Abstract
Patient: Female, 65-year-old
Final Diagnosis: Acute renal injury • COVID-19
Symptoms: Fever
Medication: —
Clinical Procedure: —
Specialty: Infectious Diseases
Objective:
Rare co-existance of disease or pathology
Background:
Acute kidney injury is one of the most common complications in patients infected with SARS-CoV-2, occurring in up to 7% of cases and increasing to 23% in patients treated in the Intensive Care Unit (ICU). The objective of this report was to describe the clinical case of a patient infected by SARS-CoV-2 who developed acute renal injury, probably secondary to this infection.
Case Report:
On 1 April 2020, a 65-year-old woman presented to the emergency service of the National Institute of Respiratory Diseases, Mexico City, with a 15-day history of dry cough and subjective fever. Finally, the following diagnoses were integrated: Acute renal injury of etiology to be determined (acute chronic kidney disease secondary to T2DM vs. acute renal injury by SARS-CoV-2) and COVID-19. The patient had a typical presentation of severe COVID-19, evidencing all the risk and severity factors for this disease. However, after being admitted to the hospital, she showed evidence of acute renal injury. Although the renal injury may have been due to microangiopathic damage caused by chronic hypertension and diabetes, it is imperative to consider the possibility that such exacerbation contributes to SARS-CoV-2 infection or synergy of multiple factors.
Conclusions:
Every aspect of this pandemic remains unclear. The formulation of hypotheses to explain the physiopathological mechanisms by which this new virus can cause mortality in infected patients may help reduce mortality rates and control the pandemic itself.
MeSH Keywords: Acute Kidney Injury, COVID-19, Diabetes Mellitus, Hypertension, SARS Virus
Background
Coronavirus disease 19 (COVID-19) has never before affected health systems worldwide, and, despite scientific and technological advances, the pandemic has not been optimally controlled. As of 9 July 2020, the World Health Organization (WHO) reported a total of 11 669 259 confirmed cases worldwide, including 539 906 deaths [1], while in Mexico during the same period, the Secretary of Health reported 275 003 confirmed cases with 32 796 deaths and an estimated worldwide mortality rate of 4.6% [2].
Acute kidney injury is one of the most common complications in patients infected with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), occurring in up to 7% of cases and increasing to 23% in patients treated in the Intensive Care Unit (ICU) [3].
Angiotensin-converting enzyme receptor 2 (ACE2) has been identified as the cellular entry point of the 3 members of the coronavirus family that have caused global health problems– Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS), and COVID-19. This receptor has also been found in cardiac, intestinal, pulmonary, hepatic, and renal cells [4].
There have been 3 mechanisms proposed through which SARSCoV-2 can injure the kidneys: 1) direct damage of kidney cells by the virus, as this new coronavirus has 10–20 times greater affinity for the ACE2 receptor; 2) cytokine-mediated injury, especially by IL2, 7, and 10, and granulocyte colony-stimulating factor, among others; and 3) by excessive activation of T lymphocytes. These pathophysiological mechanisms together with others such as a state of hypoperfusion due to hypovolemia can jointly or individually lead to acute renal injury [5].
Although the renal cells most affected in COVID-19 are in the renal tubular system, glomerular filtration is also undermined to a greater or lesser extent, which explains the disorders of both urinary sediment and metabolites such as creatinine and ureic nitrogen. Mild proteinuria is the most common sign, followed by elevation of urea nitrogen (27%) and elevation of serum creatinine (19%). Hypodensity of the renal parenchyma has been demonstrated on CT scans to be a sign of inflammation [5].
A retrospective patient cohort study by Yan Deng et al. in Wuhan, China, found that creatinine levels and the likelihood of developing acute kidney injury were significantly higher in patients who died from COVID-19 than in those who recovered [6].
Regarding guidelines for treatment of acute kidney injury in COVID-19 disease, Ronco et al. proposed that in addition to kidney-protection measures (e.g., avoiding nephrotoxic agents, monitoring of creatinine and urinary output, balance adjustment, and avoiding volume overload or hypovolemia), it is important to consider early continuous replacement therapy for kidney function as the first treatment option if conservative measures fail [7] (especially in hemodynamically unstable patients), in addition to sequential extracorporeal support of organs (ECOS) [8]. Guangchang et al. also found that adequate management of pneumonia and prevention of barotrauma provided renal protection [9].
Martínez Rojas et al. postulated that “cytokine storm”, especially involving IL-6, is the main cause of renal dysfunction. This, together with a decrease in blood volume and inflammation of the renal parenchyma, as well as microthrombosis of the renal arteries, severely impair renal functions. From this derives the therapeutic suggestion of the use of IL-6 inhibitors and extracorporeal circulation therapies to eliminate or mitigate cytokines that directly affect the kidney [10].
Finally, Ali et al. proposed that renal damage in many patients with severe SARS-CoV-2 impairs their ability to eliminate and metabolize the drugs used for their treatment and increases mortality [11]. The objective of the present report was to describe the case of a SARS-CoV-2-infected patient who developed acute renal injury, probably secondary to this infection.
Case Report
On 1 April 2020, a 65-year-old woman presented to an emergency care clinic in the National Institute of Respiratory Diseases, Mexico City, with a 15-day history of dry cough and subjective fever.
She had a 25-year history of both type 2 diabetes mellitus (treated with insulin NPH 48 International Units in the morning 20 International Units in the night) and high blood pressure (treated with Losartan 50 mg every 24 h and Amlodipine 5 mg every 24 h). She reported no history of recent travel or any contact with COVID-19-positive patients.
In the physical examination, the patient was febrile, hypotensive, tachycardic, and tachypneic. Lung auscultation revealed decreased breath sounds in both lung fields and oxygen saturation of 80% with 15 L. Due to hypoxemia and shortness of breath, we provided airway management in assisted control mode and high volumes, and ventilator parameters were established. After ventilatory parameters were stabilized, the patient was placed in prone position.
That same day, she presented a urinary volume reduction and creatinine of 6.10, revealing a kidney injury AKIN III, so treatment with furosemide was initiated, showing good response. Subsequently, she presented glucose up to 435 mg/dl, so an insulin pump was started; the patient was sent to the Intensive Care Unit due to acute respiratory syndrome and metabolic abnormalities.
A CT scan revealed patches of ground-glass opacities in both lung fields, with subpleural predominance and with a tendency to consolidation, discreetly hypodense renal parenchyma (27.34 Hounsfield units), and the renal arteries and veins showed no visible morphological alterations (Figures 1, 2).
Figure 1.

CT scan. Axial cut with the presence of ground-glass opacities, some of them with a tendency to consolidation in both lung fields, in a patient with COVID-19.
Figure 2.
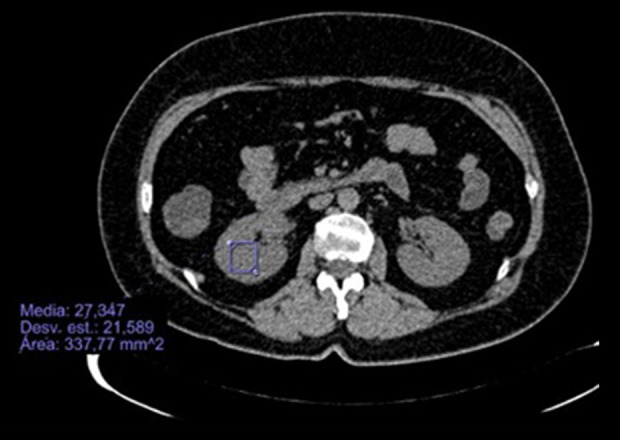
CT scan. Axial cut with slightly hypodense renal parenchyma in a patient with COVID-19.
The microbiology reports of 28 March 2020 were positive for SARS-CoV2, which was detected by real-time polymerase chain reaction (qPCR) using the BD-MAX system (Becton Dickinson Diagnostic Systems, Sparks, MD, USA) and approved by the Institute for Epidemiological Diagnosis and Reference (InDRE) according to the Berlin Protocol suggested by the WHO, and the patient was negative for AH1N1 and seasonal influenza as shown by nasopharyngeal exudate and bronchial aspiration. The blood culture of peripheral blood reported the isolation of coagulase-negative Staphylococcus. The urine culture on 29 March 2020 revealed K. pneumoniae and E. Coli, both pan-sensitive.
Blood analysis showed a clear increase in white cells and a marked lymphopenia (5.9%), D-dimer within normal ranges, elevated CPK levels (1209), clear elevation of LDH, hypoalbuminemia, and hypertriglyceridemia.
Finally, 2 diagnoses were integrated: acute renal injury of etiology to be determined (acute chronic renal injury secondary to T2DM vs. acute renal injury by SARS-CoV2) and COVID-19. The patient was treated with renal protection and supportive measures, as well as renal replacement therapy by hemodialysis.
Discussion
Despite efforts of the scientific community to understand various aspects of the COVID-19 pandemic, several factors remain unclear. The relationship between SARS-CoV-2 infection and acute kidney injury is still debated by several authors. In a patient cohort analyzed by Wang et al., the authors concluded that the infection by this new virus is not related to the occurrence of acute kidney failure [12]; however, the number of patients analyzed was low, and the fact that no genetic material of the virus was found in urine samples does not exclude the possibility that SARS-CoV-2 infection can cause kidney damage by other mechanisms such as cytokine storm or direct cytopathic effect. Xiu-Wu Pan et al. proposed that podocytes and proximal contoured tubule cells are target cells because they found overexpression of receptors was necessary for the virus to infect human cells [3]. Underexpression of ACE2 receptors at the renal level has been identified in diabetic patients [13], which could explain the acute kidney injury presented by our patient. Also, Li et al. found that early-stage viremia of this infection has a duration of 7–14 days [14], which corresponds with the time elapsed in the present case between the onset of symptoms and aggravation of respiratory injuries.
Our patient had lymphopenia, which, according to Li Tan et al., is one of the main predictors of severity and mortality in patients infected by SARS-CoV-2 [15]. No proteinuria was found, but the levels of urea and creatinine were higher than the normal range.
Our patient had a typical presentation of severe COVID-19, with all the risk factors already described for such patients; however, she was admitted due to acute renal injury, which might have been due to microangiopathic damage caused by her chronic hypertension and diabetes. It is imperative to consider that such an exacerbation could have been due to SARSCoV-2 infection, or to the synergy of multiple factors, such as damage caused by marked elevation of CPK and DHL, the state of hypotension and secondary hypoperfusion, and the bacteria isolated in the general urine test.
Finally, despite tomographic evidence of a slight decrease in renal parenchyma density reported in acute renal lesions caused by SARS-CoV-2, it is important to perform a renal biopsy or a PCR analysis of renal samples to identify the presence of the virus genetic material in the kidneys.
The initial treatment of renal failure established in the present case coincides with that recommended in the current literature on acute renal failure in COVID-19 patients [7,8], which consists of renal support and protection measures, as well as early introduction of renal replacement therapies. Interleukin 6 inhibitors were not used in our patient because it was not possible to quantify IL-6 plasma levels, and waiting for the isolation of microorganisms in blood and urine put the patient at risk for developing a serious state of infection.
Conclusions
We are still faced with an inconclusive picture of every aspect of this pandemic. The formulation of hypotheses to explain the physiopathological mechanisms through which this new virus can increase mortality in infected patients could help reduce mortality and control the pandemic itself.
This case report may provide a basis for further development and hypotheses by the medical and scientific community to understand all possible scenarios in which the virus can damage extra-pulmonary organs.
Acknowledgments
We thank the National Institute of Respiratory Diseases for providing the facilities to carry out this work and Dr. Ma. Isabel Salazar Sánchez for her contribution to virological issues.
Footnotes
Conflict of interest
None.
References:
- 1.Coronavirus Disease (COVID-19) Situation Dashboard [Internet] United Nations: World Health Organization, Esri (WHO); [Cited 2020 June 6]. Available from: https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd. [Google Scholar]
- 2.Comunicado Técnico Diario Nuevo Coronavirus en el Mundo (COVID-19) [Internet] Mexico: Secretaría de Salud; [Cited 2020 June 6]. Available from: https://www.gob.mx/cms/uploads/attachment/file/545264/Comunicado_Tecnico_Diario_COVID-19_2020.04.04.pdf [in Spanish] [Google Scholar]
- 3.Pan X, Xu D, Zhang H, et al. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: A study based on single cell transcriptome analysis. Intensive Care Med. 2020;46:1114–16. doi: 10.1007/s00134-020-06026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang F, Liang Y. The potential risk of kidney vulnerable to novel coronavirus 2019 infection [letter] Am J Physiol Renal Physiol. 2020;318(5):F1136–37. doi: 10.1152/ajprenal.00085.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang XH, Sun RH, Chen DC. [Diagnosis and treatment of COVID-19: Acute kidney injury cannot be ignored] Zhonghua Yi Xue Za Zhi. 2020;100(16):1205–8. doi: 10.3760/cma.j.cn112137-20200229-00520. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 6.Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: A retrospective study. Chin Med J (Engl) 2020;133(11):1261–67. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adapaa S, Chennab A, Ballac M, et al. COVID-19 pandemic causing acute kidney injury and impact on patients with chronic kidney disease and renal transplantation. J Clin Med Res. 2020;12(6):352–61. doi: 10.14740/jocmr4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;14:1–6. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pei GC, Zhang Z, Peng J, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31:1157–65. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez MA, Vega O, Bobadilla NA. Is the kidney a target of SARS-CoV-2? Am J Physiol Renal Physiol. 2020;318:F1454–62. doi: 10.1152/ajprenal.00160.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rismanbaf A, Zarei S. Liver and kidney injuries in COVID-19 and their effects on drug therapy. Arch Acad Emerg Med. 2020;8(1):e17. [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Li X, Chen H, et al. Coronavirus disease 19 infection does not result in acute kidney injury: An analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51(5):343–48. doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizuiri S, Ohashi Y. ACE and ACE2 in kidney disease. World J Nephrol. 2015;4(1):74–82. doi: 10.5527/wjn.v4.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li T, Lu H, Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9(1):687–90. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


