Abstract
Currently assessment of the potential immunotoxicity of a given agent involves a tiered approach for hazard identification and mechanistic studies, including observational studies, evaluation of immune function, and measurement of susceptibility to infectious and neoplastic diseases. These studies generally use costly low-throughput mammalian models. Zebrafish, however, offer an excellent alternative due to their rapid development, ease of maintenance, and homology to mammalian immune system function and development. Larval zebrafish also are a convenient model to study the innate immune system with no interference from the adaptive immune system. In this study, a respiratory burst assay (RBA) was utilized to measure reactive oxygen species (ROS) production after developmental xenobiotic exposure. Embryos were exposed to non-teratogenic doses of chemicals and at 96 hr post-fertilization, the ability to produce ROS was measured. Using the RBA, 12 compounds with varying immune-suppressive properties were screened. Seven compounds neither suppressed nor enhanced the respiratory burst; five reproducibly suppressed global ROS production, but with varying potencies: benzo[a]pyrene, 17β-estradiol, lead acetate, methoxychlor, and phenanthrene. These five compounds have all previously been reported as immunosuppressive in mammalian innate immunity assays. To evaluate whether the suppression of ROS by these compounds was a result of decreased immune cell numbers, flow cytometry with transgenic zebrafish larvae was used to count the numbers of neutrophils and macrophages after chemical exposure. With this assay, benzo[a]pyrene was found to be the only chemical that induced a change in the number of immune cells by increasing macrophage but not neutrophil numbers. Taken together, this work demonstrates the utility of zebrafish larvae as a vertebrate model for identifying compounds that impact innate immune function at non-teratogenic levels, and validates measuring ROS production and phagocyte numbers as metrics for monitoring how xenobiotic exposure alters the innate immune system.
Keywords: Chemical screen, high-throughput, phagocyte, reactive oxygen species (ROS), polycyclic aromatic hydrocarbons (PAH), endocrine disrupting compounds (EDC), lead
Introduction
Exposure to xenobiotics that alter immune function may confer susceptibility to infectious or neoplastic disease. With tens of thousands of chemicals in production globally, frameworks are needed in order to assess them for immunotoxic potential. Current practices for immunotoxicity testing consist of a low-throughput tiered approach in rodent models (Germolec et al. 2017). While Tier I involves screens to assess basic immunologic function after exposure to xenobiotics, Tier II follows in order to provide a meaningful depth of those effects by using multiple immunologic assays for apical endpoints (Germolec et al. 2017). In fact, the United States Environmental Protection Agency (U.S. EPA) Toxic Substances Control Act Health Effects Guidelines for evaluating immunotoxicity are intended only for use in rodent models, and they do not require functional assessment of myeloid cells in the innate immune system (U.S. EPA 1998). Given this, throughput is limited, and studies may often be limited by the lack of resources needed to maintain mammalian systems. While in vitro methods exist for high-throughput assessment, reliability is variable and focuses heavily on the adaptive immune system (Gehen et al. 2014; Germolec et al. 2017), leaving a gap in knowledge regarding the innate immune system. All of these points considered, there is a necessity for high-throughput animal models for innate immunotoxicity testing and hazard identification. To fill this gap, we propose the use of zebrafish (Danio rerio) as a potential animal model.
The zebrafish model has risen in popularity in recent years for laboratory use, especially with regards to toxicity studies. Zebrafish are highly fecund, with a single female producing hundreds of embryos in a single clutch that are fertilized externally (Lawrence 2016). These transparent embryos develop rapidly, allowing researchers to track development from the single-cell stage through organogenesis and into their larval state (Kimmel et al. 1995). The small size of the zebrafish embryo provides another advantage to the model, adding to its throughput capability; embryos can be placed into 96- or 384-well plates, for testing multiple chemicals and/or multiple doses on the same plate (Lantz-McPeak et al. 2015; Poureetezadi et al. 2016). By 72 hr post-fertilization (hpf), the liver of the embryonic zebrafish has developed (Wang et al. 2017) and has the ability to biotransform xenobiotics (Saad et al. 2016). This may be especially important for identification of chemicals that must be bioactivated to exert toxicity, an aspect which may be overlooked in high-throughput in vitro assays. Perhaps the most important aspect of the zebrafish model is their homology to humans; the zebrafish genome encodes orthologs to 70% of all human protein-encoding genes (Howe et al. 2013), including immunoglobulin and T-cell receptor genes that undergo RAG-mediated V(D)J recombination, Toll-like receptors, and numerous cytokines, making them an excellent model for human immunological health.
Although zebrafish are an exceptional model for toxicity studies, they have been widely neglected as a model for immunotoxicity (Planchart et al. 2016; Espenschied et al. 2018). As teleost fish, they possess both an innate immune system and an adaptive immune system capable of defending the host from pathogens, and all major immune cell lineages and pathways in zebrafish are conserved in mammalian models (reviewed in Stachura and Traver 2016; Traver and Yoder 2020). Within 24 hpf, the zebrafish has a beating heart (Kimmel et al. 1995), which aids in the circulation of immune cells in the blood. At this same time, macrophages have developed and are able to phagocytose microbes and apoptotic bodies (Herbomel et al. 1999; Willett et al. 1999; Stachura and Traver 2016). By 48 hpf, neutrophils are present within the embryo and possess the ability to migrate to sites of wounding (Willett et al. 1999; Lieschke et al. 2001; Stachura and Traver 2016) and of infection to clear pathogens (Yang et al. 2012). Lymphocytes - which comprise the adaptive immune system - are not identifiable until 3 wk post-fertilization (Willett et al. 1999; Stachura and Traver 2016) and antibody production is not fully functional until ≈ 4 wk post-fertilization (Lam et al. 2004). Thus, embryonic and larval zebrafish provide a vertebrate experimental system in which the innate immune system can be studied in vivo with no “interference” from the adaptive immune system.
Because the zebrafish lacks adaptive immunity during early development, they must rely on the innate immune system for defense against pathogens for survival during early development. One of the most useful tools in this process is the respiratory burst - the rapid production of reactive oxygen species (ROS) in response to immune stimulation. Zebrafish encode homologs to the mammalian NADPH oxidase (Weaver et al. 2016) and inducible nitric oxide synthase (Vojtech et al. 2009; Huang et al. 2014) enzymes involved in the respiratory burst. Once pathogens are phagocytosed, the NADPH oxidase complex assembles from several different proteins (reviewed by Flannagan et al. 2009). This complex then oxidizes NADPH to produce NADP+, H+, and superoxide (reviewed by Bogdan et al. 2000). Superoxide can be further acted upon to create hydrogen peroxide, hydroxyl radicals, or hypochlorous acid. These highly reactive compounds target several essential microbial molecules (proteins, lipids, and nucleic acids), disrupting their function and inducing microbial death (reviewed by Flannagan et al. 2009).
In this study, larval zebrafish were utilized to determine if exposure to 12 different xenobiotics of known/suspected immunomodulatory potential were able to modulate the production of ROS in vivo after stimulation with phorbol 12-myristate 13-acetate (PMA). For compounds shown to suppress larval ROS production, follow-up experiments were performed to determine if chemical exposure altered phagocyte number. Of the 12 compounds tested, five suppressed ROS production; of these, one altered phagocyte number. Not only does this study recapitulate findings from mammalian studies, it also demonstrates the utility of the zebrafish as a model for high-throughput hazard identification of immunotoxic compounds.
Materials and Methods
Zebrafish embryos
Adult zebrafish were maintained in a recirculating aquarium facility (Aquatic Habitats, Apopka, FL) at 28°C with a 14 hr light/10 hr dark cycle and fed a commercial grade zebrafish diet. Wild-type zebrafish were purchased from LiveAquaria (www.LiveAquaria.com) and Doctors Foster and Smith (www.drsfostersmith.com). Transgenic zebrafish lines Tg(mpx:GFP) (Renshaw et al. 2006) and Tg(mfap4:tdTomato-caax) (Walton et al. 2015) were kind gifts from Stephen Renshaw (University of Sheffield) and David Tobin (Duke University), respectively. Zebrafish embryos were obtained by natural spawning. At 2 hr post-fertilization (hpf), embryos were treated with 0.06% sodium hypochlorite (bleach [v/v]) in 10% Hanks Balanced Salt Solution (HBSS) in ultrapure water using two 5-min washes to eliminate extra-ovum microbes. The embryos were then maintained in 100-mm Petri dishes in 10% HBSS at 28°C until use. Zebrafish husbandry and experiments involving live animals were approved by the North Carolina State University Institutional Animal Care and Use Committee.
Compounds
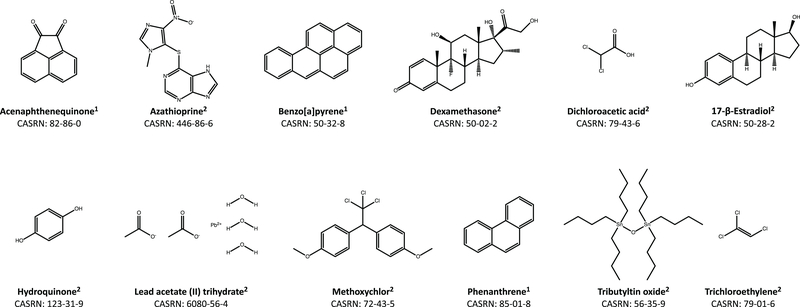
A total of 12 chemicals were selected for study (Figure 1) based on their immunomodulatory properties in mammalian models. Chemicals (20 mM stocks in DMSO [dimethyl sulfoxide; Sigma, St. Louis, MO]) were provided by Batelle (Columbus, OH) and MRI Global (Kansas City, MO). Chemicals were serially-diluted at 1:3.125 in DMSO eight times, yielding nine total stock concentrations. These stocks were then diluted at 1:250 in 10% HBSS for the zebrafish embryo treatments. Exposures of the embryos to these concentrations (80.0 μM, 25.6 μM, 8.19 μM, 2.62 μM, 839 nM, 268 nM, 85.9 nM, 27.5 nM and 8.80 nM) were evaluated in a range-finding developmental toxicity assay to determine the highest non-teratogenic concentration (aka no-observed-effect level [NOEL]) for use in the respiratory burst and flow cytometry assays.
Figure 1. Chemicals for zebrafish exposures.
Names and structures of chemicals used for zebrafish exposures. Chemicals were provided by (1) Battelle (Columbus, OH) or (2) MRI Global (Kansas City, MO). Chemical structures were generated with ChemDraw Professional (v16.0.1.4) using International Union of Pure and Applied Chemistry (IUPAC) nomenclature.
Developmental toxicity assay
At 6 hpf, zebrafish embryos were placed in 24-well plates at a density of 3 embryos/well in the 10% HBSS with chemical dilution (see above). Two wells (6 embryos total) were used for each chemical dilution. At 24, 48, and 72 hpf, a 99% HBSS/chemical solution change was achieved by two consecutive 90% media changes. At 96 hpf, embryos were observed under a light microscope and scored as dead, abnormal, or normal. For each chemical, the highest concentration exposure with all normal embryos was considered the NOEL and used as the starting (highest) concentration for subsequent respiratory burst assays (RBA).
Chemical exposure of zebrafish embryos for respiratory burst assay (RBA)
Embryos were exposed to HBSS/chemical solution from 6 hpf until the RBA was run at 96 hpf. Embryos were maintained in two 24-well plates at 3 embryos/well with three dilutions of 1:9.767 from the starting concentration determined in the developmental toxicity assay, using four concentrations in total. A total of 24 embryos/chemical concentration were treated and 48 embryos were treated with 0.4% DMSO as a negative control. HBSS/chemical solutions were replaced daily (99% exchange - see above).
Respiratory burst assay (RBA)
The RBA here was a modified version of a previously-published assay (Hermann et al. 2004; Goody et al. 2013). At 96 hpf, embryos were washed to remove compounds and re-plated into black 96-well plates with clear bottoms (#3603; Corning Inc., Corning, NY) in 100 μl of 10% HBSS (one embryo/well). In contrast to the original methods, 10% HBSS was used in place of egg water. For each chemical concentration, 16 embryos were plated in two columns of the plate; for DMSO-treated (vehicle control) embryos, 32 embryos were plated. As a positive control, 1 μl of 1 mM bisindolylmaleimide I (Bis I, a protein kinase C inhibitor; EMD Millipore, Burlington, MA), was added to 16 of the DMSO-treated embryo wells for a final concentration of 10 μM. The plate was then incubated in the dark at 28°C for 30 min before 100 μl of 10% HBSS containing 2’,7’-dichlorofluorescin-diacetate (H2DCFDA; Thermo Fisher Scientific, Waltham, MA) at a final concentration of 500 ng/ml was added to each well. Phorbol 12-myristate 13-acetate (PMA) (Sigma; final concentration of 200 ng/ml) was added to stimulate ROS production; DMSO (final concentration of 0.2% [v/v]), was employed as a vehicle control. H2DCFDA was prepared fresh for each experiment rather than making aliquots as detailed in the originally published methods. The fluorescence from each well was then read on a Fluoroskan Ascent FL plate reader (Thermo Fisher) at 28°C using excitation and emission filters set at 485 and 530 nm, respectively. Fluorescence was measured every 2.5 min over the course of 150 min. Data presented in this study represent the maximum fluorescence detected for each well over the 150-min period.
Flow cytometry
For the experiments counting neutrophils and macrophages via flow cytometry, 60 Tg(mpx:GFP) or Tg(mfap4:tdTomato-caax) embryos were exposed as previously described to each of the five compounds that were positive for ROS suppression. Due to the high number of embryos needed for flow cytometry (31–60 embryos/treatment group), only the highest dose at which ROS suppression occurred was evaluated for each chemical. Larvae that died or had physical malformations were excluded. To measure baseline autofluorescence, unexposed wild-type non-fluorescent embryos were included in all experiments.
In order to dissociate larvae into a single-cell suspension, an adapted version of a previously published protocol was used (Manoli and Driever, 2010). To include an anesthesia step, at 96 hpf, larvae were anesthetized using MS-222 (Syndel, Ferndale, WA) at a final concentration of 100 mg/L and then collected into 15-ml conical tubes. Excess media was removed, and larvae were re-suspended in 1 ml freshly-prepared deyolking buffer (856 ml molecular biology-grade water, 9 μl 2.0 M KCl, 100 μl 5.0 M NaCl, and 25 μl 0.5 M sodium bicarbonate). Larvae were then pipetted up and down to thoroughly mix and de-yolk. The larvae were then centrifuged at 400 × g for 1 min, the resulting supernatant was removed, and the larvae re-suspended in 1 ml of FACS buffer (phosphate-buffered saline containing 5% fetal bovine serum [Corning, Manassas, VA] and 100 mg MS-222/L), instead of FACSmax buffer as in the original protocol. A 40-μm cell strainer was placed atop a 50 ml conical tube and rinsed with 250 μl of FACS buffer. Larvae were then transferred to the strainer and homogenized with the rubber plunger of a 1 ml syringe. The strainer was then rinsed again into the conical tube with FACS buffer. The tube was then centrifuged (400 × g, 1 min) and placed on ice until analysis.
Flow cytometry experiments were performed in the Flow Cytometry and Cell Sorting facility at the North Carolina State University College of Veterinary Medicine. Due to laser configurations, Tg(mpx:GFP) samples were analyzed on a Becton Dickinson LSRII (Franklin Lakes, NJ) while the Tg(mfap4:tdTomato-caax) samples were analyzed on a Beckman Coulter MoFlo XDP (Brea, CA). Cells were gated based on forward-scatter and side-scatter to gate for cellular events, excluding debris, and then gated for singlet events to exclude any large cellular debris or groups of cells that were not in single-cell suspension. A final gate was drawn in the singlet population to quantify GFP+ or tdTomato+ cells (see Supplemental Figure 1 for gating strategy). Data is shown as a percentage of GFP+ or tdTomato+ cells from all singlet events. These data were analyzed using Prism software v.7.0e (GraphPad Software, La Jolla, CA, www.graphpad.com).
Statistical analyses
Statistical analyses of zebrafish RBA and flow cytometry experiments were performed using a one-way analysis of variance (ANOVA) to compare the means of results for all doses/chemicals. If there was a significant effect observed for an experiment with the one-way ANOVA (p < 0.05), a Dunnett’s post-hoc test was performed to compare each dose in that experiment to the 0.4% DMSO control (Dunnett 1955). Zebrafish RBA experiments where Bis I controls did not significantly reduce ROS (when compared to 0.4% DMSO controls) were excluded from analyses.
Results
Developmental toxicity
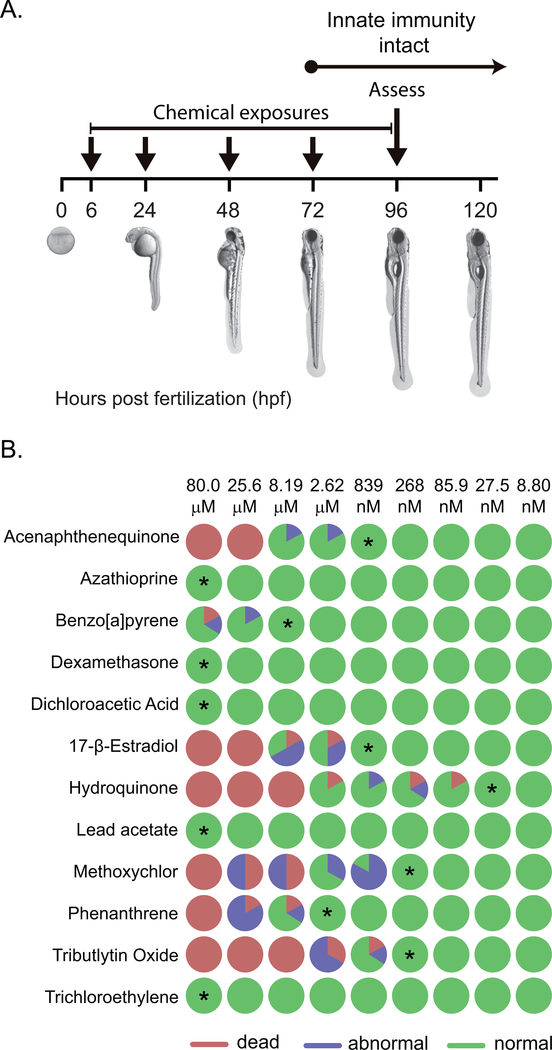
The initial goal of this project was to investigate if exposure to nonteratogenic levels of twelve structurally diverse chemicals (Figure 1) impacted ROS production in zebrafish embryos. To identify exposure levels at which embryos were viable and lacked identifiable morphological malformations, an initial range-finding study to determine a no-observed-effect level (NOEL) for developmental toxicity was performed. Embryos were exposed to various doses (8.80 nM to 80.0 μM) of each chemical, beginning at 6 hpf. Daily media changes were performed until 96 hpf upon which gross morphological assessment was performed via dissecting light microscope (Figure 2A). Among the xenobiotics, the NOEL for developmental toxicity varied (Figure 2B). Of the 12 tested compounds, five (azathioprine, dexamethasone, dichloroacetic acid, lead acetate, and trichloroethylene) had no adverse effects in the developmental toxicity assay. For these compounds in this assay, the NOEL was determined to be 80 μM. The remaining seven agents had varying potencies ranging from 27.5 nM to 8.19 μM (Figure 2B). The most potent xenobiotic identified in this assay was hydroquinone.
Figure 2. Developmental toxicity of 12 structurally-different xenobiotics.
(A) Experimental design. Zebrafish embryos were exposed to several concentrations of xenobiotics from 6 hpf to 96 hpf and monitored for gross malformation and death. (B) Summary graphic of all twelve chemicals tested at all concentrations. Six embryos were exposed to each condition. Pie charts indicate the percentage of embryos that, by 96 hpf, were dead (red), appeared malformed (abnormal, blue), or appeared normal (green). The highest concentrations for each chemical at which no adverse effects were observed (no-observed-effect level, or NOEL) are indicated by asterisks and employed as the highest doses for respiratory burst experiments in Figure 3.
Five of twelve xenobiotics tested suppressed the respiratory burst in zebrafish embryos
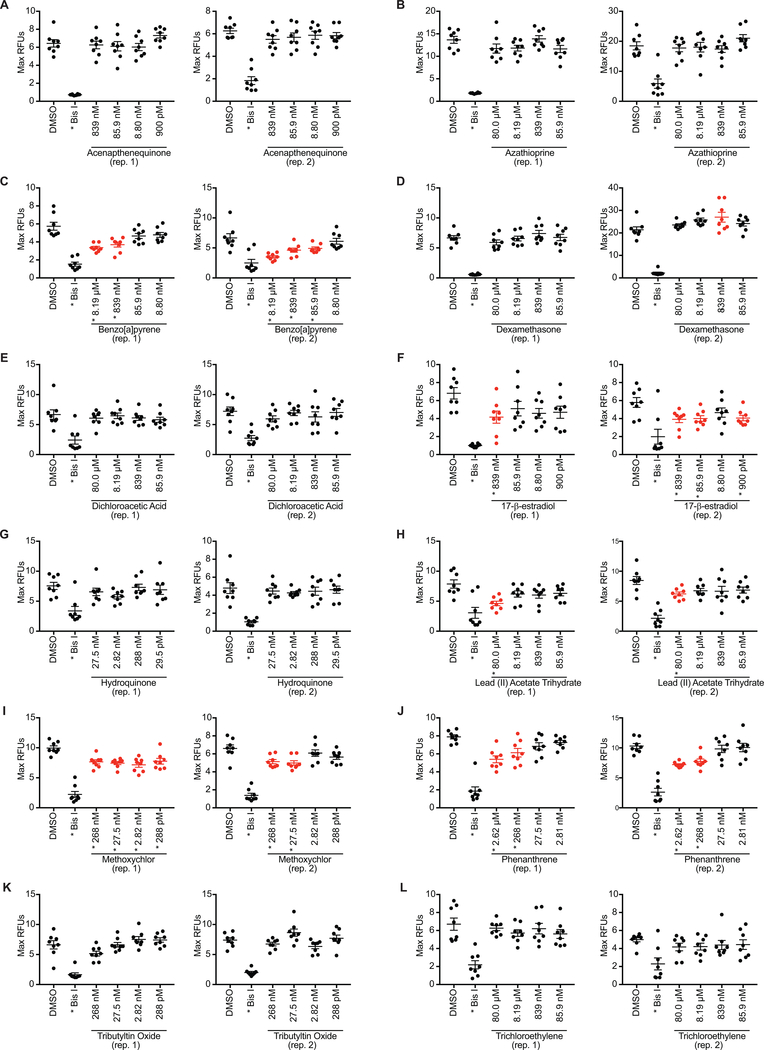
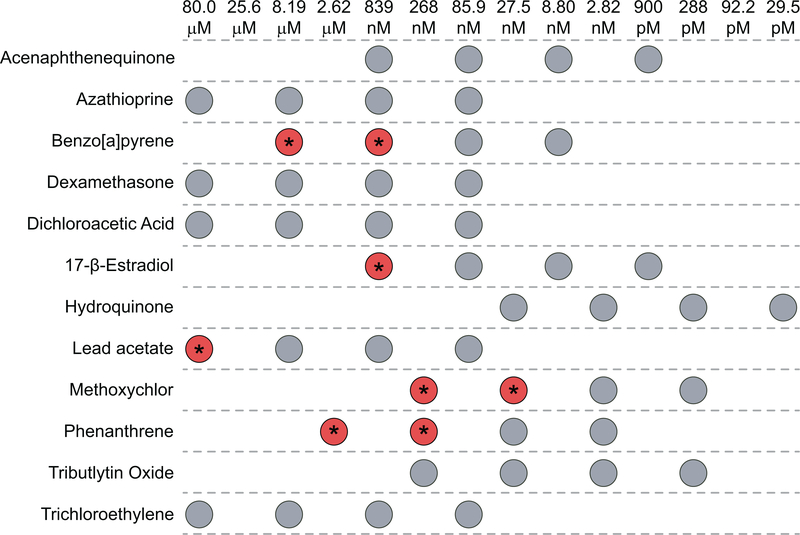
To identify compounds with potential immunosuppressive ability, an RBA using whole zebrafish embryos was employed. Embryos were exposed to multiple dilutions of non-teratogenic doses of the test xenobiotics using the dosing scheme outlined in Figure 2A and subjected to the RBA at 96 hpf. After stimulation with PMA to induce ROS production, 5 of the 12 test agents reproducibly suppressed ROS production in the RBA: 17β-estradiol, benzo[a]pyrene (BaP), lead acetate, methoxychlor, and phenanthrene (Figure 3). For each compound, a lowest-observed-effect level (LOEL) was determined to be the lowest concentration at which an effect was observed in two biological replicates (Figure 4). These five compounds had a wide range of potencies as determined by their LOEL, ranging from 27.5 nM to 80 μM. The other seven agents did not affect ROS production in this assay. No compounds were identified that enhanced ROS production.
Figure 3. ROS production in whole zebrafish larvae.
Zebrafish embryos were exposed to several concentrations of xenobiotics from 6 hpf to 96 hpf. At 96 hpf, larvae were plated into a 96 well plate, and ROS production was measured via H2DCFDA after stimulation with PMA. Maximum fluorescence of each well was used for all analyses; results of two replicate experiments are reported. (A) Acenaphthenequinone, (B) azathioprine, (C) benzo[a[pyrene, (D) dexamethasone, (E) dichloroacetic acid, (F) 17-β-estradiol, (G) hydroquinone, (H) lead (II) acetate trihydrate, (I) methoxychlor, (J) phenanthrene, (K) tributyltin oxide and (L) trichloroethylene. Bis I (10 μM; selective protein kinase C inhibitor) was used as a positive control. Significance (*p < 0.05) was determined by a one-way ANOVA with Dunnett’s post-hoc test for pairwise comparisons to the DMSO control. Red data points denote significance for tested chemicals.
Figure 4. Summary of zebrafish larval RBA results.
Data provided in Figure 3 are summarized to highlight the range of chemical doses employed in, and doses that suppressed the zebrafish RBA. Each circle indicates the dose (above) used for each chemical (left) in the RBA assay. Combinations that led to significant reproducible suppression of the zebrafish RBA are indicated by red circles with asterisks.
One of five immunosuppressive xenobiotics altered phagocyte numbers
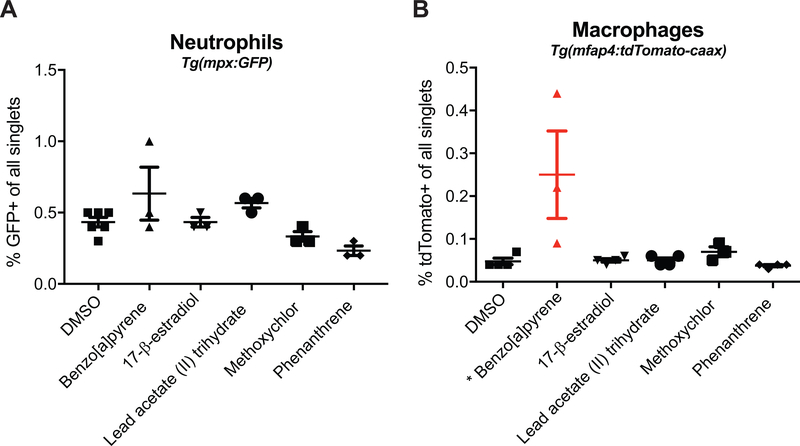
Whether the five compounds identified as immunotoxic at non-teratogenic concentrations could alter embryo levels of neutrophils/macrophages in vivo and thus, in turn, lead to the observed suppression of ROS production, was evaluated. Transgenic zebrafish embryos with fluorescent neutrophils [Tg(mpx:GFP)] (Renshaw et al. 2006) or macrophages [Tg(mfap4:tdTomato-caax)] (Walton et al. 2015) were exposed as above (Figure 2A) to the test compounds at the highest dose where ROS suppression was detected (Figures 3 and 4). At 96 hpf, larvae were homogenized to single cell suspensions and numbers of fluorescently-labeled neutrophils and macrophages were counted via flow cytometry (Supplemental Figure 1). None of the compounds tested significantly altered the percentages of neutrophils present in larvae (Figure 5A). However, exposure to 8.19 μM benzo[a]pyrene induced a slight, but significant, increase in the percentage of macrophages present in vivo (Figure 5B).
Figure 5. Benzo[a]pyrene alters macrophage number, but not neutrophil number, in whole zebrafish larvae.
Tg(mpx:GFP) or Tg(mfap4:tdTomato-caax) transgenic zebrafish embryos were exposed to highest dose of one of the five compounds that suppressed ROS production in the RBA (8.19 μM benzo[a]pyrene, 839 nM 17-β-estradiol, 80 μM lead (II) acetate trihydrate, 268 nM methoxychlor, 2.62 μM phenanthrene), or exposed to DMSO from ~6 hpf to 96 hpf. At 96 hpf, larvae were mechanically homogenized into a single cell suspension and the numbers of GFP+ and tdTomato+ cells were quantified via flow cytometry. Wild-type unexposed larvae were included in all experiments in order to measure baseline autofluorescence. Data are presented as percentage of (A) GFP+ neutrophils or (B) tdTomato+ macrophages observed in individual experiments and include at least three biological replicates per compound. Significance (*p < 0.05) was determined by one-way ANOVA with a Dunnett’s post-hoc test for pairwise compare-sons to the DMSO control. Flow cytometry gating methods are outlined in Supplemental Figure S1.
Discussion
In this study, 12 chemicals with varying levels of reported immunosuppressive properties in mammals were evaluated to investigate the potential utility of the zebrafish embryo as a viable model for screening xenobiotics for immunotoxicity. Based on studies in laboratory rodents, the compounds targeted differing immune processes with the idea of assessing the utility of the zebrafish model as a screen for a spectrum of immunotoxicities, rather than a specific effect. Due to their high fecundity and rapid development, larval zebrafish offer a unique model for high-throughput toxicology studies that would be difficult or expensive in rodent models. Larval zebrafish can also be employed in 96- or 384-well plate formats similar to in vitro systems (Lantz-McPeak et al. 2015; Poureetezadi et al. 2016), and by 72 hpf they have a functional liver capable of biotransformation that would otherwise be overlooked in a cell culture system (Saad et al. 2016; Wang et al. 2017).
Because adaptive immunity is not functional until later in development (Willett et al. 1999; Traver and Yoder 2020), the present study measured ROS production as a readout of innate immunity in larval zebrafish. Methods for measuring ROS in vivo using zebrafish embryos and larvae existed prior to this study, and they can be employed in a high-throughput manner (Hermann et al. 2004; Astin et al. 2017). One common method involves the use of H2DCFDA which offers a sensitive cost-effective way to measure ROS production. Once cellular esterases cleave the diacetate moiety of the molecule, it is trapped within the cell (Rosenkranz et al. 1992). When H2DCF is oxidized to DCF via interactions with ROS, the molecule fluoresces, an event that can be detected via microplate reader. This method, along with other ROS detection methods, has been used frequently in mammalian in vitro systems (Dahlgren and Karlsson 1999). In these systems, PMA is often used to induce ROS; PMA bypasses the cellular membrane and activates protein kinase C, leading to the phosphorylation and stimulation of the previously-noted NADPH oxidase complex (Karlsson et al. 2000). PMA successfully induces ROS production in vivo using whole zebrafish embryos and larvae, as well as ex vivo using kidney cells isolated from adult zebrafish (Hermann et al. 2004).
In the present study, the advantages of the zebrafish model were used in conjunction with the PMA-induced respiratory burst and H2DCFDA as a model for innate immune function in an assay that has been previously established (Hermann et al. 2004). This study demonstrated that five compounds that target innate immune function in mammalian systems suppress the respiratory burst in larval zebrafish. As expected, chemicals that target the adaptive immune function did not suppress the respiratory burst in zebrafish larvae which lacked functional adaptive immunity. Overall, the 12 compounds could be classified into four distinct groups based on their biological activity: (1) compounds with no observable developmental or immunotoxicity, (2) compounds with developmental but no observable immunotoxicity, (3) compounds with no observable developmental but observable immunotoxicity, and (4) compounds with both observable developmental and immunotoxicity.
Compounds with no observable developmental toxicity or immunotoxicity
The present study identified four compounds that induced no developmental toxicity and no suppression of respiratory burst, e.g., azathioprine, dexamethasone, trichloroethylene, and dichloroacetic acid. All of these compounds suppress or are implicated in suppression of the adaptive immune response (e.g., T-cell function). At 96 hpf, zebrafish larvae used in these assays do not possess functional T-cells, and as T-cell function was not a target of these assays, suppression of the respiratory burst was not anticipated.
Azathioprine is a common anti-inflammatory pharmaceutical in autoimmune disorders and organ transplantation that has been shown to inhibit T-cell activation (Patel et al. 2006). While evidence exists to indicate that azathioprine can down-regulate genes associated with the respiratory burst in liver and macrophage cell lines (Moeslinger et al. 2006; Magkoufopoulou et al. 2012), the present study did not observe a functional suppression of the respiratory burst in the zebrafish larvae.
Dexamethasone (DEX) is a glucocorticoid pharmaceutical used as an anti-inflammatory and anti-cancer therapeutic (Löwenberg et al. 2007; Burwick and Sharma 2019). Though DEX has been shown to have immunosuppressive properties in larval zebrafish - as determined by impaired wound healing and increased rates of infections (Sharif et al. 2015; Voelz et al. 2015), the current study did not observe a suppression of the respiratory burst. This outcome is in line with data in human neutrophils wherein DEX inhibited bactericidal activity and neutrophil extracellular trap (NET) formation, but did not inhibit ROS production (Wan et al. 2017). DEX has been shown to have direct action on T-cells, leading to immunosuppression (Löwenberg et al. 2007), which may indicate that T-cell signaling is required for DEX-induced immunosuppression in innate immune populations. A pharmacodynamic study of DEX in adult humans supports this hypothesis, i.e., DEX treatment resulted in reduced ROS production by neutrophils and mononuclear cells (Dandona et al. 1999). Given that larval zebrafish do not possess functional T-cells, testing this hypothesis was beyond the scope of this study. In contrast, it was reported that DEX exposure increased ROS levels in a human osteoblast cell line (Liu [L] et al. 2018), as well as in human M2 macrophages; the latter finding was unusual in that M2 macrophages are generally regarded as an anti-inflammatory (Kraaij et al. 2011).
Epidemiological studies have indicated that the solvent trichloroethylene (TCE) impacts on host adaptive immunity, resulting in autoimmunity and in T-cell suppression that is not mediated through innate immunity (Cichocki et al. 2016). Indeed, the Integrated Risk Information System (IRIS) Toxicological Report on TCE details numerous studies wherein T-cells were susceptible to dysregulation by TCE exposure (U.S. EPA 2011). However, no observations were made based on impacts upon myeloid cell populations.
Dichloroacetic acid, as a metabolite of TCE (Lash et al. 2014), may also impact on adaptive immunity. Previous studies have shown that genes related to the respiratory burst were down-regulated in the livers of mice chronically-exposed to dichloroacetic acid; similar changes were noted in a murine T cell lymphoma model (Kumar et al. 2012; Wehmas et al. 2017). Given that these findings were not identified in innate immune cells, this may indicate that dichloroacetic acid might down-regulate these genes in other tissues. Further studies should evaluate whether the above-identified genes are also down-regulated in innate immune cells and whether this could contribute to functional reductions in ROS production by immune cells.
Compounds with developmental but no observable immunotoxicity
In this study, three compounds were identified as developmentally toxic but did not alter ROS production in the zebrafish RBA, e.g., acenaphthenequinone, hydroquinone, and tributyltin oxide. In the developmental toxicity assay here, NOEL values for these compounds were similar to those previously reported (Knecht et al. 2013; Truong et al. 2014; Quevedo et al. 2019; U.S. EPA 2019). Although changes in ROS production in the zebrafish RBA were not observed here, hydroquinone has been shown to modulate ROS production in cell-based assays (Lee et al. 2007). Results from a prior study examining the impact of hydroquinone on macrophage function revealed that exposing a murine macrophage cell line to 25, 50, or 100 μM hydroquinone resulted in increased ROS production. However, this result was confounded by a simultaneous observation that LPS-induced ROS production was inhibited by 25 and 50 μM hydroquinone. Such data exemplify that the experimental context of exposure greatly influences the perceived activation or inhibition of ROS production (Lee et al. 2007).
Tributyltin oxide has been reported to impact the adaptive immune system, having been linked to suppression of natural killer cells and cytotoxic T-lymphocytes in exposed rats (Smialowicz et al. 1989). However, Kergosien and Rice (1998) found that a single low dose (but not higher doses) of tributyltin oxide resulted in enhanced macrophage secretory function and the respiratory burst in mice 6 days after intraperitoneal injection. Because the burst in a macrophage can take up to 24 hr to detect (Sponseller et al. 2016), the zebrafish RBA may not be sensitive enough to detect a macrophage respiratory burst after only 2.5 hr of PMA treatment (instead primarily detecting neutrophil respiratory bursts). This interpretation is supported by the flow cytometry data in this study wherein it was observed that there were roughly 10-fold more neutrophils at 96 hpf as compared to macrophages (see Figure 5).
A review of the literature could not identify any published studies revealing immunotoxic properties of acenaphthenequinone. The current study did not observe any induced modulations in ROS production in the zebrafish RBA. Nevertheless, further studies are still quite necessary to characterize its hazardous potential.
Compounds not developmentally toxic but immunotoxic
In this study, only one compound was not developmentally toxic but immunotoxic, i.e., lead (II) acetate trihydrate. Although the highest concentration evaluated here (80 μM) showed no signs of causing developmental toxicity, a previous report indicated that higher levels (≥ 200 μM) did cause such toxicity in zebrafish embryos (Roy et al. 2014). In the zebrafish RBA, lead acetate (II) trihydrate only suppressed ROS production at the highest dose tested, indicating that it was not a potent respiratory burst inhibitor. While this capability has previously not been fully explored, there is evidence to support the findings here; other studies have reported that lead acetate exposure resulted in decreased levels of proteins related to the respiratory burst in the brains of exposed mice (Liu [C] et al. 2013). Mishra et al. (2006) showed that, along with effects on the adaptive immune system, lead acetate exposure of a mouse macrophage cell line reduced nitric oxide production. In the context of effects on phagocyte numbers, Xu et al. (2018) reported that lead nitrate increased neutrophil numbers in exposed zebrafish larvae; no such change in neutrophil number was observed here. This difference in outcome may be due to the fact that Xu et al. focused on lead nitrate instead of lead acetate. Studies comparing the impact of lead acetate to lead nitrate, as well as those assessing both reactive oxygen and reactive nitrogen species, may be worth exploring in the future.
Compounds both developmentally toxic and immunotoxic
In this study, four compounds were identified that exhibited both developmental toxicity and immunotoxicity: BaP, 17β-estradiol, methoxychlor, and phenanthrene. Developmental toxicity of these compounds has been investigated previously, and the current findings are similar to those findings - except for that of phenanthrene (Truong et al. 2014; Fang et al. 2015; U.S. EPA 2019). Phenanthrene had variable results in previous studies; here, it was identified it as a potent developmental toxicant. The reasons for these inter-laboratory variances in outcomes might be due to differences in dosing scheme, genetics of the zebrafish strains being used (Balik-Meisner et al. 2018), or even composition of microbiota in the aquaculture facilities (Turner 2018). Such sources of potential variability between laboratories should be acknowledged until standards are agreed upon within the field.
The present study identified two polycyclic aromatic hydrocarbons, i.e., BaP and phenanthrene, that suppressed ROS production in vivo. The immunotoxicity of BaP has been widely studied to date; the data in this study adds to that wealth of knowledge. In the zebrafish RBA, BaP was shown to be a potent inhibitor. Interestingly, it was also observed that BaP caused an unexpectedly slight, but significant, increased number of macrophages in vivo. Previous studies have identified numerous genes related to the respiratory burst that are down-regulated by BaP exposure (Kann et al. 2005; Mathijs et al. 2009; Scott et al. 2011; Lizarraga et al. 2012; Fang et al. 2015); these support the current findings in the zebrafish embryos. Aside from this impact on gene expression, BaP has also been shown to inhibit ROS production in phagocytes (Zaccaria and McClure 2013) and inhibit monocytic differentiation into macrophages (van Grevenynghe et al. 2003).
In contrast to BaP, the effects of phenanthrene on the immune system have gone under-studied in mammalian models. One study noted no significant changes in the antibody responses of immunized mice exposed to phenanthrene (Silkworth et al. 1995). Conversely, phenanthrene has been studied in two freshwater fish species, revealing immunotoxic outcomes. Loughery et al. (2018) reported that phenanthrene down-regulated immune pathways after a sub-chronic developmental exposure in fathead minnow. Haque et al. (2018) noted that phenanthrene reduced immunoglobulin levels, lysozyme activity, and white blood cell count in olive flounders. In the present study, phenanthrene suppressed the respiratory burst of the zebrafish embryos. To our knowledge, this is the first study to report this outcome. The only other study related to this effect was that of Chen et al. (2016) who reported that certain phenanthrene derivatives inhibited reactive nitrogen species production in a murine macrophage cell line.
As the current work in the zebrafish supports the general conclusion that phenanthrene could suppresses the immune system of freshwater fish, future studies should work to better establish the immunomodulatory potential of phenanthrene in mammalian models. Given that both phenanthrene and BaP are polycyclic aromatic hydrocarbons (PAH) identified by the U.S. EPA as “Priority Pollutants” (Andersson and Achten 2015), and given their environmental ubiquity, future work should more fully characterize the immunotoxicity of various PAH to understand their common/disparate mechanisms of action(s) in order to reduce exposure risks and prevent adverse outcomes.
The present study also revealed that 17β-estradiol, an estrogen steroid hormone, was a potent inhibitor of the zebrafish RBA. This outcome is in line with reports that exposure of in adult male rats to 17β-estradiol resulted in a down-regulation of genes related to the respiratory burst (Razmara et al. 2005; Shih et al. 2006). Others have reviewed the roles of estrogens in inflammation, noting that pregnancy levels of 17β-estradiol are linked to decreased ROS production, potentially through decreased expression of NADPH oxidase (Straub 2007). Though the present study did not observe changes in embryo neutrophil numbers, Xu et al. (2018) reported that exposure of zebrafish larvae to a lower dose of 17β-estradiol (18 nM) significantly increased the numbers of neutrophils. This difference in observations may be a result of differences in the doses employed, differences in transgene promoters used to label the neutrophils, and/or genetic backgrounds of the transgenic larvae. It is widely accepted that 17β-estradiol is an immunomodulator of the innate and adaptive immune systems, even as an endogenous hormone (Kovats 2015; Moulton 2018); the present work with the zebrafish RBA validates these findings.
Previous studies have shown that methoxychlor is able to inhibit antibody production in rodent models (Chapin et al. 1997; Hayashi et al. 2013), indicating potential toxicity to the adaptive immune system. However, in the zebrafish RBA, methoxychlor inhibited the respiratory burst, indicating this agent also impacts innate immunity. To our knowledge, this study is the first to show that methoxychlor suppresses the respiratory burst. The current observations are supported by a previous study that showed that exposing a mouse macrophage cell line to methoxychlor reduced interferon (IFN)-β signaling after lipopolysaccharide challenge (Ohnishi et al. 2008).
Given that this study identified two known endocrine-disrupting compounds, i.e., 17β-estradiol and methoxychlor, as potent inhibitors of the respiratory burst, future studies should address mechanisms by which endocrine-disrupting compounds inhibit innate immune function, either dependent or independent of their endocrine disruption. Endocrine disrupting compounds are highly proliferative and prevalent in the environment and have been of increasing concern for decades (Acerini and Hughes 2006). This study further confirms that endocrine disruption plays a role in immune suppression.
In conclusion, the current study employed the larval zebrafish RBA as a model to identify compounds that alter the respiratory burst in vivo. From among 12 diverse compounds, five were identified with a potential to suppress innate immunity without inhibiting hematopoiesis. As these agents were known/predicted to be immunotoxic in mammalian models, this supports the validity of the larval zebrafish RBA as a screening tool that could be used with other high throughput and/or in vitro and in silico methods as part of a defined approach to assess potential immunotoxicity. Of the five, BaP unexpectedly led to increased macrophage numbers in vivo, thereby warranting follow-up mechanistic studies.
Summary
A goal of this study was to determine if results from the zebrafish RBA might be comparable to those from mammalian models. It does not elude us that comparing rodent and cell culture models to zebrafish embryos is not straightforward; the differences among models, species, and experimental designs are readily acknowledged. Nevertheless, it is proposed here that the zebrafish RBA could be a reproducible high-throughput in vivo tool for screening and prioritizing potentially immunotoxic compounds prior to evaluations in more costly rodent studies. The zebrafish embryo model can also be used to evaluate the impact of chemical exposure on other innate immune functions, such as in vivo chemotaxis and phagocytosis by macrophages and neutrophils. Though beyond the scope of this study, it will be of interest to determine if inhibition of the respiratory burst is a true indicator of in situ immune suppression and increased host susceptibility to infectious diseases by these agents. Given that this assay focused on myeloid cell function, it helps fill a gap in the current paradigm under the Toxic Substances Control Act (TSCA) wherein currently only lymphocytes are being investigated for immunotoxic effects from test agents. It is anticipated that this assay can be used for hazard identification and risk evaluation processes and to better inform decision makers in the regulation of toxic chemicals with innate immunotoxic potential.
Supplementary Material
Acknowledgements
The authors thank Stephen Renshaw (University of Sheffield) and David Tobin (Duke University) for the transgenic zebrafish lines. Thanks are also extended to Javid Mohammed (NC State University) for the assistance in the flow cytometry experiments, and to John Rawls (Duke University) for helpful discussions. The authors thank Nisha Sipes (NIEHS) for a thoughtful and comprehensive review of this manuscript.
Funding
This work was supported by the National Institute of Environmental Health Sciences under contracts HHSN273201400195P and HHSN273201500234P, and the NC State University Center for Human Health and the Environment under NIH grant P30ES025128. D.W.P. was supported by a NIH Biotechnology Traineeship (T32 GM008776). D.A.T. was supported by a NIH Comparative Medicine and Translational Research Traineeship (T32 OD011130) and by a Careers in Immunology Fellowship from the American Association of Immunologists.
Footnotes
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content of this manuscript.
References
- Acerini C, and Hughes I. 2006. Endocrine-disrupting chemicals: A new and emerging public health problem? Arch. Dis. Childhood 91:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J, and Achten C. 2015. Time to say goodbye to the 16 EPA PAHs? Toward an up-to-date use of PACs for environmental purposes. Polycyclic Aromatic Compounds 35:330–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astin J, Keerthisinghe P, Du L, Sanderson L, Crosier K, Crosier P, Hall C. 2017. Innate immune cells and bacterial infection in zebrafish. Meth. Cell Biol 138:31–60. [DOI] [PubMed] [Google Scholar]
- Balik-Meisner M, Truong L, Scholl E, La Du J, Tanguay R, Reif D. 2018. Elucidating gene-by-environment interactions associated with differential susceptibility to chemical exposure. Environ. Health Perspect 126:067010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C, Röllinghoff M, Diefenbach A. 2000. Reactive oxygen and reactive nitrogen intermediates in innate and specific Immunity. Curr. Opin. Immunol 12:64–76. [DOI] [PubMed] [Google Scholar]
- Burwick N, and Sharma S. 2019. Glucocorticoids in multiple myeloma: Past, present, and future. Annals of Hematology. 98:19–28. [DOI] [PubMed] [Google Scholar]
- Chapin R, Harris M, Davis B, Ward S, Wilson R, Mauney M, Lockhart A, Smialowicz R, Moser V, Burka L, et al. 1997. The effects of perinatal/juvenile methoxychlor exposure on adult rat nervous, immune, and reproductive system function. Fundam. Appl. Toxicol 40:138–157. [DOI] [PubMed] [Google Scholar]
- Chen L, Shen X, Hu B, Lin Y, Igbe I, Zhang C, Zhang G, Yuan X, Wang F. 2016. Nitric oxide production inhibition and mechanism of phenanthrene analogs in LPS-stimulated RAW264.7 macrophages. Bioorg. Med. Chem. Lett 26:2521–2525. [DOI] [PubMed] [Google Scholar]
- Cichocki J, Guyton K, Guha N, Chiu W, Rusyn I, Lash L. 2016. Target organ metabolism, toxicity, and mechanisms of trichloroethylene and perchloroethylene: Key similarities, differences, and data gaps. J. Pharmacol. Exp. Ther 359:110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren C, and Karlsson A. 1999. Respiratory burst in human neutrophils. J. Immunol. Meth 232:3–14. [DOI] [PubMed] [Google Scholar]
- Dandona P, Mohanty P, Hamouda W, Aljada A, Kumbkarni Y, Garg R. 1999. Effect of dexamethasone on reactive oxygen species generation by leukocytes and plasma IL-10 concentrations: A pharmacodynamic study. Clin. Pharmacol. Ther 66:58–65. [DOI] [PubMed] [Google Scholar]
- Dunnett C 1955. A multiple comparison procedure for comparing several treatments with a control. J. Am. Stat. Assoc 50:1096–1121. [Google Scholar]
- Espenschied S, Tighe R, Gowdy K. 2018. Flow cytometry for the immunotoxicologist. Meth. Mol. Biol 1803:183–197. [DOI] [PubMed] [Google Scholar]
- Fang X, Corrales J, Thornton C, Clerk T, Scheffler B, Willett K. 2015. Transcriptomic changes in zebrafish embryos and larvae following B[a]P exposure. Toxicol. Sci 146:395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannagan R, Cosío G, Grinstein S. 2009. Anti-microbial mechanisms of phagocytes and bacterial evasion strategies. Nat. Rev. Microbiol 7:355–366. [DOI] [PubMed] [Google Scholar]
- Gehen S, Blacker A, Boverhof D, Hanley T, Hastings C, Ladics G, Lu H, O’Neal F. 2014. Retrospective evaluation of the impact of functional immunotoxicity testing on pesticide hazard identification and risk assessment. Crit. Rev. Toxicol 44:407–419. [DOI] [PubMed] [Google Scholar]
- Germolec D, Luebke R, Rooney A, Shipkowski K, Vandebriel R, van Loveren H. 2017. Immunotoxicology: A brief history, current status and strategies for future immunotoxicity assessment. Curr. Opin. Toxicol. 5:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goody M, Peterman E, Sullivan C, Kim C. 2013. Quantification of the respiratory burst response as an indicator of innate immune health in zebrafish. J. Visualized Exp 79:e50667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M, Eom H, Rhee J. 2018. Waterborne phenanthrene modulates immune, biochemical, and anti-oxidant parameters in the bloods of juvenile olive flounder. Toxicol. Environ. Health Sci 10:194–202. [Google Scholar]
- Hayashi K, Fukuyama T, Ohnuma A, Tajima Y, Kashimoto Y, Yoshida T, Kosaka T. 2013. Immunotoxicity of the organochlorine pesticide methoxychlor in female ICR, BALB/c, and C3H/He mice. J. Immunotoxicol 10:119–124. [DOI] [PubMed] [Google Scholar]
- Herbomel P, Thisse B, Thisse C. 1999. Ontogeny and behavior of early macrophages in the zebrafish embryo. Development 126:3735–3745. [DOI] [PubMed] [Google Scholar]
- Hermann A, Millard P, Blake S, Kim C. 2004. Development of a respiratory burst assay using zebrafish kidneys and embryos. J. Immunol. Meth 292:119–129. [DOI] [PubMed] [Google Scholar]
- Howe K, Clark M, Torroja C, Torrance J, Berthelot C, Muffato M, Collins J, Humphray S, McLaren K, Matthews L, et al. 2013. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Feng C, Hung H, Chakraborty C, Chen C, Chen W, Jean Y, Wang H, Sung C, Sun Y, et al. 2014. A novel zebrafish model to provide mechanistic insights into the inflammatory events in carrageenan-induced abdominal edema. PloS One 9:e104414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann S, Huang M, Estes C, Reichard J, Sartor M, Xia Y, Puga A. 2005. Arsenite-induced aryl hydrocarbon receptor nuclear translocation results in additive induction of Phase I genes and synergistic induction of Phase II genes. Mol. Pharmacol 68:336–346. [DOI] [PubMed] [Google Scholar]
- Karlsson A, Nixon J, McPhail L. 2000. Phorbol Myristate acetate induces neutrophil NADPH-oxidase activity by two separate signal transduction pathways: Dependent or independent of phosphatidylinositol 3-kinase. J. Leukocyte Biol 67:396–404. [DOI] [PubMed] [Google Scholar]
- Kergosien D, and Rice C. 1998. Macrophage secretory function is enhanced by low doses of tributyltin oxide (TBTO), but not tributyltin chloride (TBTCl). Arch. Environ. Contam. Toxicol 34:223–228. [DOI] [PubMed] [Google Scholar]
- Kimmel C, Ballard W, Kimmel S, Ullmann B, Schilling T. 1995. Stages of embryonic development of the zebrafish. Devel. Dynamics 203:253–310. [DOI] [PubMed] [Google Scholar]
- Knecht A, Goodale B, Truong L, Simonich M, Swanson A, Matzke M, Anderson K, Waters K, Tanguay R. 2013. Comparative developmental toxicity of environmentally-relevant oxygenated PAHs. Toxicol. Appl. Pharmacol 271:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats S 2015. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol 294:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaij M, van der Kooij S, Reinders M, Koekkoek K, Rabelink T, van Kooten C, Gelderman K. 2011. Dexamethasone increases ROS production and T-cell suppressive capacity by anti-inflammatory macrophages. Mol. Immunol 49:549–557. [DOI] [PubMed] [Google Scholar]
- Kumar A, Kant S, Singh S. 2012. Novel molecular mechanisms of anti-tumor action of dichloroacetate against T-cell lymphoma: Implication of altered glucose metabolism, pH homeostasis and cell survival regulation. Chem.-Biol. Interact 199:29–37. [DOI] [PubMed] [Google Scholar]
- Lam S, Chua H, Gong Z, Lam T, Sin Y. 2004. Development and maturation of immune system in zebrafish, Danio rerio: A Gene expression profiling, in situ hybridization and immunological study. Develop. Comp. Immunol 28:9–28. [DOI] [PubMed] [Google Scholar]
- Lantz-McPeak S, Guo X, Cuevas E, Dumas M, Newport G, Ali S, Paule M, Kanungo J. 2015. Developmental toxicity assay using high content screening of zebrafish embryos. J. Appl. Toxicol 35:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash L, Chiu W, Guyton K, Rusyn I. 2014. Trichloroethylene biotransformation and its role in mutagenicity, carcinogenicity and target organ toxicity. Mutat. Res 762:22–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C 2016. New frontiers for zebrafish management. Meth. Cell Biol 135:483–508. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim J, Lee Y, Shin W, Chun T, Rhee M, Cho J. 2007. Hydroquinone, a reactive metabolite of benzene, reduces macrophage-mediated immune responses. Molecules Cells 23:198–206. [PubMed] [Google Scholar]
- Lieschke G, Oates A, Crowhurst M, Ward A, Layton J. 2001. Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood 98:3087–3096. [DOI] [PubMed] [Google Scholar]
- Liu C, Zheng G, Cheng C, Sun J. 2013. Quercetin protects mouse brain against lead-induced neurotoxicity. J. Agric. Food Chem 61:7630–7635. [DOI] [PubMed] [Google Scholar]
- Liu W, Zhao Z, Na Y, Meng C, Wang J, Bai R. 2018. Dexamethasone-induced production of reactive oxygen species promotes apoptosis via endoplasmic reticulum stress and autophagy in MC3T3-E1 Cells. Intl. J. Mol. Med 41:2028–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizarraga D, Gaj S, Brauers KJ, Timmermans L, Kleinjans JC, van Delft JHM. 2012. B[a]P-induced changes in microRNA-mRNA networks. Chem. Res. Toxicol 25:838–849. [DOI] [PubMed] [Google Scholar]
- Loughery J, Kidd K, Mercer A, Martyniuk C. 2018. Part B: Morphometric and transcriptomic responses to sub-chronic exposure to the polycyclic aromatic hydrocarbon phenanthrene in the fathead minnow (Pimephales promelas). Aquat. Toxicol 199:77–89. [DOI] [PubMed] [Google Scholar]
- Löwenberg M, Verhaar A, van den Brink G, Hommes D. 2007. Glucocorticoid signaling: A non-genomic mechanism for T-cell immunosuppression. Trends Mol. Med 13:158–163. [DOI] [PubMed] [Google Scholar]
- Magkoufopoulou C, Claessen S, Tsamou M, Jennen D, Kleinjans J, van Delft J. 2012. A trans-criptomics-based in vitro assay for predicting chemical genotoxicity in vivo. Carcinogenesis. 33:1421–1429. [DOI] [PubMed] [Google Scholar]
- Manoli M, and Driever W. 2012. Fluorescence-activated cell sorting (FACS) of fluorescently-tagged cells from zebrafish larvae for RNA isolation. Cold Spring Harbor Protoc. 2012:pdb.prot069633. [DOI] [PubMed] [Google Scholar]
- Mathijs K, Brauers K, Jennen D, Boorsma A, van Herwijnen M, Gottschalk R, Kleinjans J, van Delft J. 2009. Discrimination for genotoxic and non-genotoxic carcinogens by gene expression profiling in primary mouse hepatocytes improves with exposure time. Toxicol. Sci 112:374–384. [DOI] [PubMed] [Google Scholar]
- Mishra K, Chauhan U, Naik S. 2006. Effect of lead exposure on serum immunoglobulins and reactive nitrogen and oxygen intermediate. Human Exp. Toxicol 25 :661–665. [DOI] [PubMed] [Google Scholar]
- Moeslinger T, Friedl R, Spieckermann P. 2006. Inhibition of inducible nitric oxide synthesis by azathioprine in a macrophage cell line. Life Sci. 79:374–381. [DOI] [PubMed] [Google Scholar]
- Moulton V 2018. Sex hormones in acquired immunity and autoimmune disease. Front. Immunol 9:2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T, Yoshida T, Igarashi A, Muroi M, Tanamoto K. 2008. Effects of possible endocrine disruptors on MyD88-independent TLR4 signaling. FEMS Immunol. Med. Microbiol 52:293–295. [DOI] [PubMed] [Google Scholar]
- Patel A, Swerlick R, McCall C. 2006. Azathioprine in dermatology: The past, the present, and the future. J. Am. Acad. Dermatol 55:369–389. [DOI] [PubMed] [Google Scholar]
- Planchart A, Mattingly C, Allen D, Ceger P, Casey W, Hinton D, Kanungo J, Kullman S, Tal T, Bondesson M, et al. 2016. Advancing toxicology research using high-throughput toxicology with small fish models. ALTEX 33:435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poureetezadi S, Cheng C, Chambers J, Drummond B, Wingert R. 2016. Prostaglandin signaling regulates nephron segment patterning of renal progenitors during zebrafish kidney development. eLife 5:e17551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo C, Behl M, Ryan K, Paules R, Alday A, Muriana A, Alzualde A. 2019. Detection and prioritization of developmentally neurotoxic and/or neurotoxic compounds using zebrafish. Toxicol. Sci 168:225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razmara A, Krause D, Duckles S. 2005. Testosterone Augments endotoxin-mediated cerebro-vascular inflammation in male rats. Am. J. Physiol 289:H1843–1850. [DOI] [PubMed] [Google Scholar]
- Renshaw S, Loynes C, Trushell D, Elworthy S, Ingham P, Whyte M. 2006. A transgenic zebrafish model of neutrophilic inflammation. Blood 108:3976–3978. [DOI] [PubMed] [Google Scholar]
- Rosenkranz A, Schmaldienst S, Stuhlmeier K, Chen W, Knapp W, Zlabinger G. 1992. A microplate assay for the detection of oxidative products using 2’,7’-dichlorofluorescin-diacetate. J. Immunol. Meth 156:39–45. [DOI] [PubMed] [Google Scholar]
- Roy N, DeWolf S, Schutt A, Wright A, Steele L. 2014. Neural alterations from lead exposure in zebrafish. Neurotoxicol. Teratol 46:40–48. [DOI] [PubMed] [Google Scholar]
- Saad M, Cavanaugh K, Verbueken E, Pype C, Casteleyn C, van Ginneken C, van Cruchten S. 2016. Xenobiotic metabolism in the zebrafish: A review of the spatiotemporal distribution, modulation and activity of cytochrome P450 families 1 to 3. Toxicol. Sci 41:1–11. [DOI] [PubMed] [Google Scholar]
- Scott D, Devonshire A, Adeleye Y, Schutte M, Rodrigues M, Wilkes T, Sacco M, Gribaldo L, Fabbri M, Coecke S, et al. 2011. Inter- and intra-laboratory study to determine the reproduce-bility of toxicogenomics datasets. Toxicology 290:50–58. [DOI] [PubMed] [Google Scholar]
- Sharif F, Steenbergen P, Metz J, Champagne D. 2015. Long-lasting effects of dexamethasone on immune cells and wound healing in the zebrafish. Wound Repair Regen. 23:855–865. [DOI] [PubMed] [Google Scholar]
- Shih H, Lin C, Lee T, Lee W, Hsu C. 2006. 17β-Estradiol inhibits subarachnoid hemorrhage-induced inducible nitric oxide synthase gene expression by interfering with the NF-κB trans-activation. Stroke 37:3025–3031. [DOI] [PubMed] [Google Scholar]
- Silkworth J, Lipinskas T, Stoner C. 1995. Immunosuppressive potential of several polycyclic aromatic hydrocarbons (PAHs) found at a Superfund site: New model used to evaluate additive interactions between benzo[a]pyrene and TCDD. Toxicology 105:375–386. [DOI] [PubMed] [Google Scholar]
- Smialowicz R, Riddle M, Rogers R, Luebke R, Copeland C. 1989. Immunotoxicity of tributyltin oxide in rats exposed as adults or pre-weanlings. Toxicology 57:97–111. [DOI] [PubMed] [Google Scholar]
- Sponseller B, Clark S, Gilbertie J, Wong D, Hepworth K, Wiechert S, Chandramani P, Sponseller B, Alcott C, Bellaire B, et al. 2016. Macrophage effector responses of horses are influ-enced by expression of CD154. Vet. Immunol. Immunopathol 180:40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachura D, and Traver D. 2016. Cellular dissection of zebrafish hematopoiesis. Meth. Cell Biol 133:11–53. [DOI] [PubMed] [Google Scholar]
- Straub R 2007. The complex role of estrogens in inflammation. Endocrine Rev. 28:521–574. [DOI] [PubMed] [Google Scholar]
- Traver D, and Yoder J. 2020. Chapter 19 - Immunology In: The Zebrafish in Biomedical Research (Cartner S, Eisen J, Farmer S, Guillemin K, Kent M, Sanders G, Eds.). New York: Academic Press, pp. 191–216. [Google Scholar]
- Truong L, Reif D, St Mary L, Geier M, Truong H, Tanguay R. 2014. Multi-dimensional in vivo hazard assessment using zebrafish. Toxicol. Sci 137:212–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P 2018. The Role of the Gut Microbiota on Animal Model Reproducibility. Animal Models Exp. Med 1:109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (United States Environmental Protection Agency). 1998. Health Effects Test Guidelines OPPTS 870.7800 Immunotoxicity. Available at https://www.epa.gov/test-guide-lines-pesticides-and-toxic-substances/series-870-health-effects-test-guidelines. Accessed 17 June 2019.
- U.S. EPA (United States Environmental Protection Agency). 2011. Toxicological Review of Trichloroethylene. Available at https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?-substance_nmbr=199. Accessed 17 June 2019.
- U.S. EPA (United States Environmental Protection Agency). 2019. ToxCast & Tox21 Summary Files from invitroDBv3.2. Exploring ToxCast Data: Downloadable Data. 2019. https://www.epa.gov/chemical-research/toxicity-forecaster-toxcasttm-data. Accessed 17 June 2019.
- van Grevenynghe J, Rion S, Le Ferrec E, Le Vee M, Amiot L, Fauchet R, Fardel O. 2003. Polycyclic aromatic hydrocarbons inhibit differentiation of human monocytes into macrophages. J. Immunol 170:2374–2381. [DOI] [PubMed] [Google Scholar]
- Voelz K, Gratacap R, Wheeler R. 2015. A zebrafish larval model reveals early tissue-specific innate immune responses to Mucor circinelloides. Dis. Models Mech 8:1375–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtech L, Sanders G, Conway C, Ostland V, Hansen J. 2009. Host immune response and acute disease in a zebrafish model of Francisella pathogenesis. Infect. Immun. 77:914–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton E, Cronan M, Beerman R, Tobin D. 2015. The macrophage-specific promoter mfap4 allows live, long-term analysis of macrophage behavior during mycobacterial infection in zebrafish. PloS One 10:e0138949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Miller S, Ober E, Sadler K. 2017. Making it new again: Insight into liver development, regeneration, and disease from zebrafish research. Curr. Topics Develop. Biol 124:161–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan T, Zhao Y, Fan F, Hu R, Jin X. 2017. Dexamethasone inhibits S. aureus-induced neutrophil extracellular pathogen-killing mechanism, possibly through Toll-like receptor regulation. Front. Immunol 8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C, Leung Y, Suter D. 2016. Expression dynamics of NADPH oxidases during early zebrafish development. J. Comp. Neurol 524:2130–2141. [DOI] [PubMed] [Google Scholar]
- Wehmas L, DeAngelo A, Hester S, Chorley B, Carswell G, Olson G, George M, Carter J, Eldridge S, Fisher A, et al. 2017. Metabolic disruption early in life is associated with latent carcinogenic activity of dichloroacetic acid in mice. Toxicol. Sci 159:354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett C, Cortes A, Zuasti A, Zapata A. 1999. Early hematopoiesis and developing lymphoid organs in the zebrafish. Develop. Dynamic 214:323–336. [DOI] [PubMed] [Google Scholar]
- Xu H, Zhang X, Li H, Li C, Huo X, Hou L, Gong Z. 2018. Immune response induced by major environmental pollutants through altering neutrophils in zebrafish larvae. Aquat. Toxicol 201:99–108. [DOI] [PubMed] [Google Scholar]
- Yang C, Cambier C, Davis J, Hall C, Crosier P, Ramakrishnan L. 2012. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocy-tosed from infected macrophages. Cell Host Microbe 12:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccaria K, and McClure P. 2013. Using immunotoxicity information to improve cancer risk assessment for polycyclic aromatic hydrocarbon mixtures. Intl. J. Toxicol 32:236–250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.