Abstract
Case series
Patients: Male, 36-year-old • Male, 47-year-old • Male, 78-year-old
Final Diagnosis: COVID-19 • pneumomediastinum • subcutaneous emphysema
Symptoms: Respiratory distress • shortness of breath
Medication: —
Clinical Procedure: —
Specialty: Critical Care Medicine • Pulmonology
Objective:
Rare co-existance of disease or pathology
Background:
Novel Coronavirus 2019 (COVID-19) has been in the spotlight since the first cases were reported in December 2019. COVID-19 has been found to cause severe acute respiratory distress syndrome and, more uncommonly, subcutaneous emphysema and pneumomediastinum. We present a case series of 3 patients with COVID-19 infection managed in the Intensive Care Unit and found to have subcutaneous emphysema and pneumomediastinum on chest imaging.
Case Reports:
We present a case series of 3 men, ages 36, 47, and 78 years, diagnosed with COVID-19 via RT-PCR, found to have severe acute respiratory distress syndrome, and managed in the Intensive Care Unit. Two patients described in this case series were mechanically ventilated on low positive end-expiratory pressures and developed subcutaneous emphysema and pneumomediastinum on chest imaging, and 1 patient developed subcutaneous emphysema prior to intubation. Each of these patients had a more eventful hospital course and worse outcomes than most COVID-19 infected patients.
Conclusions:
Subcutaneous emphysema and pneumomediastinum in COVID-19 patients have been rarely reported and is poorly understood. In our institution, we have found the diagnosis of subcutaneous emphysema and pneumomediastinum in COVID-19 patients is associated with unfavorable outcomes and worse prognosis.
MeSH Keywords: Coronavirus; COVID-19; Pneumomediastinum, Diagnostic; Subcutaneous Emphysema
Background
Novel Coronavirus 2019 (COVID-19), also known as severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), causes severe pneumonia and acute respiratory distress syndrome (ARDS), with a high mortality rate [1]. Subcutaneous emphysema is not commonly seen in this infection and can be suggestive of more serious disease [2]. We present a cases series of patients diagnosed with COVID-19 and found to have sub-cutaneous emphysema and pneumomediastinum associated with a more complicated hospital course.
Case Reports
First case
A 36-year-old man with a history of poorly controlled type 1 diabetes mellitus (hemoglobin A1c on admission 15.1%) presented to the Emergency Department complaining of worsening shortness of breath, fevers, and dry cough of 1-week duration. On examination, he was evidently short of breath but not using accessory muscles and was able to speak in full sentences. His laboratory tests on admission were significant for a C-reactive protein of 20.34 mg/dL (normal value: 0.0–0.74 mg/dL), D-dimer of 6298 ng/mL (normal value: <500 ng/mL), and procalcitonin level of 0.52 ng/mL (normal value: <0.05 ng/mL). His arterial blood gas showed a pH of 7.293, partial pressure of carbon dioxide (pCO2) of 18.8 mmHg, pO2 of 90.4 mmHg, and oxygen saturation of 94.3%. A chest X-ray was performed, showing bilateral ground-glass opacities and diffuse infiltrates worse at the bilateral lung bases, as well as evidence of subcutaneous emphysema (Figure 1). He was diagnosed with COVID-19 using reverse-transcriptase polymerase chain reaction (RTPCR) assay and was also found to have anion-gap metabolic acidosis due to diabetic ketoacidosis (blood glucose level on admission 410 mg/dL). He was started on insulin infusion and intravenous fluids for ketoacidosis. He was also started on azithromycin, vitamin C, zinc sulfate, and hydroxychloroquine for COVID-19.
Figure 1.
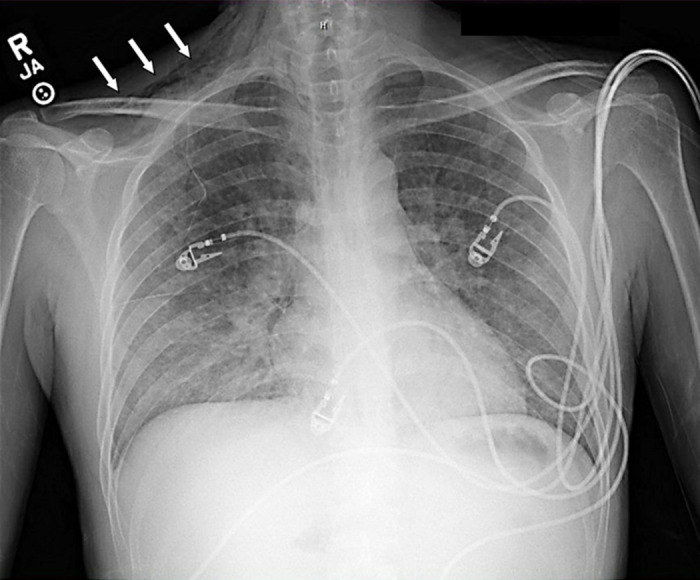
Chest X-ray of the first patient showing subcutaneous emphysema (denoted by arrows) prior to intubation.
While in the Emergency Department, he became hypoxic with an oxygen saturation of 82% on room air and was placed on supplemental oxygen. His condition continued to worsen, and he was subsequently intubated and transferred to the Intensive Care Unit. On day 3, he remained in critical condition and was given 1 dose of tocilizumab. He was also started on high-dose solumedrol for a 7-day course. On day 5, a computed tomography (CT) scan of the chest was obtained, showing bilateral ground-glass opacities, pneumomediastinum, and moderatesized subcutaneous emphysema (Figure 2). His positive endexpiratory pressure (PEEP) was 6 cmH2O and peak pressure was 30. By day 7, his subcutaneous emphysema had improved, and on day 8 it was no longer present. Due to worsening and severe ARDS, the decision was made to place him in prone position for 18 hours a day. After minimal improvement and on high ventilator settings, on day 9 he began veno-venous extracorporeal membrane oxygenation at 3 liters per minute and pump speed of 4100 rpm. Despite aggressive management, his condition deteriorated, and he ultimately died due to his condition on day 15 of hospitalization.
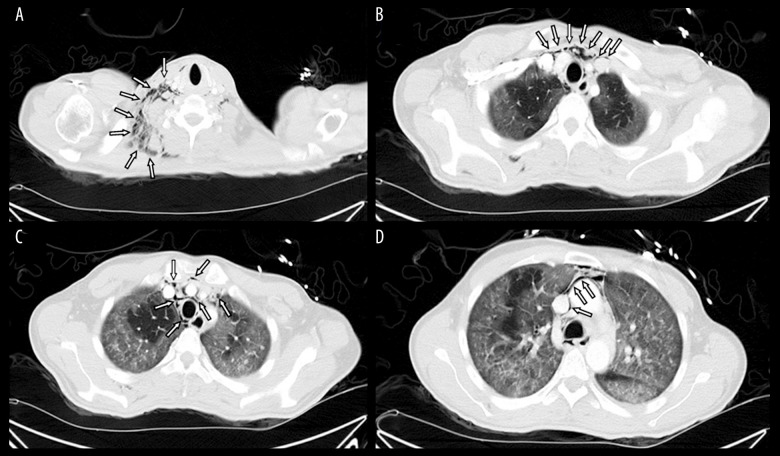
Figure 2.
(A–D) Chest computed tomography of the first patient showing subcutaneous emphysema and pneumomediastinum (denoted by arrows).
Second Case
A 47-year-old man with a past medical history of drug abuse (now on methadone) and alcohol abuse was admitted for acute hypoxic respiratory failure. At an urgent care center, he had tested positive for COVID-19 a few days prior to admission following complaints of shortness of breath and fevers for 1 week. His vitals on admission were blood pressure of 135/70 mmHg, temperature 100.9°F (38.2°C), heart rate of 110 beats per minute, oxygen saturation of 75% on room air, and respiratory rate of 28 breaths per minute. He was in mild-to-moderate distress on exam, his pulse O2 improved initially to the low 90s on 15 liters non-rebreather mask, then started declining, and he became hypoxic to the mid 80s on 60 liters and fraction of inspired oxygen (FiO2) of 100% via Optiflow machine. He was subsequently intubated for progressive hypoxemia, and his saturation dropped to the low 60s. His initial ventilator setting was 100% FiO2 and PEEP of 12 cmH2O. X-rays taken after intubation and on the next day showed the endotracheal tube was in place, with no subcutaneous emphysema or pneumomediastinum visible (Figure 3).
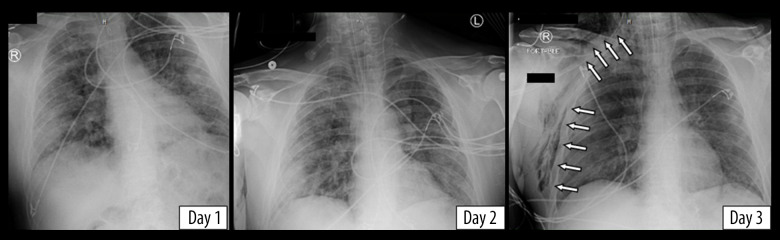
Figure 3.
Chest X-rays of the second patient showing new-onset subcutaneous emphysema on day 3 (denoted by arrows).
He had elevated inflammatory markers with ferritin 493 ng/mL, CRP 3.02 ng/mL, D-dimer 6867, and LDH 389 U/L and was started on a 5-day course of hydroxychloroquine, azithromycin, and zinc sulfate. His PaO2/FiO2 ratio (P/F ratio) was 85, so the decision was made to prone him for 18 hours and he received 1 dose of 8 mg/kg of tocilizumab. The following day, his P/F ratio improved to 207, so his ventilator setting was changed to 80% FiO2 and PEEP stayed at 12 cmH2O. On day 3, a chest X-ray showed a right subcutaneous emphysema and pneumomediastinum and his PEEP was subsequently decreased from 12 to 10 (Figure 3).
Over the course of the next 2 days, his PEEP was weaned down to 8 cmH2O, and a chest X-ray on day 6 showed significant improvement of the subcutaneous emphysema. On day 8, the patient had a right spontaneous apical pneumothorax, for which a chest tube was placed. His respiratory status continued to improve, and he was ultimately extubated on day 13 and was transferred to the general medical floors for further management.
Third case
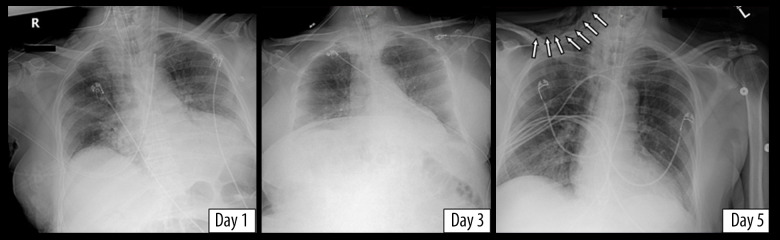
A 78-year-old man with a history of hypertension and hyper-lipidemia presented to our facility complaining of shortness of breath and confusion. In the Emergency Department, his vital signs were a temperature of 99°Ft, heart rate 126 beats per minute, blood pressure 126/72 mmHg, and oxygen saturation 89% on room air. He was intubated in the Emergency Department, and he tested positive for COVID-19 the following day. He was started on hydroxychloroquine, azithromycin, zinc sulfate, and vitamin C for a 5-day course. On day 3, his mean arterial pressure dropped to <60 mmHg, so he was started on norepinephrine infusion for hemodynamic stability. He remained on a FiO2 of 100% and PEEP of 5 cmH2O. A portable chest X-ray on day 5 showed subcutaneous emphysema and pneumomediastinum, which were not seen on the chest X-rays taken on day 1 and 3 (Figure 4). He remained in critical condition, eventually requiring the addition of 2 more vasopressors to maintain a mean arterial pressure of >60 mmHg. On day 8, the decision was made at the request of his family to withdraw care and pursue comfort measures, and the patient died.
Figure 4.
Chest X-rays of the third patient showing new-onset subcutaneous emphysema on day 5 (denoted by arrows).
Discussion
Subcutaneous emphysema can be spontaneous, secondary to trauma or infection, or iatrogenic secondary to barotrauma or medical procedures [3]. Iatrogenic subcutaneous emphysema and pneumomediastinum, although rare, can occur following endotracheal intubation and is usually evident within 24 hours after intubation [4,5]. Mechanisms proposed to explain such complications include tracheal mucosal tear, over-inflation of the endotracheal tube cuff, and congenital dehiscence in the mucosa [6]. Barotrauma, another iatrogenic cause of subcutaneous emphysema in mechanically ventilated patients, has only been reported when plateau pressure exceeds 35 cmH2O [7]. Our patients did not develop subcutaneous emphysema within 24 hours of intubation and did not have plateau pressure above 35 cmH2O (Figures 5–7).
Figure 5.
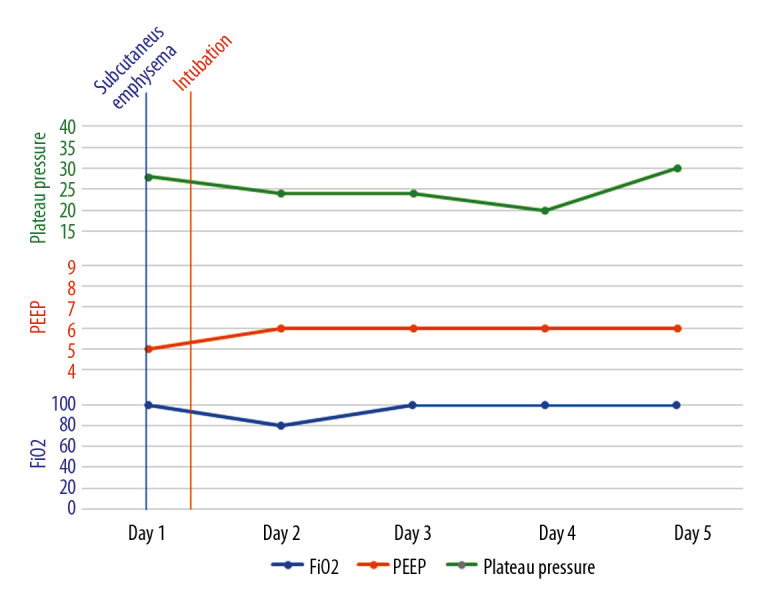
A graph showing the timeline of events and ventilator parameters for the first patient.
Figure 6.
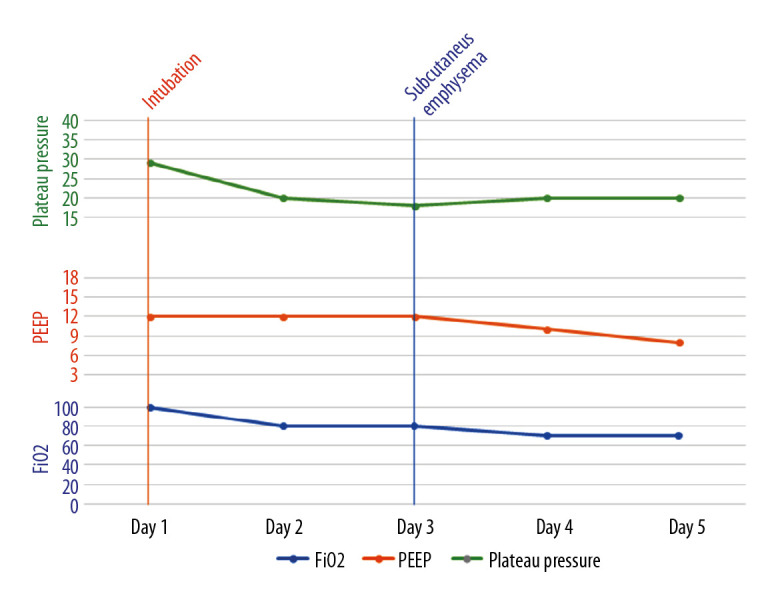
A graph showing the timeline of events and ventilator parameters for the second patient.
Figure 7.
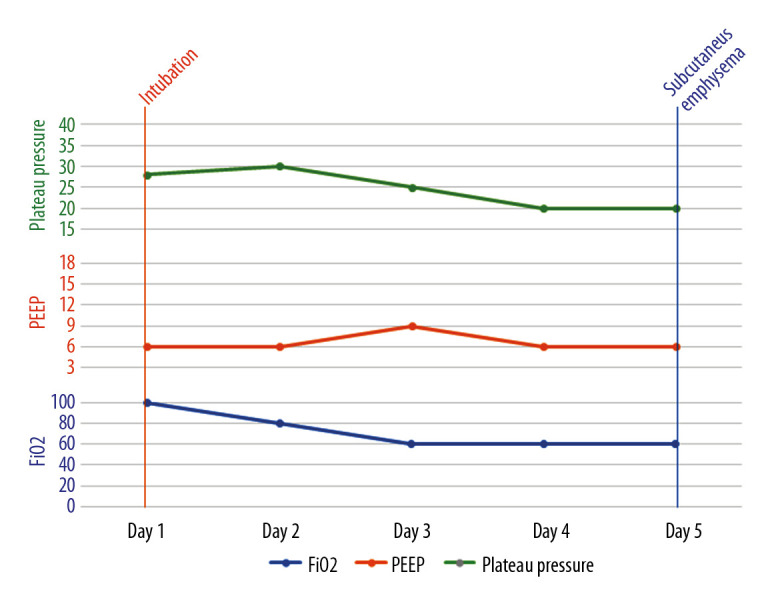
A graph showing the timeline of events and ventilator parameters for the third patient.
Spontaneous subcutaneous emphysema and pneumomediastinum have been reported as uncommon clinical findings in COVID-19 patients [2,8,9], and it has been suspected to be the sequelae of alveolar membrane rupture secondary to direct infection of pneumocytes type I and II [8]. In general, sub-cutaneous emphysema causes mild symptoms and resolves on its own, but can be life-threatening if expanding into the chest wall, and can lead to worsening respiratory acidosis and failure [3,10].
Chu et al. reported that subcutaneous emphysema and pneumomediastinum can complicate severe acute respiratory syndrome (SARS), and that in this subset of patients, the development of these pathologies indicated worse disease and patients requiring more aggressive management [11]. The coincidence of also seeing this clinical manifestation in COVID-19 may indicate a similar viral pathogenesis [12]. Our 3 patients had unfavorable outcomes compared with patients with similar profiles in our institution, which may suggest that subcutaneous emphysema is a predictor of poor prognosis. However, larger case-controlled studies are needed to determine if a correlation between COVID-19 disease severity and subcutaneous emphysema and pneumomediastinum does in fact exist.
Conclusions
Subcutaneous emphysema and pneumomediastinum can be a spontaneous condition seen in COVID-19 patients. In non-COVID-19 patients, it is commonly self-limited; however, COVID-19 patients with subcutaneous emphysema and pneumomediastinum seem to have more complicated hospital courses. Larger studies are warranted to investigate this possible correlation.
Footnotes
Conflicts of interest
None.
References:
- 1.Zhai P, Ding Y, Wu X, et al. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020;55(5):105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou C, Gao C, Xie Y, Xu M. COVID-19 with spontaneous pneumomediastinum. Lancet Infect Dis. 2020;20(4):510. doi: 10.1016/S1473-3099(20)30156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aghajanzadeh M, Dehnadi A, Ebrahimi H, et al. Classification and management of subcutaneous emphysema: A 10-year experience. Indian J Surg. 2015;77(Suppl. 2):673–77. doi: 10.1007/s12262-013-0975-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandey M, Jain A, Mehta A, Sharma M. Endotracheal intubation related massive subcutaneous emphysema and tension pneumomediastinum resulting in cardiac arrest. J Postgrad Med. 2003;49(2):188–89. [PubMed] [Google Scholar]
- 5.Kaloud H, Smolle-Juettner FM, Prause G, List WF. Iatrogenic ruptures of the tracheobronchial tree. Chest. 1997;112(3):774–78. doi: 10.1378/chest.112.3.774. [DOI] [PubMed] [Google Scholar]
- 6.Jo YY, Park WY, Choi E, et al. Delayed detection of subcutaneous emphysema following routine endotracheal intubation – A case report. Korean J Anesthesiol. 2010;59(3):220–23. doi: 10.4097/kjae.2010.59.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boussarsar M, Thierry G, Jaber S, et al. Relationship between ventilatory settings and barotrauma in the acute respiratory distress syndrome. Intensive Care Med. 2002;28(4):406–13. doi: 10.1007/s00134-001-1178-1. [DOI] [PubMed] [Google Scholar]
- 8.Kolani S, Houari N, Haloua M, et al. Spontaneous pneumomediastinum occurring in the SARS-COV-2 infection. IDCases. 2020;21:e00806. doi: 10.1016/j.idcr.2020.e00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Gao R, Zheng Y, Jiang L. COVID-19 with spontaneous pneumothorax,pneumomediastinum and subcutaneous emphysema. J Travel Med. 2020 doi: 10.1093/jtm/taaa062. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck PL, Heitman SJ, Mody CH. Simple construction of a subcutaneous catheter for treatment of severe subcutaneous emphysema. Chest. 2002;121(2):647–49. doi: 10.1378/chest.121.2.647. [DOI] [PubMed] [Google Scholar]
- 11.Chu CM, Leung YY, Hui JYH, et al. Spontaneous pneumomediastinum in patients with severe acute respiratory syndrome. Eur Respir J. 2004;23(6):802–4. doi: 10.1183/09031936.04.00096404. [DOI] [PubMed] [Google Scholar]
- 12.Petrosillo N, Viceconte G, Ergonul O, et al. COVID-19, SARS and MERS: Are they closely related? Clin Microbiol Infect. 2020;26(6):729–34. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]