Abstract
Patient: Male, 29-year-old
Final Diagnosis: Acute respiratory distress syndrome (ARDS) • COVID-19 •multi organ failure/septic shock • pneumothorax
Symptoms: Cough • dyspnea • fatigue • myalgia
Medication:—
Clinical Procedure: Mechanical ventilation • thoracentesis
Specialty: Critical Care Medicine
Objective:
Unknown ethiology
Background:
COVID-19 patients that develop acute respiratory distress syndrome (ARDS) “CARDS” behave differently compared to patients with classic forms of ARDS. Recently 2 CARDS phenotypes have been described, Type L and Type H. Most patients stabilize at the milder form, Type L, while an unknown subset progress to Type H, resembling full-blown ARDS. If uncorrected, phenotypic conversion can induce a rapid downward spiral towards progressive lung injury, vasoplegia, and pulmonary shrinkage, risking ventilator-induced lung injury (VILI) known as the “VILI vortex”. No cases of in-hospital phenotypic conversion have been reported, while ventilation strategies in these patients differ from the lung-protective approaches seen in classic ARDS.
Case Report:
A 29-year old male was admitted with COVID-19 pneumonia complicated by severe ARDS, multi-organ failure, cytokine release syndrome, and coagulopathy during his admission. He initially resembled CARDS Type L case, although refractory hypoxemia, fevers, and a high viral burden prompted conversion to Type H within 8 days. Despite ventilation strategies, neuromuscular blockade, inhalation therapy, and vitamin C, he remained asynchronous to the ventilator with volumes and pressures beyond accepted thresholds, eventually developing a fatal tension pneumothorax.
Conclusions:
Patients that convert to Type H can quickly enter a spiral of hypoxemia, shunting, and dead-space ventilation towards full-blown ARDS. Understanding its nuances is vital to interrupting phenotypic conversion and entry into VILI vortex. Tension pneumothorax represents a poor outcome in patients with CARDS. Further research into monitoring lung dynamics, modifying ventilation strategies, and understanding response to various modes of ventilation in CARDS are required to mitigate these adverse outcomes.
MeSH Keywords: Coronavirus; COVID-19; Pneumothorax; Respiration, Artificial; Respiratory Distress Syndrome, Adult; Ventilator-Induced Lung Injury
Background
The cluster of pneumonia cases associated with the novel coronavirus (COVID-19) or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that emerged from Wuhan, China and spread rapidly across continents was labeled by the World Health Organization (WHO) as a global pandemic. As of May 19, 2020, 1.5 million cases of COVID-19 were present in the United States (US), with roughly 85 400 deaths [1]. COVID-19 pneumonia seems to behave differently from other viral types of pneumonia, with large swings in respiratory functioning, inferring that not all previous practices can be adopted, and new strategies are needed to mitigate the high mortality rates (79% to 86%) seen in advanced cases [2,3]. Supplemental oxygen use was seen in 38.9% of infected patients, with 28.7% requiring mechanical ventilation and less than 1% requiring advanced therapies such as extracorporeal membrane oxygenation (ECMO); however, these numbers may likely be underrepresented with preventative measures such as social distancing and stay-at-home executive orders leading to reluctance and delay in receiving care [4]. The progression of COVID-19 to ARDS (“CARDS”) represents a life-threatening sequela, with its ability to lower blood oxygenation levels and induce systemic hypoxemia and multi-organ failure [3,5]. Despite CARDS meeting the Berlin Diagnostic Criteria, its trajectory is characterized by severe hypoxemia with near-normal respiratory compliance, unlike its classic form [5,6]. Patients with CARDS can present within a broad spectrum from perceived normal breathing (“silent hypoxemia”) to floored respiratory compromise with a wide array of overlapping features in between [6]. Recently, 2 CARDS phenotypes have been parsed out, Type L and Type H, with each one having its distinct pathophysiological pathway. Understanding these nuances are vital to providing appropriate treatment and avoiding sub-optimal outcomes [5]. We present a case of CARDS with subsequent sequelae and numerous challenges in management. We aim to strengthen the existing literature, explore the CARDS phenotypes, and discuss therapeutic and ventilator strategies to counteract the unique lung injury seen in COVID-19 pneumonia that progress to ARDS.
Case Report
A 29-year-old male with a history of asthma, previous gunshot wound, and obesity, presented to the hospital with dyspnea, cough, fatigue, and myalgias. He used tobacco products and worked at an auto-parts manufacturing unit. On arrival, he was febrile, tachycardic, and tachypneic requiring supplemental oxygen. He appeared ill with a high work of breathing and a productive cough. Workup revealed lymphopenia to 1.4 k/uL, thrombocytopenia to 121 000 k/uL, and an unremarkable chest radiograph (Figure 1); the patient was transferred to the intensive care unit (ICU) with a high suspicion for COVID-19. Testing for SARS-CoV-2 was completed using a nasopharyngeal swab transported in an M4 viral tube to the State Department of Health and Human Services. Samples were tested on the SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) Abbott ID NOW™ point-of-care system under the Food and Drug Administration (FDA) emergency use authorization (EUA). Computed tomography (CT) of the chest could not be performed due to concern for virus transmission and environmental contamination due to high demands and short downtime for decontamination. By day 3, the patient required higher flow rates on a non-rebreather mask, and by day 4, persistent fevers, tachypnea, and new consolidative changes in the right middle and lower lung zones were noted. He was empirically started on broad-spectrum antibiotics with hydroxychloroquine. Foregoing Bi-PAP or C-PAP due to concerns for aerosolization, he was placed on mechanical ventilation. His chest x-ray by day 8 revealed extensive consolidative infiltrates bilaterally and a PaO2/FiO2 (PF) ratio of 59 consistent with severe acute respiratory distress syndrome (ARDS) (Figure 2).
Figure 1.
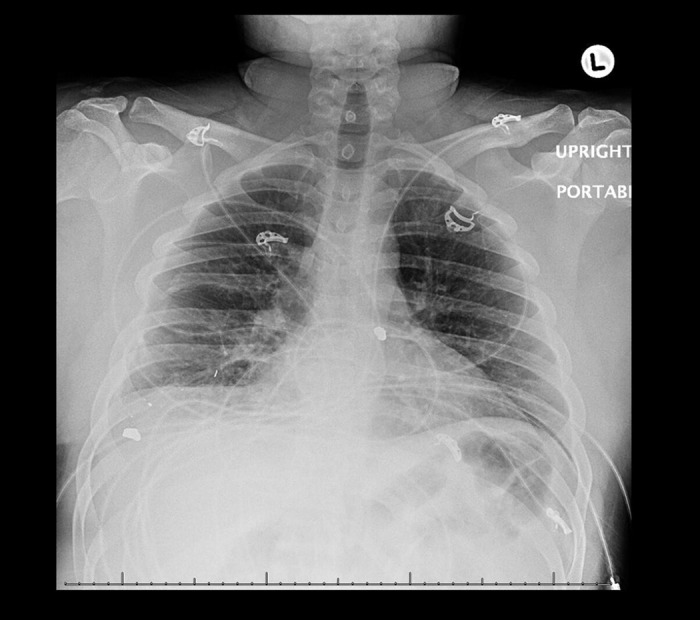
Chest radiograph revealing mild bilateral interstitial changes consistent with CARDS Type L on day 1.
Figure 2.
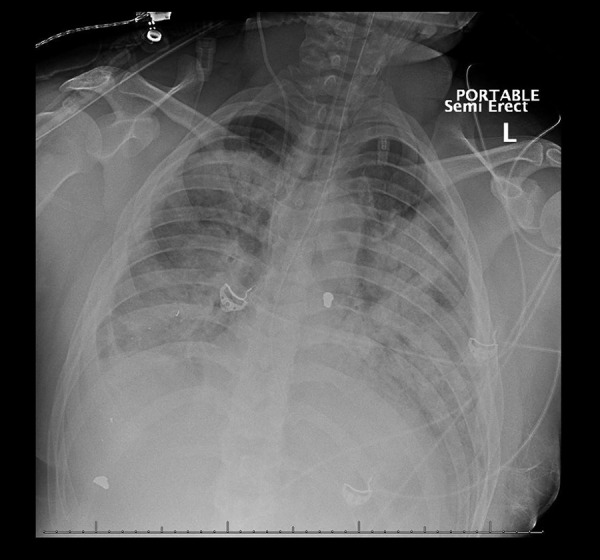
Chest radiograph revealing extensive bilateral consolidations consistent with CARDS Type H on day 8.
Testing for SARS-CoV-2 came back positive, confirming COVID-19 pneumonia. Over the following days, he went into septic shock requiring vasopressor support, while previously sent cytokine labs, including an interleukin-6 (IL-6) of 46 pg/mL, were consistent with cytokine release syndrome. He was given a dose of tocilizumab 400 mg. On day 10, extensive acute deep vein thromboses (DVTs) was discovered in his left upper extremity with d-dimer levels over 10 µg/mL. His previous prophylactic dose of enoxaparin was increased to a therapeutic dose. His fevers did not abate, requiring cooling, neuromuscular blockade, and deep sedation. ARDS strategies, including low tidal volumes, proning, recruitment maneuvers, diuretics, nitric oxide, and vitamin C, were used despite his rising pressures. During these periods, he exhibited high plateau pressures, often over 30 cm of H2O. Worsening status prompted consideration of transfer to a specialized ECMO center. However, surrounding centers had limited the inflow of patients adhering to strict infection control measures, while judicious resource allocation and logistical challenges made transportation un-feasible. On day 17, after a sudden episode of desaturation, a chest x-ray revealed left-sided tension pneumothorax in the left mid and lower lung fields (Figure 3) with a chest tube draining 700 mL of serosanguineous fluid mixed with blood clots intermittently blocking output with persistent air leaks. Fluid characteristics were not obtained, and his overall clinical trajectory began declining. A family discussion was held to discuss his poor prognosis and address the goals of care. His code status was changed to do-not-resuscitate (DNR) with an emphasis on comfort measures. He eventually desaturated and went into asystole, passing away after spending 20 days in the hospital. Trends in oxygenation in our patient can be seen in Table 1.
Figure 3.
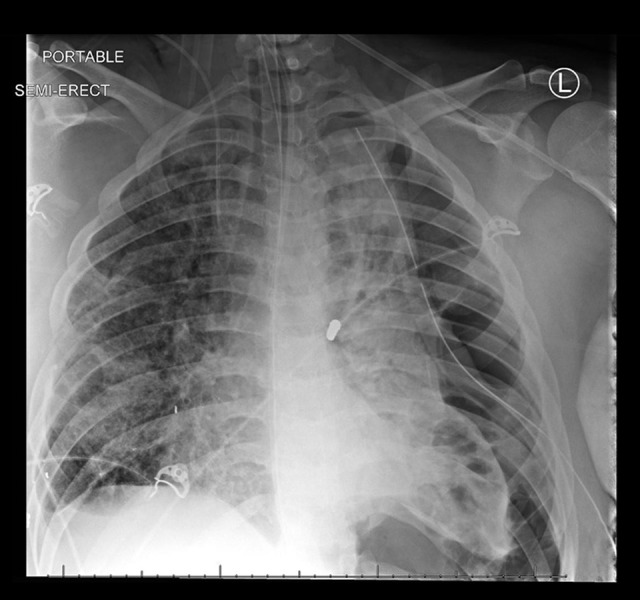
Chest radiograph revealing pneumothorax with near collapse of the left lung and placement of a chest tube on day 18 of admission.
Table 1.
Trends in oxygenation and ventilation.
| Day of admission | Oxygen delivery | FiO2 (%)/flow rate (L/min) | Tidal volume (mL) | PEEP | Respiratory rate (breaths/min) | pH | PaO2 | O2saturation (%) | PaCO2 | HCO3 | PaO2/FiO2 (PF) ratio | Radiographic findings | CARDS phenotype |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Nasal cannula | 2L– 6L | N/A | N/A | 28–32 | N/A | N/A | 90 | N/A | 29 | N/A | Mild bilateral interstitial changes (Figure 1) | Type L |
| Day 3 | Non-rebreather | 12L | N/A | N/A | 34–47 | N/A | N/A | 90 | N/A | 23 | N/A | Mild bilateral interstitial changes | Type L |
| Day 4 | Volume control– Assist control | 100 | 500 | 8 | 14 | 7.36 | 43 | 76.3 | 51 | 28.1 | 43 | Interval worsening of airspace consolidation in the right mid and lower lung zone | Type L |
| Day 8 | Volume control– Assist control | 80 | 370 | 14 | 30 | 7.33 | 47 | 79.1 | 60 | 31.1 | 59 | Bilateral extensive consolidated infiltrate concerning for multifocal pneumonia (Figure 2) | Type H |
| Day 13 | Volume control– Assist control | 100 | 500 | 20 | 32 | 7.48 | 118 | 98.5 | 44 | 32.5 | 118 | Worsening of bilateral infiltrates. Interval development of moderate size left pleural effusion | Type H |
| Day 17 | Volume control– Assist control | 90 | 420 | 18 | 26 | 7.30 | 60 | 87.2 | 82 | 39.2 | 67 | Interval development of moderate tension pneumothorax in left mid/lower lung field with bilateral alveolar infiltrates (Figure 3) | Type H |
| Day 18 | Volume control– Assist control | 100 | 450 | 18 | 32 | 7.38 | 68 | 92.9 | 61 | 35.1 | 68 | Left pneumothorax with chest tube placed. Worse compared to prior with near collapse of the left lung. Left lung base consolidation | Type H |
Discussion
ARDS can be mitigated by opening collapsed alveoli with higher positive end-expiratory pressures (PEEPs), recruiting maneuvers, or proning. In contrast, these high pressures are poorly tolerated, leading to the use of low tidal volumes to minimize ventilator-induced lung injury (VILI). Despite this, high rates of barotrauma were reported from the previous SARS epidemic [7]. COVID-19 patients complicated by ARDS (“CARDS”) can present despite lacking traditional risk factors such as advancing age, pre-existing co-morbidities, or an advanced lung pathology [5]. Despite the initial insult being the inoculation of SARS-CoV-2, CARDS can occur from injuries from either the gas or vascular side of the alveoli [6].
Approaching CARDS from the gas side
CARDS is defined by 2 phenotypes based on its clinical trajectory. “Type L” has a relatively compensated clinical state while “Type H” resembles full-blown ARDS (Table 2). Our patient, initially a Type L, had scattered infiltrates with rising minute ventilation over days. Type L patients are perceived to be breathing normally (“silent hypoxemia”) and often respond to supplemental oxygen. At the same time, deep swings in respiration can induce patient self-inflicted lung injury (P-SILI), triggering an inflammation cascade and a rapid downward spiral towards progressive pulmonary injury and pulmonary shrinkage, known as the ‘VILI vortex’ towards full-blown ARDS [6]. Evidence of transformation to Type H by day 8 was noted by his rising plateau and driving pressures, lower static compliance, and low PF ratios signifying a bulkier lung. Refractory hypoxia, fevers, and systemic insults led to a dependency on high PEEPs and FiO2 to maintain oxygen saturation above 88%.
Table 2.
Characteristics of COVID-19 pneumonia (CARDS) Type L and H.
| COVID-19 Pneumonia, Type L | Property | COVID-19 Pneumonia, Type H |
|---|---|---|
| Low | Elastance | High |
| Normal-to-high | Compliance | Low |
| Normal | Gas volume | Decreased |
| Low ventilation-to-perfusion (V/Q) ratio due to vasoplegia and loss of hypoxic vasoconstriction | Primary driver of hypoxia | Right-to-left shunt due to perfusion of non-aerated regions affected by edema and high pressures |
| Normal-to-low | Lung weight | High |
| Low with sparse non-aerated regions | Lung recruitability/PEEP response | Increased with more non-aerated regions |
| Limited interstitial ground-glass opacities along subpleural planes and lung fissures | Computed tomography (CT) | Extensive consolidations |
Approaching CARDS from the vascular side
Post-mortem studies reveal numerous thromboses in COVID-19 patients with d-dimer serving as a surrogate marker of pulmonary endothelial damage, promoting ventilation-perfusion mismatches and subsequent hypoxemia [5,6,8]. DVTs were noted in over 50% of patients, suggesting that coagulopathy may be an independent risk factor for poor prognosis [9]. Our patient reflected coagulopathy and vasoplegia with rising d-dimer levels in the days leading up to his DVT, while the development of cytokine release syndrome and coagulopathy signaled his growing disease burden. Once this occurs, vasoregulation is altered due to the failure of hypoxic vasoconstriction from the endothelial damage resulting in significant hypoxemia. If the respiratory drive is not altered by oxygen administration, the generated inspiratory increases transpulmonary pressures across vascular channels, risking VILI. This can also serve as a learning point, that early intubation strategies can help minimize the powerful respiratory effort leading to vasoplegia. If uncorrected, high plateau pressures reflecting high trans alveolar pressures can increase thoracic compliance leading to high oxygen and PEEP requirements that can redirect blood to damaged parts with high permeability affecting hemodynamics and contributing to Type H conversion.
Pneumothorax in CARDS lungs
The accepted thresholds for VILI protection include a plateau pressure of 30 cmH2O and a driving pressure of 15 cmH2O [10]. Protecting CARDS lungs involves aspects of lung-protective ventilation while ensuring that the vascular side of the alveoli does not incur further damage. Oxygen strategies in these patients are summarized in Table 3. Surrogate measures to monitor work of breathing have been proposed and may help guide therapy with esophageal manometry for inspiratory or central venous pressure (CVP) swings. At the same time, interrupters of the VILI vortex include early intubation, sedation, or paralysis with the use of lower PEEP (8–10 cmH2O) [6,11]. After consistently cycling through the VILI vortex, our patient developed spontaneous pneumothorax with a 50% drop in his PF ratio because of his high plateau pressures, respiratory swings, and inflammation. The incidence of pneumothorax has been reported to be roughly 6% in COVID-19 patients [12], although these represented spontaneous cases from the community, unlike our case which was a consequence of progressive lung injury and the inability to liberate the patient from the VILI vortex. The development of pneumothorax after intubation can portend a poor prognosis in patients with CARDS [12–14].
Table 3.
Oxygen strategies in COVID-19 pneumonia (CARDS) Type L and Type H.
| COVID-19 Pneumonia, Type L | Time Frame | COVID-19 Pneumonia, Type H |
|---|---|---|
| Nasal cannula, high-flow nasal cannula, continuous positive airway pressure (CPAP), non-invasive positive pressure ventilation (NIPPV), or awake proning to avoid increased respiratory efforts | Pre-intubation | Nasal cannula, high-flow nasal cannula, continuous positive airway pressure (CPAP), non-invasive positive pressure ventilation (NIPPV), or awake proning to avoid increased respiratory efforts |
| Lower positive end-expiratory pressures (PEEP) (<10 cmH2 O), tidal volumes (7–9 mL/kg), maintain gas exchange and fluid balances. Proning only as a rescue maneuver. extracorporeal membrane oxygenation (ECMO) in severe COVID-19 patients | Mechanical ventilation | Higher positive end-expiratory pressures (PEEP) (<15 cmH2O), tidal volumes (5–7 mL/kg), maintain gas exchange and fluid balances. Initiate proning and extracorporeal membrane oxygenation (ECMO) if parameters met. Manage these patients as full-blown ARDS |
| Avoid vigorous spontaneous increases in breathing, pressure swings with breathing trials towards the ending of the weaning process. The goal is to avoid ventilator-induced lung injury (VILI) and worsening edema. Initiate careful weaning measures | Weaning | Avoid vigorous spontaneous increases in breathing, pressure swings with breathing trials towards the ending of the weaning process. The goal is to avoid ventilator-induced lung injury (VILI) and worsening edema. Initiate careful weaning measures |
Importance of categorizing CARDS lungs
Categorizing lung injury from COVID-19 into CARDS Type L or Type H can help unwanted practices and initiate targeted ventilator approaches to correct the underlying mismatch. The Surviving Sepsis Campaign recommended that mechanically ventilated COVID-19 patients be managed similarly to other patients with respiratory failure in the ICU, although with the growing body of evidence, CARDS is displaying a distinct course [15]. The 3 essential contributors to CARDS and entry into the VILI vortex are 1) SARS-CoV-2 burden, 2) ventilator responsiveness, and 3) time of symptom onset [6]. The paucity of reported cases brings into light the importance of isolated reports in guiding therapy in the current climate. This case represents the first reported CARDS patient that developed a pneumothorax as a consequence of his phenotype conversion. In previous cases of SARS patients, pneumothorax was noted at 14–37 days after the initial diagnosis [16], suggesting that a sustained period of lung inflammation serves as a pre-requisite, a similar time course as our patient Recently a scoring system was proposed to predict the risk of developing critical illness in COVID-19, allowing early interventions and resource allocation to mitigate the high disease burden [17].
Conclusions
COVID-19 patients that develop ARDS (“CARDS”) come in 2 phenotypes: Type L and H. Type L is often stable while Type H presents like full-blown ARDS. These patients require different ventilator strategies with the goal of avoiding conversion to Type H and limiting VILI. In these cases, pneumothorax may represent an indicator of a poor outcome.
Footnotes
Conflict of interests
None.
References:
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–34. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dondorp AM, Hayat M, Aryal D, et al. Respiratory support in novel corona-virus disease (COVID-19) patients, with a focus on resource-limited settings. Am J Trop Med Hyg. 2020;102:1191–97. doi: 10.4269/ajtmh.20-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geier MR, Geier DA. Respiratory conditions in coronavirus disease 2019 (COVID-19): important considerations regarding novel treatment strategies to reduce mortality. Med Hypotheses. 2020;140:109760. doi: 10.1016/j.mehy.2020.109760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Simone G, Mancusi C. COVID-19: Timing is important. Eur J Intern Med. 2020 doi: 10.1016/j.ejim.2020.04.019. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;7(6):435–44. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 6.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fowler RA. Critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:367–73. doi: 10.1001/jama.290.3.367. [DOI] [PubMed] [Google Scholar]
- 8.Rello J, Storti E, Belliato M, Serrano R. Clinical phenotypes of SARS-CoV-2: Implications for clinicians and researchers. Eur Respir J. 2020;55:2001028. doi: 10.1183/13993003.01028-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wichmann D, Sperhake J-P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020 doi: 10.7326/L20-1206. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Tonetti T, Vasques F, Rapetti F, et al. Driving pressure and mechanical power: New targets for VILI prevention. Ann Transl Med. 2017;5(14):286. doi: 10.21037/atm.2017.07.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walling Pt, Savege Tm. A comparison of oesophageal and central venous pressures in the measurement of transpulmonary pressure change. Br J Anaesth. 1976;48(5):475–79. doi: 10.1093/bja/48.5.475. [DOI] [PubMed] [Google Scholar]
- 12.Yao W, Wang T, Jiang B, et al. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: Lessons learnt and international expert recommendations. Br J Anaesth. 2020 doi: 10.1016/j.bja.2020.03.026. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rohailla S, Ahmed N, Gough K. SARS-CoV-2 infection associated with spontaneous pneumothorax. CMAJ. 2020;2020:E510. doi: 10.1503/cmaj.200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Gao R, Zheng Y, Jiang L. COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema. J Travel Med. 2020. [Online ahead of print] [DOI] [PMC free article] [PubMed]
- 15.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: Guidelines on the management of critically ill adults with Coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46(5):854–87. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sihoe ADL, Wong RHL, Lee ATH, et al. Severe acute respiratory syndrome complicated by spontaneous pneumothorax. Chest. 2004;125(6):2345–51. doi: 10.1378/chest.125.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.2033. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


