophobic N-alkylated imino sugars is independent of the inhibition of protein and lipid glycosylation. Biochem. J. 2003;374:307–314. doi: 10.1042/BJ20030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.L., Lachica R., Sayce A.C., Williams J.P., Bapat M., Dwek R., Beatty P.R., Harris E., Zitzmann N. Liposome-mediated delivery of iminosugars enhances efficacy against dengue virus in vivo. Antimicrob. Agents Chemother. 2012;56:6379–6386. doi: 10.1128/AAC.01554-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.L., Tyrrell B.E., Zitzmann N. Mechanisms of antiviral activity of iminosugars against dengue virus. Adv. Exp. Med. Biol. 2018;1062:277–301. doi: 10.1007/978-981-10-8727-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.L., Hill M.L., Brun J., Pountain A., Sayce A.C., Zitzmann N. Iminosugars counteract the downregulation of the interferon gamma receptor by dengue virus. Antivir. Res. 2019;170:104551. doi: 10.1016/j.antiviral.2019.104551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M., Helenius A. Glycoproteins form mixed disulphides with oxidoreductases during folding in living cells. Nature. 1999;402:90–93. doi: 10.1038/47062. [DOI] [PubMed] [Google Scholar]
- Mondotte J.A., Lozach P.Y., Amara A., Gamarnik A.V. Essential role of dengue virus envelope protein N glycosylation at asparagine-67 during viral propagation. J. Virol. 2007;81:7136–7148. doi: 10.1128/JVI.00116-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossenta M., Marchese S., Poggianella M., Slon Campos J.L., Burrone O.R. Role of N-glycosylation on Zika virus E protein secretion, viral assembly and infectivity. Biochem. Biophys. Res. Commun. 2017;492:579–586. doi: 10.1016/j.bbrc.2017.01.022. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S., Kim B.S., Chipman P.R., Rossmann M.G., Kuhn R.J. Structure of West Nile virus. Science. 2003;302:248. doi: 10.1126/science.1089316. [DOI] [PubMed] [Google Scholar]
- Muylaert I.R., Chambers T.J., Galler R., Rice C.M. Mutagenesis of the N-linked glycosylation sites of the yellow fever virus NS1 protein: effects on virus replication and mouse neurovirulence. Virology. 1996;222:159–168. doi: 10.1006/viro.1996.0406. [DOI] [PubMed] [Google Scholar]
- Nelson D.R., Zeuzem S., Andreone P., Ferenci P., Herring R., Jensen D.M., Marcellin P., Pockros P.J., Rodriguez-Torres M., Rossaro L., Rustgi V.K., Sepe T., Sulkowski M., Thomason I.R., Yoshida E.M., Chan A., Hill G. Balapiravir plus peginterferon alfa-2a (40KD)/ribavirin in a randomized trial of hepatitis C genotype 1 patients. Ann. Hepatol. 2012;11:15–31. [PMC free article] [PubMed] [Google Scholar]
- Neufeldt C.J., Cortese M., Acosta E.G., Bartenschlager R. Rewiring cellular networks by members of the Flaviviridae family. Nat. Rev. Microbiol. 2018;16:125–142. doi: 10.1038/nrmicro.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D.C., Coquard V., Priestman D.A., te Vruchte D.J., Sillence D.J., Dwek R.A., Platt F.M., Butters T.D. Analysis of fluorescently labeled glycosphingolipid-derived oligosaccharides following ceramide glycanase digestion and anthranilic acid labeling. Anal. Biochem. 2004;331:275–282. doi: 10.1016/j.ab.2004.03.051. [DOI] [PubMed] [Google Scholar]
- Nguyen N.M., Tran C.N., Phung L.K., Duong K.T., Huynh Hle A., Farrar J., Nguyen Q.T., Tran H.T., Nguyen C.V., Merson L., Hoang L.T., Hibberd M.L., Aw P.P., Wilm A., Nagarajan N., Nguyen D.T., Pham M.P., Nguyen T.T., Javanbakht H., Klumpp K., Hammond J., Petric R., Wolbers M., Nguyen C.T., Simmons C.P. A randomized, double-blind placebo controlled trial of balapiravir, a polymerase inhibitor, in adult dengue patients. J. Infect. Dis. 2013;207:1442–1450. doi: 10.1093/infdis/jis470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto S., Speranzini V., Hashimoto T., Noshi T., Yamaguchi H., Kawai M., Kawaguchi K., Uehara T., Shishido T., Naito A., Cusack S. Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci. Rep. 2018;8:9633. doi: 10.1038/s41598-018-27890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op De Beeck A., Cocquerel L., Dubuisson J. Biogenesis of hepatitis C virus envelope glycoproteins. J. Gen. Virol. 2001;82:2589–2595. doi: 10.1099/0022-1317-82-11-2589. [DOI] [PubMed] [Google Scholar]
- Ouzounov S., Mehta A., Dwek R.A., Block T.M., Jordan R. The combination of interferon alpha-2b and n-butyl deoxynojirimycin has a greater than additive antiviral effect upon production of infectious bovine viral diarrhea virus (BVDV) in vitro: implications for hepatitis C virus (HCV) therapy. Antivir. Res. 2002;55:425–435. doi: 10.1016/s0166-3542(02)00075-x. [DOI] [PubMed] [Google Scholar]
- Paul D., Bartenschlager R. Flaviviridae replication organelles: oh, what a tangled web we weave. Annu. Rev. Virol. 2015;2:289–310. doi: 10.1146/annurev-virology-100114-055007. [DOI] [PubMed] [Google Scholar]
- Pavlovic D., Neville D.C., Argaud O., Blumberg B., Dwek R.A., Fischer W.B., Zitzmann N. The hepatitis C virus p7 protein forms an ion channel that is inhibited by long-alkyl-chain iminosugar derivatives. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6104–6108. doi: 10.1073/pnas.1031527100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S.T., Buck M.D., Plummer E.M., Penmasta R.A., Batra H., Stavale E.J., Warfield K.L., Dwek R.A., Butters T.D., Alonzi D.S., Lada S.M., King K., Klose B., Ramstedt U., Shresta S. An iminosugar with potent inhibition of dengue virus infection in vivo. Antivir. Res. 2013;98:35–43. doi: 10.1016/j.antiviral.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Petrova E., Gracias S., Beauclair G., Tangy F., Jouvenet N. Uncovering flavivirus host dependency factors through a genome-wide gain-of-function screen. Viruses. 2019;11(1):68. doi: 10.3390/v11010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S., Dudek J., Zimmermann R., Forster F. Organization of the native ribosome-translocon complex at the mammalian endoplasmic reticulum membrane. Biochim. Biophys. Acta. 2016;1860:2122–2129. doi: 10.1016/j.bbagen.2016.06.024. [DOI] [PubMed] [Google Scholar]
- Platt F.M., Neises G.R., Dwek R.A., Butters T.D. N-butyldeoxynojirimycin is a novel inhibitor of glycolipid biosynthesis. J. Biol. Chem. 1994;269:8362–8365. [PubMed] [Google Scholar]
- Platt F.M., Neises G.R., Karlsson G.B., Dwek R.A., Butters T.D. N-butyldeoxygalactonojirimycin inhibits glycolipid biosynthesis but does not affect N-linked oligosaccharide processing. J. Biol. Chem. 1994;269:27108–27114. [PubMed] [Google Scholar]
- Plummer E., Buck M.D., Sanchez M., Greenbaum J.A., Turner J., Grewal R., Klose B., Sampath A., Warfield K.L., Peters B., Ramstedt U., Shresta S. Dengue virus evolution under a host-targeted antiviral. J. Virol. 2015;89:5592–5601. doi: 10.1128/JVI.00028-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokidysheva E., Zhang Y., Battisti A.J., Bator-Kelly C.M., Chipman P.R., Xiao C., Gregorio G.G., Hendrickson W.A., Kuhn R.J., Rossmann M.G. Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Cell. 2006;124:485–493. doi: 10.1016/j.cell.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Puschnik A.S., Marceau C.D., Ooi Y.S., Majzoub K., Rinis N., Contessa J.N., Carette J.E. A small-molecule oligosaccharyltransferase inhibitor with pan-flaviviral activity. Cell Rep. 2017;21:3032–3039. doi: 10.1016/j.celrep.2017.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnak J.R., Charles P.C., Padmanabhan R., Irie K., Hoke C.H., Burke D.S. Functional and antigenic domains of the dengue-2 virus nonstructural glycoprotein NS-1. Virology. 1988;163:93–103. doi: 10.1016/0042-6822(88)90236-x. [DOI] [PubMed] [Google Scholar]
- Qu X., Pan X., Weidner J., Yu W., Alonzi D., Xu X., Butters T., Block T., Guo J.T., Chang J. Inhibitors of endoplasmic reticulum alpha-glucosidases potently suppress hepatitis C virus virion assembly and release. Antimicrob. Agents Chemother. 2011;55:1036–1044. doi: 10.1128/AAC.01319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekharan S., Bonotto R.M., Kazungu Y., Alves L.N., Poggianella M., Orellana P.M., Skoko N., Polez S., Marcello A. Repurposing of miglustat to inhibit the coronavirus severe acquired respiratory syndrome SARS-CoV-2. 2020. [DOI]
- Rathore A.P., Paradkar P.N., Watanabe S., Tan K.H., Sung C., Connolly J.E., Low J., Ooi E.E., Vasudevan S.G. Celgosivir treatment misfolds dengue virus NS1 protein, induces cellular pro-survival genes and protects against lethal challenge mouse model. Antivir. Res. 2011;92:453–460. doi: 10.1016/j.antiviral.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Richard A.S., Zhang A., Park S.J., Farzan M., Zong M., Choe H. Virion-associated phosphatidylethanolamine promotes TIM1-mediated infection by Ebola, dengue, and West Nile viruses. Proc. Natl. Acad. Sci. U. S. A. 2015;112:14682–14687. doi: 10.1073/pnas.1508095112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S.K., Cooksley G., Dore G.J., Robson R., Shaw D., Berns H., Hill G., Klumpp K., Najera I., Washington C. Robust antiviral activity of R1626, a novel nucleoside analog: a randomized, placebo-controlled study in patients with chronic hepatitis C. Hepatology. 2008;48:398–406. doi: 10.1002/hep.22321. [DOI] [PubMed] [Google Scholar]
- Sadat M.A., Moir S., Chun T.W., Lusso P., Kaplan G., Wolfe L., Memoli M.J., He M., Vega H., Kim L.J.Y., Huang Y., Hussein N., Nievas E., Mitchell R., Garofalo M., Louie A., Ireland D.C., Grunes C., Cimbro R., Patel V., Holzapfel G., Salahuddin D., Bristol T., Adams D., Marciano B.E., Hegde M., Li Y., Calvo K.R., Stoddard J., Justement J.S., Jacques J., Priel D.A.L., Murray D., Sun P., Kuhns D.B., Boerkoel C.F., Chiorini J.A., Di Pasquale G., Verthelyi D., Rosenzweig S.D. Glycosylation, hypogammaglobulinemia, and resistance to viral infections. N. Engl. J. Med. 2014;370:1615–1625. doi: 10.1056/NEJMoa1302846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai A., Claire M.S., Faulk K., Govindarajan S., Emerson S.U., Purcell R.H., Bukh J. The p7 polypeptide of hepatitis C virus is critical for infectivity and contains functionally important genotype-specific sequences. Proc. Natl. Acad. Sci. U. S. A. 2003;100:11646–11651. doi: 10.1073/pnas.1834545100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidis G., McDougall W.M., Meraner P., Perreira J.M., Portmann J.M., Trincucci G., John S.P., Aker A.M., Renzette N., Robbins D.R., Guo Z., Green S., Kowalik T.F., Brass A.L. Identification of zika virus and dengue virus dependency factors using functional genomics. Cell Rep. 2016;16:232–246. doi: 10.1016/j.celrep.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Sayce A.C., Alonzi D.S., Killingbeck S.S., Tyrrell B.E., Hill M.L., Caputo A.T., Iwaki R., Kinami K., Ide D., Kiappes J.L., Beatty P.R., Kato A., Harris E., Dwek R.A., Miller J.L., Zitzmann N. Iminosugars inhibit dengue virus production via inhibition of ER alpha-glucosidases--not glycolipid processing enzymes. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallus T., Jaeckh C., Feher K., Palma A.S., Liu Y., Simpson J.C., Mackeen M., Stier G., Gibson T.J., Feizi T., Pieler T., Muhle-Goll C. Malectin: a novel carbohydrate-binding protein of the endoplasmic reticulum and a candidate player in the early steps of protein N-glycosylation. Mol. Biol. Cell. 2008;19:3404–3414. doi: 10.1091/mbc.E08-04-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schul W., Liu W., Xu H.Y., Flamand M., Vasudevan S.G. A dengue fever viremia model in mice shows reduction in viral replication and suppression of the inflammatory response after treatment with antiviral drugs. J. Infect. Dis. 2007;195:665–674. doi: 10.1086/511310. [DOI] [PubMed] [Google Scholar]
- Shrimal S., Cherepanova N.A., Gilmore R. Cotranslational and posttranslocational N-glycosylation of proteins in the endoplasmic reticulum. Semin. Cell Dev. Biol. 2015;41:71–78. doi: 10.1016/j.semcdb.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Becher P., Bukh J., Gould E.A., Meyers G., Monath T., Muerhoff S., Pletnev A., Rico-Hesse R., Smith D.B., Stapleton J.T., Ictv Report C. ICTV virus taxonomy profile: flaviviridae. J. Gen. Virol. 2017;98:2–3. doi: 10.1099/jgv.0.000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone M.I., Mares L.J., Eveleens C.A., McCluskey A., Pappin B.B., Kiefel M.J., Houston T.A. Back to (non-)Basics: an update on neutral and charge-balanced glycosidase inhibitors. Mini Rev. Med. Chem. 2018;18:812–827. doi: 10.2174/1389557517666171002161325. [DOI] [PubMed] [Google Scholar]
- Simsek E., Mehta A., Zhou T., Dwek R.A., Block T. Hepatitis B virus large and middle glycoproteins are degraded by a proteasome pathway in glucosidase-inhibited cells but not in cells with functional glucosidase enzyme. J. Virol. 2005;79:12914–12920. doi: 10.1128/JVI.79.20.12914-12920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somnuke P., Hauhart R.E., Atkinson J.P., Diamond M.S., Avirutnan P. N-linked glycosylation of dengue virus NS1 protein modulates secretion, cell-surface expression, hexamer stability, and interactions with human complement. Virology. 2011;413:253–264. doi: 10.1016/j.virol.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler K., Allison S.L., Schalich J., Heinz F.X. Proteolytic activation of tick-borne encephalitis virus by furin. J. Virol. 1997;71:8475–8481. doi: 10.1128/jvi.71.11.8475-8481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavale E.J., Vu H., Sampath A., Ramstedt U., Warfield K.L. In vivo therapeutic protection against influenza A (H1N1) oseltamivir-sensitive and resistant viruses by the iminosugar UV-4. PloS One. 2015;10 doi: 10.1371/journal.pone.0121662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann E., Penin F., Kallis S., Patel A.H., Bartenschlager R., Pietschmann T. Hepatitis C virus p7 protein is crucial for assembly and release of infectious virions. PLoS Pathog. 2007;3:e103. doi: 10.1371/journal.ppat.0030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiasny K., Heinz F.X. Flavivirus membrane fusion. J. Gen. Virol. 2006;87:2755–2766. doi: 10.1099/vir.0.82210-0. [DOI] [PubMed] [Google Scholar]
- Takashita E., Kawakami C., Morita H., Ogawa R., Fujisaki S., Shirakura M., Miura H., Nakamura K., Kishida N., Kuwahara T., Mitamura K., Abe T., Ichikawa M., Yamazaki M., Watanabe S., Odagiri T., On Behalf Of The Influenza Virus Surveillance Group Of J. Detection of influenza A(H3N2) viruses exhibiting reduced susceptibility to the novel cap-dependent endonuclease inhibitor baloxavir in Japan, December 2018. Euro Surveill. 2019;24(3):1800698. doi: 10.2807/1560-7917.ES.2019.24.3.1800698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashita E., Kawakami C., Ogawa R., Morita H., Fujisaki S., Shirakura M., Miura H., Nakamura K., Kishida N., Kuwahara T., Ota A., Togashi H., Saito A., Mitamura K., Abe T., Ichikawa M., Yamazaki M., Watanabe S., Odagiri T. Influenza A(H3N2) virus exhibiting reduced susceptibility to baloxavir due to a polymerase acidic subunit I38T substitution detected from a hospitalised child without prior baloxavir treatment, Japan, January 2019. Euro Surveill. 2019;24(12):1900170. doi: 10.2807/1560-7917.ES.2019.24.12.1900170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam D.T., Ngoc T.V., Tien N.T., Kieu N.T., Thuy T.T., Thanh L.T., Tam C.T., Truong N.T., Dung N.T., Qui P.T., Hien T.T., Farrar J.J., Simmons C.P., Wolbers M., Wills B.A. Effects of short-course oral corticosteroid therapy in early dengue infection in Vietnamese patients: a randomized, placebo-controlled trial. Clin. Infect. Dis. 2012;55:1216–1224. doi: 10.1093/cid/cis655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricou V., Minh N.N., Van T.P., Lee S.J., Farrar J., Wills B., Tran H.T., Simmons C.P. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Neglected Trop. Dis. 2010;4:e785. doi: 10.1371/journal.pntd.0000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermehren J., Park J.S., Jacobson I.M., Zeuzem S. Challenges and perspectives of direct antivirals for the treatment of hepatitis C virus infection. J. Hepatol. 2018;69(5):1178–1187. doi: 10.1016/j.jhep.2018.07.002. [DOI] [PubMed] [Google Scholar]
- Volkmar N., Thezenas M.L., Louie S.M., Juszkiewicz S., Nomura D.K., Hegde R.S., Kessler B.M., Christianson J.C. The ER membrane protein complex promotes biogenesis of sterol-related enzymes maintaining cholesterol homeostasis. J. Cell Sci. 2019;132 doi: 10.1242/jcs.223453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorndam V., Mathews J.H., Barrett A.D., Roehrig J.T., Trent D.W. Molecular and biological characterization of a non-glycosylated isolate of St Louis encephalitis virus. J. Gen. Virol. 1993;74(Pt 12):2653–2660. doi: 10.1099/0022-1317-74-12-2653. [DOI] [PubMed] [Google Scholar]
- Warfield K.L., Plummer E., Alonzi D.S., Wolfe G.W., Sampath A., Nguyen T., Butters T.D., Enterlein S.G., Stavale E.J., Shresta S., Ramstedt U. A novel iminosugar UV-12 with activity against the diverse viruses influenza and dengue (novel iminosugar antiviral for influenza and dengue) Viruses. 2015;7:2404–2427. doi: 10.3390/v7052404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield K.L., Barnard D.L., Enterlein S.G., Smee D.F., Khaliq M., Sampath A., Callahan M.V., Ramstedt U., Day C.W. The iminosugar UV-4 is a broad inhibitor of influenza A and B viruses ex vivo and in mice. Viruses. 2016;8:71. doi: 10.3390/v8030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield K.L., Plummer E.M., Sayce A.C., Alonzi D.S., Tang W., Tyrrell B.E., Hill M.L., Caputo A.T., Killingbeck S.S., Beatty P.R., Harris E., Iwaki R., Kinami K., Ide D., Kiappes J.L., Kato A., Buck M.D., King K., Eddy W., Khaliq M., Sampath A., Treston A.M., Dwek R.A., Enterlein S.G., Miller J.L., Zitzmann N., Ramstedt U., Shresta S. Inhibition of endoplasmic reticulum glucosidases is required for in vitro and in vivo dengue antiviral activity by the iminosugar UV-4. Antivir. Res. 2016;129:93–98. doi: 10.1016/j.antiviral.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield K.L., Schaaf K.R., DeWald L.E., Spurgers K.B., Wang W., Stavale E., Mendenhall M., Shilts M.H., Stockwell T.B., Barnard D.L., Ramstedt U., Das S.R. Lack of selective resistance of influenza A virus in presence of host-targeted antiviral, UV-4B. Sci. Rep. 2019;9:7484. doi: 10.1038/s41598-019-43030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Rathore A.P., Sung C., Lu F., Khoo Y.M., Connolly J., Low J., Ooi E.E., Lee H.S., Vasudevan S.G. Dose- and schedule-dependent protective efficacy of celgosivir in a lethal mouse model for dengue virus infection informs dosing regimen for a proof of concept clinical trial. Antivir. Res. 2012;96:32–35. doi: 10.1016/j.antiviral.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Chan K.W., Dow G., Ooi E.E., Low J.G., Vasudevan S.G. Optimizing celgosivir therapy in mouse models of dengue virus infection of serotypes 1 and 2: the search for a window for potential therapeutic efficacy. Antivir. Res. 2016;127:10–19. doi: 10.1016/j.antiviral.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Bowden T.A., Wilson I.A., Crispin M. Exploitation of glycosylation in enveloped virus pathobiology. Biochim. Biophys. Acta Gen. Subj. 2019;1863:1480–1497. doi: 10.1016/j.bbagen.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch S., Miller S., Romero-Brey I., Merz A., Bleck C.K., Walther P., Fuller S.D., Antony C., Krijnse-Locker J., Bartenschlager R. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe. 2009;5:365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby K., Taylor D., Patel D., Ahmed P., Tyms A.S. Action of celgosivir (6 O-butanoyl castanospermine) against the pestivirus BVDV: implications for the treatment of hepatitis C. Antivir. Chem. Chemother. 2004;15:141–151. doi: 10.1177/095632020401500304. [DOI] [PubMed] [Google Scholar]
- Whitby K., Pierson T.C., Geiss B., Lane K., Engle M., Zhou Y., Doms R.W., Diamond M.S. Castanospermine, a potent inhibitor of dengue virus infection in vitro and in vivo. J. Virol. 2005;79:8698–8706. doi: 10.1128/JVI.79.14.8698-8706.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehorn J., Nguyen C.V.V., Khanh L.P., Kien D.T.H., Quyen N.T.H., Tran N.T.T., Hang N.T., Truong N.T., Hue Tai L.T., Cam Huong N.T., Nhon V.T., Van Tram T., Farrar J., Wolbers M., Simmons C.P., Wills B. Lovastatin for the treatment of adult patients with dengue: a randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 2016;62:468–476. doi: 10.1093/cid/civ949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichit S., Jittmittraphap A., Hidari K.I., Thaisomboonsuk B., Petmitr S., Ubol S., Aoki C., Itonori S., Morita K., Suzuki T., Suzuki Y., Jampangern W. Dengue virus type 2 recognizes the carbohydrate moiety of neutral glycosphingolipids in mammalian and mosquito cells. Microbiol. Immunol. 2011;55:135–140. doi: 10.1111/j.1348-0421.2010.00293.x. [DOI] [PubMed] [Google Scholar]
- Winkler G., Maxwell S.E., Ruemmler C., Stollar V. Newly synthesized dengue-2 virus nonstructural protein NS1 is a soluble protein but becomes partially hydrophobic and membrane-associated after dimerization. Virology. 1989;171:302–305. doi: 10.1016/0042-6822(89)90544-8. [DOI] [PubMed] [Google Scholar]
- Woodhouse S.D., Smith C., Michelet M., Branza-Nichita N., Hussey M., Dwek R.A., Zitzmann N. Iminosugars in combination with interferon and ribavirin permanently eradicate noncytopathic bovine viral diarrhea virus from persistently infected cells. Antimicrob. Agents Chemother. 2008;52:1820–1828. doi: 10.1128/AAC.01181-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.F., Lee C.J., Liao C.L., Dwek R.A., Zitzmann N., Lin Y.L. Antiviral effects of an iminosugar derivative on flavivirus infections. J. Virol. 2002;76:3596–3604. doi: 10.1128/JVI.76.8.3596-3604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.Y., Jan J.T., Ma S.H., Kuo C.J., Juan H.F., Cheng Y.S., Hsu H.H., Huang H.C., Wu D., Brik A., Liang F.S., Liu R.S., Fang J.M., Chen S.T., Liang P.H., Wong C.H. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc. Natl. Acad. Sci. U. S. A. 2004;101:10012–10017. doi: 10.1073/pnas.0403596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap S.S.L., Nguyen-Khuong T., Rudd P.M., Alonso S. Dengue virus glycosylation: what do we know? Front. Microbiol. 2017;8:1415. doi: 10.3389/fmicb.2017.01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida E., Kunimoto D., Lee S.S. Results of a phase II dose ranging study of orally administered celgosivir as monotherapy in chronic hepatitis C genotype-1 patients. Gastroenterology. 2006;130:A784. [Google Scholar]
- Yoshii K., Yanagihara N., Ishizuka M., Sakai M., Kariwa H. N-linked glycan in tick-borne encephalitis virus envelope protein affects viral secretion in mammalian cells, but not in tick cells. J. Gen. Virol. 2013;94:2249–2258. doi: 10.1099/vir.0.055269-0. [DOI] [PubMed] [Google Scholar]
- Yu I.M., Zhang W., Holdaway H.A., Li L., Kostyuchenko V.A., Chipman P.R., Kuhn R.J., Rossmann M.G., Chen J. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science. 2008;319:1834–1837. doi: 10.1126/science.1153264. [DOI] [PubMed] [Google Scholar]
- Yu L., Nomaguchi M., Padmanabhan R., Markoff L. Specific requirements for elements of the 5' and 3' terminal regions in flavivirus RNA synthesis and viral replication. Virology. 2008;374:170–185. doi: 10.1016/j.virol.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.M., Holdaway H.A., Chipman P.R., Kuhn R.J., Rossmann M.G., Chen J. Association of the pr peptides with dengue virus at acidic pH blocks membrane fusion. J. Virol. 2009;83:12101–12107. doi: 10.1128/JVI.01637-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Gill T., Wang L., Du Y., Ye H., Qu X., Guo J.T., Cuconati A., Zhao K., Block T.M., Xu X., Chang J. Design, synthesis, and biological evaluation of N-alkylated deoxynojirimycin (DNJ) derivatives for the treatment of dengue virus infection. J. Med. Chem. 2012;55:6061–6075. doi: 10.1021/jm300171v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zai J., Mei L., Wang C., Cao S., Fu Z.F., Chen H., Song Y. N-glycosylation of the premembrane protein of Japanese encephalitis virus is critical for folding of the envelope protein and assembly of virus-like particles. Acta Virol. 2013;57:27–33. doi: 10.4149/av_2013_01_27. [DOI] [PubMed] [Google Scholar]
- Zakaria M.K., Carletti T., Marcello A. Cellular targets for the treatment of flavivirus infections. Front. Cell Infect. Microbiol. 2018;8:398. doi: 10.3389/fcimb.2018.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Chipman P.R., Corver J., Johnson P.R., Zhang Y., Mukhopadhyay S., Baker T.S., Strauss J.H., Rossmann M.G., Kuhn R.J. Visualization of membrane protein domains by cryo-electron microscopy of dengue virus. Nat. Struct. Biol. 2003;10:907–912. doi: 10.1038/nsb990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.B., Tian L.Q., Li Y.M., Liao Y.F., Li J., Bing F.H. Protective effect of homonojirimycin from Commelina communis (dayflower) on influenza virus infection in mice. Phytomedicine. 2013;20:964–968. doi: 10.1016/j.phymed.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Zhang R., Miner J.J., Gorman M.J., Rausch K., Ramage H., White J.P., Zuiani A., Zhang P., Fernandez E., Zhang Q., Dowd K.A., Pierson T.C., Cherry S., Diamond M.S. A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nature. 2016;535:164–168. doi: 10.1038/nature18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Jia R., Shen H., Wang M., Yin Z., Cheng A. Structures and functions of the envelope glycoprotein in flavivirus infections. Viruses. 2017;9 doi: 10.3390/v9110338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Guo F., Comunale M.A., Mehta A., Sehgal M., Jain P., Cuconati A., Lin H., Block T.M., Chang J., Guo J.T. Inhibition of endoplasmic reticulum-resident glucosidases impairs severe acute respiratory syndrome coronavirus and human coronavirus NL63 spike protein-mediated entry by altering the glycan processing of angiotensin I-converting enzyme 2. Antimicrob. Agents Chemother. 2015;59:206–216. doi: 10.1128/AAC.03999-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzmann N., Mehta A.S., Carrouee S., Butters T.D., Platt F.M., McCauley J., Blumberg B.S., Dwek R.A., Block T.M. Imino sugars inhibit the formation and secretion of bovine viral diarrhea virus, a pestivirus model of hepatitis C virus: implications for the development of broad spectrum anti-hepatitis virus agents. Proc. Natl. Acad. Sci. U. S. A. 1999;96:11878–11882. doi: 10.1073/pnas.96.21.11878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.