Abstract
Purpose
The lysozyme 2 (Lyz2 or LysM) cre mouse is extensively used to achieve genetic manipulation in myeloid cells and it has been widely employed in retinal research. However, LysM has been recently described to be expressed in brain neurons and there is a debate on whether it is also expressed by resident microglia in addition to infiltrating macrophages.
Methods
We examined LysM-cre recombination in retinal tissue using a LysM-cre/tdTomato reporter mouse together with immunolabeling for several retinal cell markers. We further compared LysM-cre tdTomato recombination with that of Cdh5-cre driver, which is expressed in both endothelial and hematopoietic cells.
Results
LysM-cre was strongly expressed in most microglia/resident macrophages in neonatal retinas (P8) and to a lesser extent in microglia of adult retinas. In addition, there was some neuronal recombination (8 %) of LysM-cre specifically in adult retinal ganglion cells and amacrine cells. After retinal ischemia-reperfusion injury, LysM-cre was strongly expressed in microglia/infiltrating macrophages. Cdh5-cre was expressed in endothelial and myeloid cells of P8 pups retinas. Unexpectedly, Cdh5 showed additional expression in adult mouse retinal ganglion cells and brain neurons.
Conclusions
LysM-cre is expressed in macrophages and a subset of microglia together with a small but significant recombination of LysM-cre in the retinal neurons of adult mice. Cdh5 also showed some neuronal expression in both retina and brain of adult mice. These findings should be taken into consideration when interpreting results from central nervous system research using LysM-cre and Cdh5-cre mice.
Keywords: Cdh5-cre, LysM-cre, macrophages, microglia, neurons, retina, tdTomato
The retina is part of the central nervous system (CNS) and is considered to be a window to the brain. Many researchers use the accessibility of the retina and the ease of in vivo imaging in live animals to study neurovascular changes in different CNS disorders.1 Like the brain, the retina is protected by a blood–retina barrier that prevents immune cell infiltration under normal healthy conditions. Therefore, the retina is considered an immune-privileged organ.2 While peripheral organs rely on the infiltration of circulating white blood cells to fight infections, the retina, like the brain, has its own surveillance immune cells called microglia. Under steady-state conditions, the eye also contains resident macrophages mainly in the iris/ciliary body, cornea, choroid, and along the retinal blood vessels (perivascular macrophages).3 These resident macrophages as well as the microglia are believed to originate from yolk sac precursors during embryonic development. This is in contrast to the macrophages that originate from circulating monocytes and infiltrate the retina under disease conditions, and thus are bone marrow-derived.4,5 In recent years, research has focused on finding markers that can differentiate between microglia and macrophages with the aim to better understand their differential roles in CNS diseases. Among these markers, TMEM119 and P2Y12 have been described to specifically label microglia, thus differentiating them from macrophages.6,7 Microglia play a major role in the CNS response to infection or injury, yet studies have identified an important role of peripherally derived macrophages and infiltrating myeloid cells under CNS disease conditions.8–11
In this report, we characterize the retinal and brain expression of two cre promoters that are widely used in CNS research, lysozyme 2 (or LysM) and cadherin 5 (VE-cadherin). LysM is widely used to modulate gene expression in myeloid cells. It encodes lysozyme in mice, an antimicrobial enzyme that hydrolyzes the bacterial cell wall and hence is involved in the innate immune response.12,13 Therefore, LysM-cre mice are commonly used to dissect the role of myeloid cells in CNS diseases. Several recent retina studies have used the LysM-cre driver mouse for gene deletion in myeloid cells.14–25 However, the use of LysM-cre mice to target genes in macrophages versus microglia has been a topic of major debate. Moreover, it has been reported recently that the LysM promoter is also expressed in brain neurons.26–28 On the other hand, the Cdh5 gene encodes the calcium-dependent cell adhesion molecule, cadherin 5 (VE-cadherin), which is expressed in both endothelial cells and hematopoietic cells.29 VE-cadherin is a major component of the endothelial cell adherens junction and plays an important role in vascular permeability and angiogenesis.30–32 Therefore, Cdh5-cre is widely used for endothelial gene deletion as well as for lineage tracing studies.33,34
In this imaging study, we examined the expression of the LysM and Cdh5 promoters in neonatal and adult mouse retinas and brains under normal conditions and after retinal ischemia-reperfusion injury using tdTomato (Lys/td) reporter mice. The tdTomato reporter mouse line allows for identification of cells expressing the cre recombinase of choice through the expression of the highly fluorescent protein, tdTomato, that can be visualized by fluorescence imaging.35,36
Materials and Methods
Generation of Reporter Mice of LysM-cre and Cdh5-cre
Animal work was performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and was approved by the institutional animal care and use committee (Animal Welfare Assurance no. A3307-01). Reporter tdTomato mouse (Jax stock# 007914) under a CAG promoter (CMV enhancer, chicken beta-Actin promoter, and rabbit beta-Globin splice acceptor site) was used in this study. This mouse has a loxP-flanked STOP cassette to prevent transcription of the red fluorescent protein, tdTomato. When bred to mice expressing cre recombinase under a certain promoter, the offspring will have the STOP cassette deleted in the cells expressing cre resulting in strong red fluorescence. Heterozygous LysM-cre (Jax stock# 004781) or Cdh5-cre (Jax stock# 006137) female mice were crossed to tdTomato male reporter mouse (Jax stock# 007914) to generate LysM/tdTomato (Lys/td) or Cdh5/tdTomato (Cdh5/td) that express the tdTomato under LysM-cre and Cdh5-cre, respectively. Sex of the breeding cre and reporter mice was chosen based on availability in our colony. Being highly fluorescent, the tdTomato mouse is a highly sensitive tool to detect cre recombination.
Tissue Collection and Imaging
Neonatal (P8) or adult mice (12–14 weeks old) were anesthetized with ketamine/xylazine mixture and transcardially perfused with phosphate-buffered saline. Eyeballs, brain, liver, and spleen were collected and fixed in 4% paraformaldehyde overnight. Retina flat mounts or cross-sections were prepared as previously described.25 Immunostaining was performed using primary antibodies listed in the Table as previously described.25 Alexa Fluor 488 and 647 secondary conjugated antibodies were used to allow for triple labeling. Images were taken using fluorescent (Keyence) or confocal (Zeiss LSM 780) microscopy. As needed, brightness and contrast adjustments were conducted using Zen blac64k software to reduce background fluorescence. Videos were created from retina flat mount Z-stack images (inner to outer side of the retina) using ImageJ software. Quantification was conducted on flat mount images using ImageJ software as previously described.25
Table.
Antibody Information
| Catalog | ||||
|---|---|---|---|---|
| Antibody | No. | Company | Dilution | Experiment |
| NeuN | MAB377 | Millipore, MA | 1:200 | Immunostaining |
| Brn3a | SC-31984 | Santa Cruz, TX | 1:200 | Immunostaining |
| anti-TUJ1 | 801202 | BioLegend, CA | 1:200 | Immunostaining |
| GFAP | Z0334 | Dako, CA | 1:200 | Immunostaining |
| Iba1 | 019-19741 | Wako, VA | 1:200 | Immunostaining |
| P2Y12 | AS-55043A | Anaspec Inc, CA | 1:200 | Immunostaining |
| SMI32 | 801701 | BioLegend, CA | 1:200 | Immunostaining |
| ChAT | AB144P | Millipore, MA | 1:200 | Immunostaining |
| CRALBP | Sc-28193 | Santa Cruz, TX | 1:200 | Immunostaining |
| PKCα | Sc-208 | Santa Cruz, TX | 1:200 | Immunostaining |
| Calbindin | C9848 | Sigma-Aldrich, MO | 1:200 | Immunostaining |
Mouse Retinal Ischemia-Reperfusion Injury Model
Adult mice (10 to 12 weeks old) were subjected to retinal ischemia-reperfusion (IR) injury. The right eye was subjected to IR injury as previously described, whereas the left eye served as sham control.25 IR injury was induced by inserting a needle connected to an elevated saline reservoir into the anterior chamber to raise the intraocular pressure (IOP) to 110 mm Hg for 60 minutes. The mice were sacrificed at 2, 5, 7, and 10 days after IR injury.
Results
LysM Shows Strong Expression in Retinal Microglia of Mouse Pups
We crossed LysM-cre mice to floxed tdTomato mice to generate a LysM-cre/tdTomato (Lys/td) mouse line in which cells expressing the LysM-cre promoter can be identified by fluorescent or confocal microscopic imaging of the highly fluorescent tdTomato protein (Fig. 1A). As expected, and previously reported, tdTomato was expressed in splenic macrophages and liver Kupffer cells, which confirmed the LysM recombination in our Lys/td mice (Supplementary Fig. S1A).
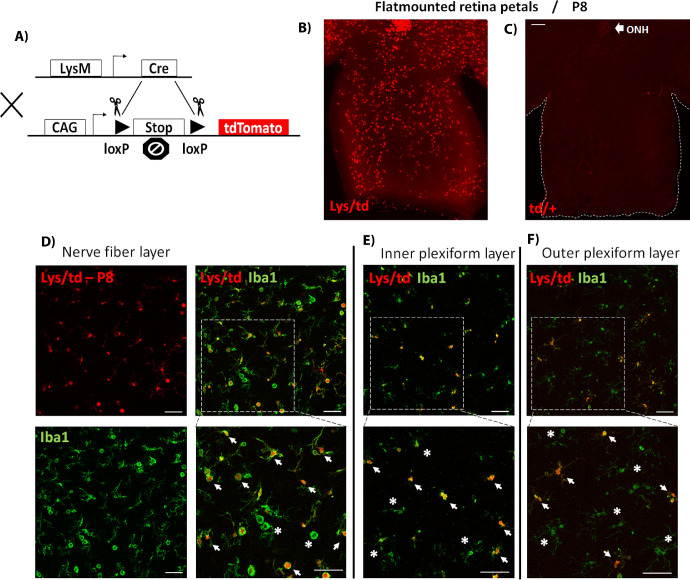
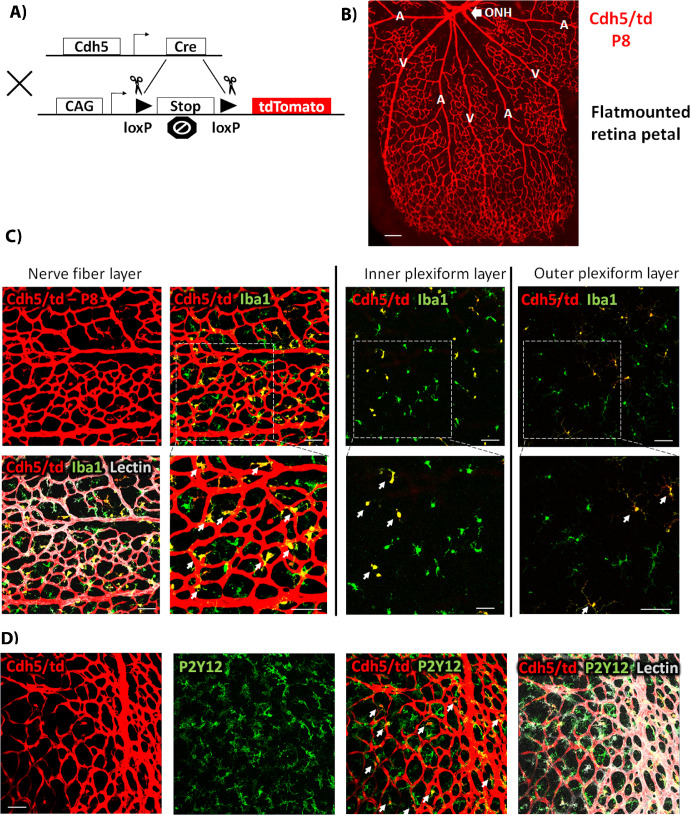
Figure 1.
(A) Diagram showing cre-lox-mediated expression of the tdTomato highly fluorescent protein. The recombination allows for excision of the STOP codon, thus leading to tdTomato expression under the CAG (CMV enhancer, chicken beta-Actin promoter, and rabbit beta-Globin splice acceptor site) promoter in cells expressing LysM-cre. (B, C) Flat-mounted retina petals from P8 Lys/td and td/+ mice, respectively, showing tdTomato expression only in the Lys/td retinas. (D–F) Colocalization of Lys/td expression and Iba1 in the NFL, IPL, and OPL, respectively. ONH, optic nerve head. Arrows denote colocalization; asterisks show areas of no colocalization. N = 4 per group. Scale bar: 100 µm.
Examination of P8 retinas from Lys/td mouse pups showed strong expression of tdTomato protein throughout the retina flat mount (Fig. 1B, and supplementary video 1). Although the Lys/td retinas displayed a LysM-cre mediated expression of tdTomato, floxed tdTomato mice did not show any red fluorescence (Fig. 1C). Colocalization studies showed strong expression of tdTomato in myeloid cells (microglia/macrophages) with near complete colocalization with the general myeloid cell marker, Iba1 on the retina surface (nerve fiber layer) (Fig. 1D). All the tdTomato-positive cells were also positive for Iba1, but a few Iba1-positive cells were negative for tdTomato. Looking at two other layers of the retina (inner plexiform layer [IPL], and outer plexiform layer [OPL]) using Z-stack images, all the tdTomato-positive cells were also positive for Iba1; however, more Iba1-positive cells were negative for tdTomato compared with the nerve fiber layer (NFL) (Figs. 1E, 1F). We then performed colabeling with P2y12, a specific marker for microglia. Immunolabelling with P2y12 showed a very similar expression pattern to the Iba1 labelling. Again, tdTomato showed more colocalization with P2y12 in the NFL (Supplementary Fig. S1B), with less colocalization in the IPL and OPL (data not shown).
LysM is Expressed in a Subset of Retinal Microglia as well as Neurons in the Adult Mouse Retina
We next examined the pattern of Lys/td recombination in the adult mouse retina (12-14 weeks old). There was no expression of tdTomato in the floxed tdTomato (td/+) adult mouse retina (Fig. 2A). Unexpectedly, retina cross-section and flat mount imaging of Lys/td retinas showed expression of tdTomato in large round cell bodies in both the ganglion cell and inner nuclear layers (Figs. 2B, 2C, and supplementary video 2). We next performed colocalization experiments to identify the type of cells expressing tdTomato. Costaining for the general neuronal marker, neuronal nuclei (NeuN), showed colocalization with tdTomato indicating that the large round cells expressing tdTomato in the ganglion cell layer (GCL) and inner nuclear layer (INL) are neurons (Figs. 2D, 2F). Retina flat mount image quantification showed expression of tdTomato in 8 ± 1% (mean ± standard error [SE], n = 5) of NeuN-positive cells (Fig. 2E). As expected, Lys/td showed colocalization with Iba1-positive retinal microglia/macrophages and choroidal macrophages (Figs. 2D, 2G). Quantification showed that 40 ± 5% (mean ± SE, n = 5) of Iba1-positive cells showed tdTomato expression in control uninjured retina flat mounts (Fig. 2E). Lys/td also colocalized with the microglia specific marker P2Y12 (Supplementary Fig. S1C). In addition, LysM-cre was expressed in perivascular as well as iris/ciliary body cells that were positive for Iba1 but largely negative for P2Y12 (Supplementary Figs. S1D-H: S1F is a retina cross-section while the rest are retina flatmount images).
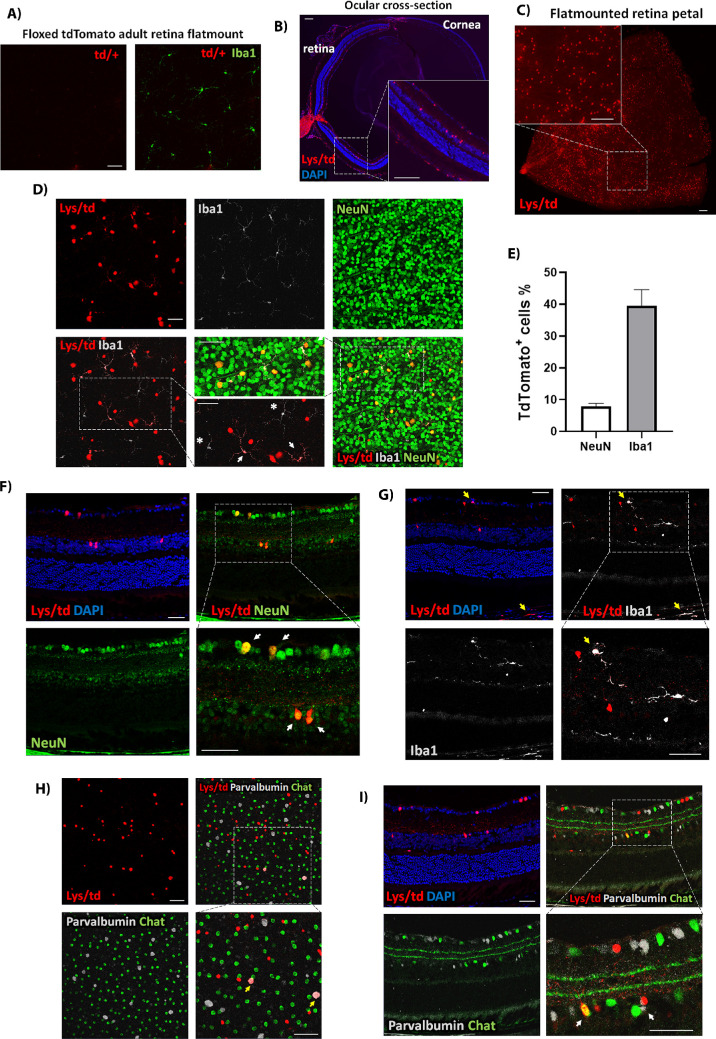
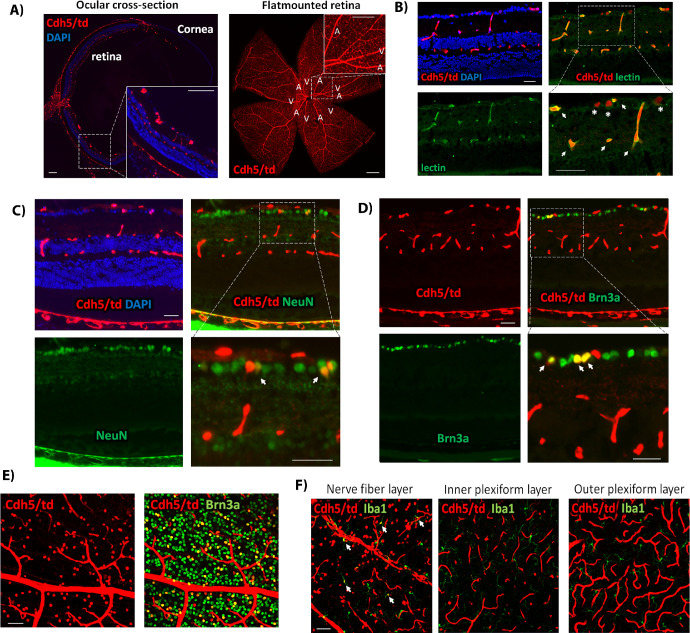
Figure 2.
(A) Representative td/+ adult retina flat mount image labeled with Iba1 showing no expression of tdTomato. (B) Ocular cross-section from Lys/td adult mouse. (C) Cut wing from a Lys/td adult retina flat mount. (D) Lys/td retina flat mount taken at the GCL layer showing colabeling with NeuN and Iba1. (E) Quantification of the percent of colabeled tdTomato-positive cells from the total number of NeuN and Iba1-positive cells. Graph is presented as mean ± SE, N = 5 per group. (F) Retina cross-section showing colocalization of Lys/td and NeuN. (G) Retina cross-section showing colabeling of Lys/td and Iba1. (H) Retina flat mount image taken at the INL showing colabeling of Lys/td with parvalbumin and ChAT. (I) Retina cross-section showing colabeling of Lys/td with parvalbumin and ChAT. Arrows denote colocalization; asterisks showing areas of no colocalization. N = 5. Scale bar: 100 µm.
We further studied the expression of tdTomato in different retinal neuronal cell types using flat mount and cross-section immunolabeling. TdTomato expression in the ganglion cell layer showed colocalization with the ganglion cell marker Brn3a (Supplementary Fig. S2A [flat mount], S2B [cross-section]). Interestingly, there was no colocalization with the amacrine cell marker, ChAT, in the GCL yet there was some colocalization with another amacrine cell marker, parvalbumin, which also marks retinal ganglion cells, RGCs (Supplementary Fig. S2C). Furthermore, tdTomato was also found in RGC axons as labeled with SMI32 and Tuj1 axonal markers (Supplementary Fig. S2D).
In the INL, tdTomato showed colocalization with ChAT and parvalbumin, suggesting that the cells expressing tdTomato in the INL are amacrine cells (Figs. 2H, 2I). Immunolabelling for Müller, bipolar, and horizontal cells markers (CRALBP, PKCα, and calbindin) did not show colocalization with tdTomato-positive cells (Supplementary Fig. S2B, E, F). There was also no colocalization with the astrocyte marker, GFAP (Supplementary Fig. S2F).
LysM is Strongly Expressed in Activated Microglia/Macrophages After Retinal Ischemia-Reperfusion Injury
To assess the impact of injury on Lys/td expression, we subjected Lys/td mice to retinal IR injury. Analysis of retina flat mounts showed numerous Iba1-positive cells that were also positive for tdTomato. The double-positive cells displayed a round ameboid morphology at 2 days after IR that coincides with infiltration of peripheral monocytes/macrophages (Fig. 3A). At 5 and 7 days after injury, these cells displayed an elongated morphology that extended radially from the center of the retina toward the periphery (Figs. 3B, 3C). At 10 days, the double-positive cells displayed enlarged soma and extended processes (Fig. 3D).
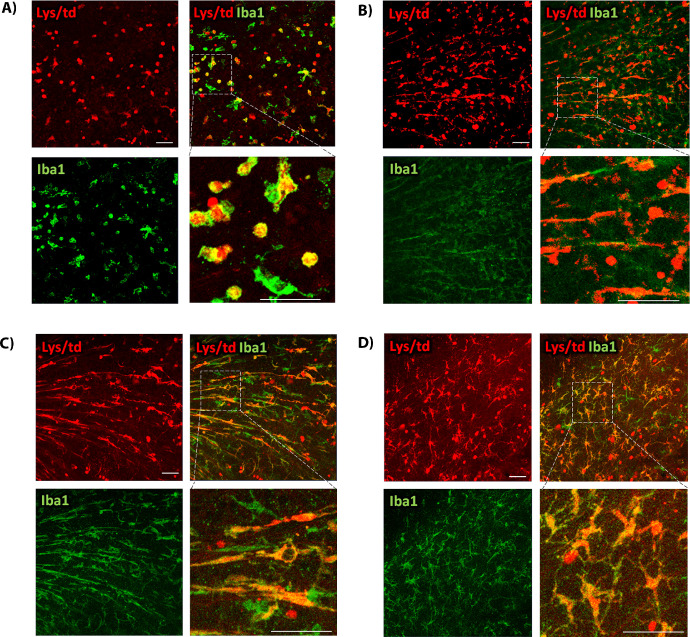
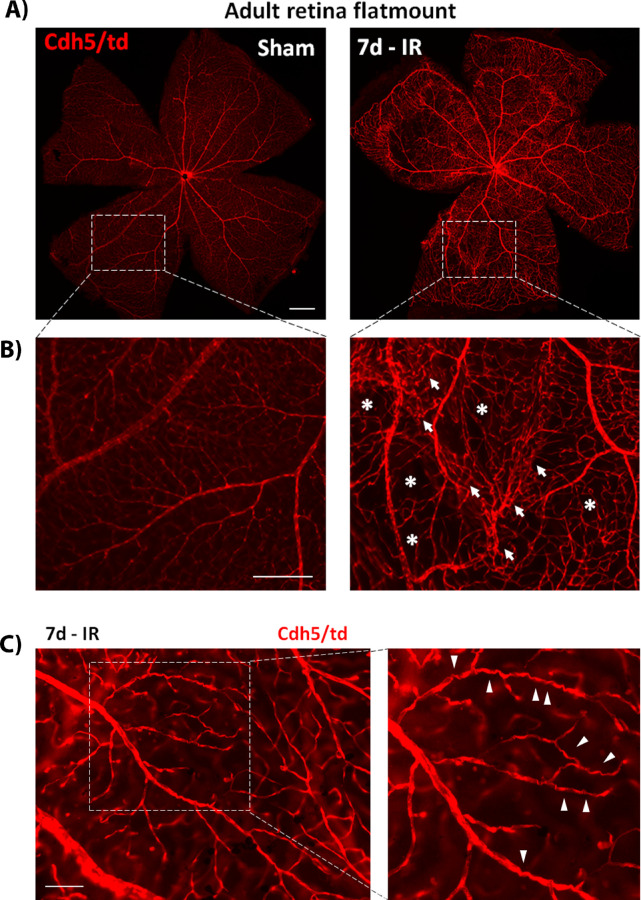
Figure 3.
(A) Flat mount colabeling of Lys/td with Iba1 at 48 hours after IR showing increases in double-positive cells that have a round amoeboid morphology. (B, C) Lys/td colabeling with Iba1 at 5 and 7 days, respectively, after IR showing increases in double-positive cells that assume an elongated morphology extending along the retina surface from the center to the periphery of the retina. (D) Lys/td co-labeling with Iba1 at 10 days after IR showing double-positive cells that exhibit elongated processes. N = 2 per group. Scale bar: 100 µm.
LysM is Expressed in Brain Neurons of Adult Mice but not Pups
Because we did not see neuronal expression of Lys/td in retinas from P8 pups, we examined the brains of these pups to determine the distribution of Lys/td. As we observed in the retinas, there was no neuronal expression of Lys/td in brain neurons at P8. However, Lys/td expression strongly colocalized with Iba1-positive cells (Figs. 4A, 4B, 4C). As previously reported,26–28 we found strong Lys/td signal in brain neurons of adult mice (Figs. 4D, 4E, 4F). The signal was very strong in cerebellar neurons. In addition, Lys/td was expressed in cortical neurons and to a lesser extent in hippocampal neurons as detected by NeuN colabeling (not shown). In addition, there was some colocalization with Iba1-positive microglia/macrophages (Figs. 4G, 4H).
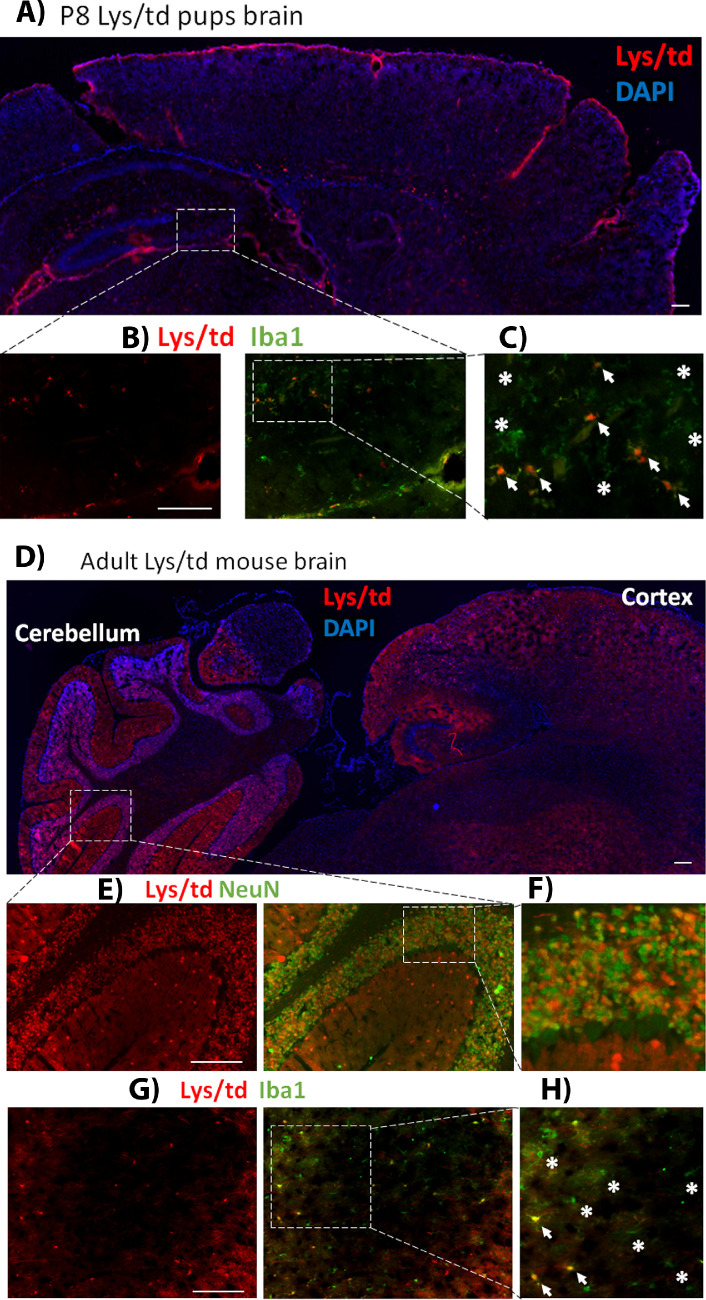
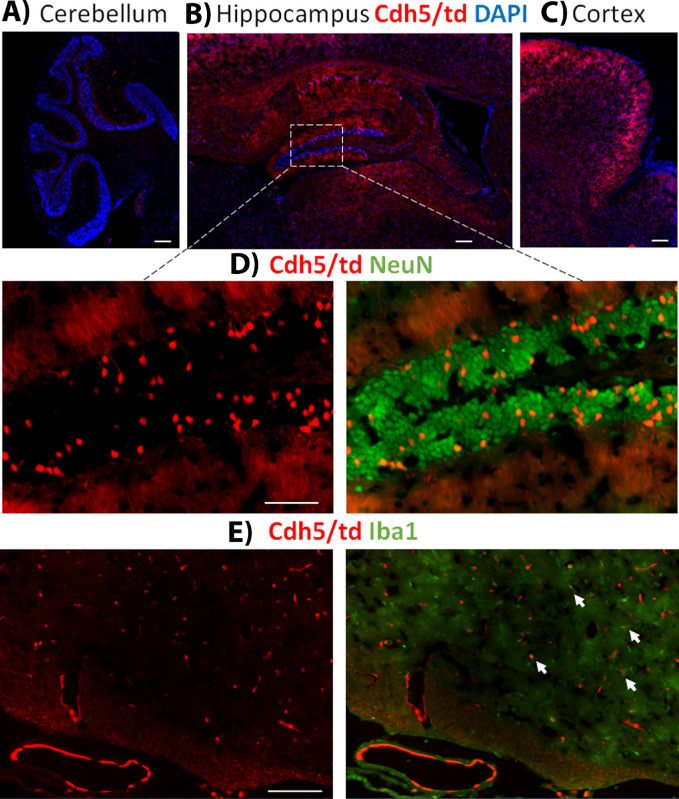
Figure 4.
(A) Image of P8 Lys/td mouse pup brain showing cerebral cortex and hippocampus. (B) Magnified image of the P8 Lys/td brain showing colocalization with Iba1. (C) Further magnified inset from the hippocampus showing points of colocalization (arrows); asterisks showing cells that are only Iba1-positive. (D) Lys/td adult mouse brain cerebral cortex and cerebellum showing expression of tdTomato with a stronger expression in the cerebellum. (E) Magnified image showing colabeling with NeuN in cerebellar cortex. (F) Further magnified inset showing colocalization of tdTomato and NeuN. (G) Adult Lys/td brain striatum image with Iba1 colabeling. (H) Magnified inset showing colocalization of tdTomato with Iba1 (arrows); asterisks showing area of no colocalization. N = 2. Scale bar: 100 µm.
Cdh5 is Expressed in Endothelial Cells and Some Myeloid Cells in Neonatal Mouse Retinas
We further crossed floxed tdTomato mice to VE-cadherin (cadherin 5 or Cdh5) cre mice to generate Cdh5/td mice (Fig. 5A). Cdh5 is an endothelial specific promoter widely used to knockout protein expression in endothelial cells using the cre-lox system. However, it has also been shown to be expressed in some hematopoietic cells.29 As expected, Cdh5/td showed strong expression in the superficial vascular plexus of P8 retinas. The intermediate and deep vascular plexuses are not yet developed at this age (Figs. 5B, 5C, supplementary video 3).37 There was also some expression of Cdh5 in Iba1-positive (Fig. 5C) as well as P2y12-positive cells (Fig. 5D).
Figure 5.
(A) Diagram of Cdh5/td mouse expression of tdTomato in cells expressing Cdh5-cre. (B) Expression of tdTomato in retina petal of P8 Cdh5/td mouse. A, artery; V, vein; ONH, optic nerve head. Arteries are identified by having fewer branches compared with veins that have multiple small branches. (C) Expression of Cdh5/td in the NFL, IPL, and OPL and colabeling with lectin and Iba1. (D) Colabeling of Cdh5/td with P2Y12, a microglia specific marker. N = 4. Scale bar: 100 µm.
Cdh5 is Expressed in a Subset of Ganglion Cells in Addition to Endothelial Cells in the Adult Mouse Retina
We next examined the Cdh5/td recombination in the adult mouse retina. There was a strong expression of Cdh5/td in the choriocapillaris and the three layers of retinal blood vessels as confirmed with lectin colocalization (Figs. 6A, 6B). To our surprise, Cdh5/td also showed some neuronal expression as demonstrated by double labeling with NeuN and Brn3a. However, the pattern was different from the Lys/td (supplementary video 4). Furthermore, Cdh5/td-positive neurons were only found in the GCL and were not seen in the INL (Figs. 6C-6E) as confirmed by flat mount and cross-section immunolabelling studies. Furthermore, there was also some expression of Cdh5 in Iba1-positive cells in the NFL of the adult retina (Fig. 6F).
Figure 6.
(A) Cdh5/td adult mouse ocular cross-section and retina flat mount. (B) Retina cross-section showing colocalization of Cdh5/td and lectin (arrows). Asterisks showing tdTomato-positive cells that are negative for lectin. (C, D) Retina cross-section showing colabeling of Cdh5/td and the neuronal markers, NeuN and Brn3a. Arrows point to colocalization in ganglion cells. (E) Images of Cdh5/td retina flat mount GCL colabeling with Brn3a. (F) Images of Cdh5/td at the NFL, IPL, and OPL colabeled with Iba1. Arrows indicate colocalization. N = 4. Scale bar: 100 µm.
Cdh5 Expression is Increased in Retinal Vessels After IR Injury
To examine the effect of retinal ischemia on Cdh5 expression, we subjected Cdh5/td mice to IR injury and collected the retinas at 7 days after injury. IR-injured retinas showed increased expression of Cdh5 in vasculature compared with the sham retina (Fig. 7A). Furthermore, the vasculature in the IR retinas exhibited distorted morphology with areas of vessel clusters and tortuosity, and regions of vascular regression (Figs. 7B, 7C, supplementary video 5).
Figure 7.
(A) Representative Cdh5/td adult flat mounts of sham- and IR-injured retinas collected 7 days after injury. IR-injured retinas showing stronger expression of Cdh5/td as compared with shams. (B) Magnified images showing distorted vessel network after injury with vessel clusters (arrows) and regions of reduced vessel density (asterisks). (C) Higher magnification image of IR-injured retina showing some tortuous vessels. N = 3. Scale bar: 100 µm.
Cdh5 is Expressed in Brain Neurons of Adult Mice
Because we detected Cdh5 expression in adult retinal neurons, we wanted to examine if there is neuronal expression in the adult brain as well. Indeed, Cdh5 showed strong expression in brain neurons in addition to the endothelial cells (Figs. 8A, 8B, 8C, 8D). The expression pattern was different from that of LysM in the adult brain, with a stronger expression of Cdh5 in the hippocampus and cortical neurons and no expression in the cerebellum neurons. There was also some expression in Iba1-positive cells (Fig. 8E).
Figure 8.
(A) Sagittal section image of the cerebellum of Cdh5/td adult brain showing weak expression of tdTomato. (B) Sagittal section image of Cdh5/td adult brain hippocampus area showing strong expression of tdTomato. (C) Sagittal section image of Cdh5/td adult brain prefrontal cortex showing strong expression of tdTomato. (D) Magnified images of the Cdh5/td adult brain hippocampus with NeuN colabeling. (E) Image of the Cdh5/td adult brain striatum with Iba1 colabeling. Arrows point to colocalization with Iba1-positive cells. N = 2. Scale bar: 100 µm.
Discussion
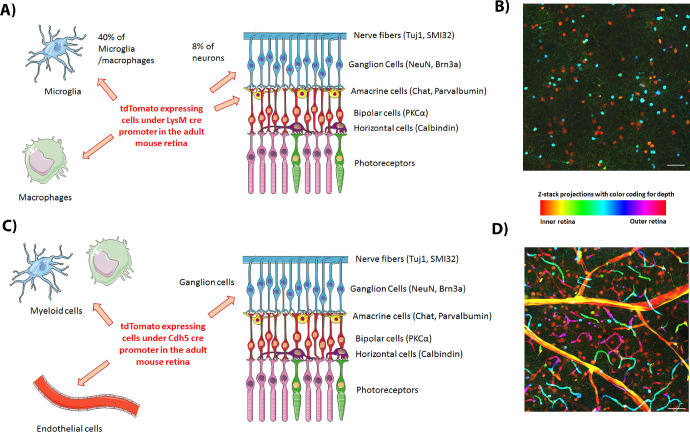
In this report, we characterized LysM-cre recombination in the mouse retina using the tdTomato reporter mouse. We show LysM expression in retinal macrophages and subsets of retinal microglia and neurons. The neuronal expression was only present in adult retina neurons and was absent in pup retinas. The neonate retinas also showed many Lys/td-positive microglia associated with the superficial vascular plexus. The neuronal expression was evident in the ganglion and the inner retinal neurons and represented 8% of the NeuN-positive cells (Fig. 9). We further examined the td expression under a different promoter, Cdh5-cre, that is specific for endothelial and hematopoietic cells. Cdh5/td showed expression in the retina vessels with some expression in Iba1-positive microglia/macrophages. Interestingly, Cdh5/td neuronal expression was also seen in adult but not pup retinas and it was restricted to retinal ganglion cells (Fig. 9).
Figure 9.
(A) Diagram summarizing the expression of LysM-cre in adult uninjured mouse retina. (B) Color-coded depth Z-stack projection of Lys/td adult mouse retina flat mount image showing neuronal expression of tdTomato in RGCs (orange) and INL neurons (turquoise) in addition to microglia/macrophages (green and pink). (C) Diagram summarizing the expression of Cdh5-cre in adult uninjured mouse retina. (D) Color-coded depth Z-stack projection of Cdh5/td adult mouse retina flat mount image showing expression of tdTomato in RGCs (orange) in addition to blood vessels (endothelium, visible across the color spectrum). Diagrams were created using Servier medical art https://smart.servier.com/.
We observed a stronger expression of Lys/td in retinal microglia/macrophages at base line at P8 and in the adult retinas subjected to IR injury compared with uninjured adult retinas which only showed 40% expression in Iba1-positive cells. By P8, the growth of the superficial vascular plexus is complete, while the intermediate and deep vascular plexuses are not yet developed, thus the retina is still undergoing remodeling and maturation.37 At this age, the retina cells undergo developmental apoptosis which has been suggested to impart a disease-related gene signature to microglia cells.38 In line with our data, the increased LysM expression in microglia from P7 pups compared with adult retinas has been recently observed using high-throughput RNA sequencing.38
LysM-cre has been reported before to be expressed in 30% of retinal microglia.18 This is also the case with microglia in other parts of the CNS such cortex, cerebellum, hippocampus, and spinal cord which showed 30% to 40% recombination efficacy of LysM-cre.39 In adult retinas, we found that LysM/tdTomato was expressed in 40% of Iba1-positive cells under normal conditions. It should be taken into consideration that Iba1 is not an exclusive marker for microglia, and some of the Iba1-positive cells are likely to be macrophages. Under normal conditions, macrophages are present in the eye mainly in the iris/ciliary body, cornea, choroid, and along the retinal vessels (perivascular macrophages).3 Indeed, we found strong expression of tdTomato in Iba1-positive cells in the ciliary body and perivascular macrophages and these cells were largely negative for the microglia marker P2y12.
The retinal IR injury model has been shown to stimulate the infiltration of peripheral blood-borne myeloid cells into the retina.40 Therefore, we used this model to examine Lys/td expression in infiltrating myeloid cells. The activated Lys/td and Iba1 double-positive cells attained different morphology at different time points after IR. The cells were ameboid early on at 2 days after injury and displayed enlarged soma and extended processes at day 10. At days 5 and 7 postinjury, these infiltrating monocytes/macrophages appeared to associate with GCL nerve fibers, suggesting that they were engulfing dying neurons.41,42 Interestingly, Cdh5/td IR retinas did not show strong expression of td in microglia/macrophages but rather the vascular expression was augmented. The IR injury seemed to increase the Cdh5 promoter activity leading to a stronger tdTomato signal in the vasculature. The vessels showed a distorted and tortuous morphology similar to the clinical phenotype seen with retinal vascular occlusion. In line with this, increased expression of Cdh5 in the vasculature after injury has been previously reported in the heart and the brain as well.43,44 The increased Cdh5 expression after ischemic injury could be related to the barrier disruption. The significance of this increase in Cdh5 expression is yet to be elucidated.
The LysM promoter has been reported to be expressed in mouse brain and spinal cord neurons.26–28 More specifically, it has been shown to be expressed in motor cortex neurons and their axons, as well as in neurons of the hippocampus, and the molecular and Purkinje cell layers of the cerebellum where it colocalized with parvalbumin, a marker of GABA-ergic interneurons.26–28 In the retina, Lys/td colocalized with parvalbumin, which marks some ganglion as well as some amacrine cells. In addition to its antimicrobial and immune modulatory effects,13 lysozyme has been shown to play a role in Alzheimer's disease by clearing β-amyloid aggregates.45 Lysozyme has also been recently shown to be involved in neuropathic pain.46 These diverse actions could explain the purpose of LysM expression by adult neurons.
Although VE-cadherin is generally thought to be expressed in endothelial and hematopoietic cells, another calcium-dependent cell adhesion molecule, N-cadherin, is expressed in neurons and mediates neuronal cell-cell interaction.47 In line with our data, VE-cadherin has also been shown to be expressed in brain neurons.48 The expression of LysM and Cdh5 in ganglion cells highlight the diversity of these neurons and their ability to modify their gene expression in the adult retina.
Retinas of LysM-cre and Cdh5-cre reporter mice have been studied before with no specific reporting of neuronal expression.14,17–21,24 However, most of the previous studies were conducted on pups. One interesting article that used both LysM-cre and Cdh5-cre mice crossed to floxed tdTomato mice showed a very similar expression pattern to our study.19 Cdh5-td was present in round, neuron-like cells in the GCL in addition to vessels, whereas LysM-cre showed neuron-like positive cells in the GCL and INL. The authors did colocalization studies for F4/80 and concluded that the LysM-cre-positive cells are of myeloid/hematopoietic origin. However, they did not perform colocalization experiments with neuronal markers and the study was not aimed to specifically characterize these mice. Similarly, another article using LysMeYFP mice also showed enhanced yellow fluorescent protein (eYFP) expression in round cells at the GCL and INL at steady state but the authors did not perform colabeling studies.49 In our hands, Lys/td-positive microglia/macrophages showed a ramified morphology. This suggested to us that the round cells that lacked processes were neurons, which we confirmed with colocalization studies using different neuronal markers. Another study using LysMeYFP reporter mouse showed expression of eYFP in neuron-like cells in the GCL and INL but again colabeling with neuronal markers was not performed.24
In conclusion, this article provides important information to the scientific community regarding the use of LysM-cre and Cdh5-cre mice in retina research. Based on our findings, LysM is highly expressed in microglia of neonatal retinas. In adult retinas, LysM is expressed in a subset of microglia and in infiltrating macrophages responding to ischemic injury. Cdh5 is expressed in the vascular endothelium and some microglia/macrophages. There is some neuronal recombination of LysM and Cdh5 in adult retinas that should be considered when interpreting results from retina studies using LysM-cre or Cdh5-cre knockout mouse line. Genes of infiltrating immune cells after retinal injury can be efficiently targeted using the LysM-cre mouse line provided that small neuronal recombination is taken into consideration. CX3CR1, another promoter that is widely used to target protein expression in microglia/nonparenchymal macrophages, has also been shown to be expressed in brain neurons.50 Therefore, there is a need for new cre-recombinase promoters that can specifically target microglia as well as macrophage populations in the retina to better dissect the role of these cells under healthy and diseased conditions.
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health (NIH R01-EY11766 [RBC and RWC], NIH R01-EY028569 [SPN]), the Department of Veterans Affairs, Veterans Health Administration (RBC), Office of Research and Development, Biomedical Laboratory Research and Development (BX001233 [RBC]), National Institutes of Health K99 award (1K99EY029373-01A1 [AYF]), and the Culver Vision Discovery Institute at Augusta University. RBC is the recipient of a Research Career Scientist Award from the Department of Veterans Affairs. The contents do not represent the views of the Department of Veterans Affairs or the United States Government. The authors alone are responsible for the content and writing of the paper.
Authors’ Contribution: AYF conceived the study, performed the experiments, and drafted the manuscript; ZX performed the breeding protocol and colony maintenance; PSN provided the tdTomato mouse and critically revised the manuscript; RWC provided the Cdh5-cre mouse and critically revised the manuscript; and RBC directed the study and critically revised the manuscript.
Disclosure: A.Y. Fouda, None; Z. Xu, None; S.P. Narayanan, None; R.W. Caldwell, None; R.B. Caldwell, None
Supplementary Materials
Supplementary Video S1. LysM/td P8 retina flat mount (Z-stack images from the inner to the outer side of the retina).
Supplementary Video S2. LysM/td adult retina flat mount (Z-stack images from the inner to the outer side of the retina).
Supplementary Video S3. Cdh5/td P8 retina flat mount (Z-stack images from the inner to the outer side of the retina).
Supplementary Video S4. Cdh5/td adult retina flat mount (Z-stack images from the inner to the outer side of the retina).
Supplementary Video S5. Cdh5/td adult retina - 7 days IR (Z-stack images from the inner to the outer side of the retina).
References
- 1. London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013; 9: 44–53. [DOI] [PubMed] [Google Scholar]
- 2. Zhou R, Caspi RR. Ocular immune privilege. F1000 Biology Reports. 2010; 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chinnery HR, McMenamin PG, Dando SJ. Macrophage physiology in the eye. Pflugers Archiv. 2017; 469: 501–515. [DOI] [PubMed] [Google Scholar]
- 4. Goldmann T, Wieghofer P, Jordao MJ, et al.. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016; 17: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kierdorf K, Masuda T, Jordao MJC, Prinz M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat Rev Neurosci. 2019; 20: 547–562. [DOI] [PubMed] [Google Scholar]
- 6. Bennett ML, Bennett FC, Liddelow SA, et al.. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci USA. 2016; 113: E1738–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haynes SE, Hollopeter G, Yang G, et al.. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006; 9: 1512–1519. [DOI] [PubMed] [Google Scholar]
- 8. Paschalis EI, Lei F, Zhou C, et al.. The role of microglia and peripheral monocytes in retinal damage after corneal chemical injury. Am J Pathol. 2018; 188: 1580–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greenhalgh AD, Zarruk JG, Healy LM, et al.. Peripherally derived macrophages modulate microglial function to reduce inflammation after CNS injury. PLoS Biol. 2018; 16: e2005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karlen SJ, Miller EB, Wang X, Levine ES, Zawadzki RJ, Burns ME. Monocyte infiltration rather than microglia proliferation dominates the early immune response to rapid photoreceptor degeneration. J Neuroinflamm. 2018; 15: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okunuki Y, Mukai R, Pearsall EA, et al.. Microglia inhibit photoreceptor cell death and regulate immune cell infiltration in response to retinal detachment. Proc Natl Acad Sci. 2018; 115: E6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cross M, Renkawitz R. Repetitive sequence involvement in the duplication and divergence of mouse lysozyme genes. EMBO J. 1990; 9: 1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ragland SA, Criss AK. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017; 13: e1006512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bremer D, Pache F, Gunther R, et al.. Longitudinal intravital imaging of the retina reveals long-term dynamics of immune infiltration and its effects on the glial network in experimental autoimmune uveoretinitis, without evident signs of neuronal dysfunction in the ganglion cell layer. Front Immunol. 2016; 7: 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen M, Lechner J, Zhao J, et al.. STAT3 activation in circulating monocytes contributes to neovascular age-related macular degeneration. Curr Mole Med. 2016; 16: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen M, Zhao J, Ali IHA, et al.. Cytokine signaling protein 3 deficiency in myeloid cells promotes retinal degeneration and angiogenesis through arginase-1 up-regulation in experimental autoimmune uveoretinitis. Am J Pathol. 2018; 188: 1007–1020. [DOI] [PubMed] [Google Scholar]
- 17. Chen X, Kezic JM, Forrester JV, et al.. In vivo multi-modal imaging of experimental autoimmune uveoretinitis in transgenic reporter mice reveals the dynamic nature of inflammatory changes during disease progression. J Neuroinflamm. 2015; 12: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dejda A, Mawambo G, Daudelin JF, et al.. Neuropilin-1-expressing microglia are associated with nascent retinal vasculature yet dispensable for developmental angiogenesis. Invest Ophthalmol Vis Sci. 2016; 57: 1530–1536. [DOI] [PubMed] [Google Scholar]
- 19. Huang W, Li Q, Amiry-Moghaddam M, et al.. Critical endothelial regulation by LRP5 during retinal vascular development. PloS One. 2016; 11: e0152833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haupt F, Krishnasamy K, Napp LC, et al.. Retinal myeloid cells regulate tip cell selection and vascular branching morphogenesis via Notch ligand Delta-like 1. Scient Rep. 2019; 9: 9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liyanage SE, Fantin A, Villacampa P, et al.. Myeloid-derived vascular endothelial growth factor and hypoxia-inducible factor are dispensable for ocular neovascularization–brief report. Arterioscler Thromb Vasc Biol. 2016; 36: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vahatupa M, Cordova ZM, Barker H, et al.. Furin deficiency in myeloid cells leads to attenuated revascularization in a mouse-model of oxygen-induced retinopathy. Exp Eye Res. 2018; 166: 160–167. [DOI] [PubMed] [Google Scholar]
- 23. Ban N, Lee TJ, Sene A, et al.. Impaired monocyte cholesterol clearance initiates age-related retinal degeneration and vision loss. JCI Insight 2018; 3: e120824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gardner PJ, Liyanage SE, Cristante E, et al.. Hypoxia inducible factors are dispensable for myeloid cell migration into the inflamed mouse eye. Scient Rep. 2017; 7: 40830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fouda AY, Xu Z, Shosha E, et al.. Arginase 1 promotes retinal neurovascular protection from ischemia through suppression of macrophage inflammatory responses. Cell Death Dis. 2018; 9: 1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Orthgiess J, Gericke M, Immig K, et al.. Neurons exhibit Lyz2 promoter activity in vivo: implications for using LysM-Cre mice in myeloid cell research. Eur J Immunol. 2016; 46: 1529–1532. [DOI] [PubMed] [Google Scholar]
- 27. Ferro A, Qu W, Lukowicz A, Svedberg D, Johnson A, Cvetanovic M. Inhibition of NF-κB signaling in IKKβF/F;LysM Cre mice causes motor deficits but does not alter pathogenesis of Spinocerebellar ataxia type 1. PLoS One. 2018; 13: e0200013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neul JL. Data generated by the Neul Lab: LysM Cre induces sporadic recombination within neurons. 2019. http://neullab.com/html/data.html. Accessed March 18, 2020.
- 29. Payne S, De Val S, Neal A. Endothelial-specific Cre mouse models. Arterioscler Thromb Vasc Biol. 2018; 38: 2550–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dejana E, Orsenigo F. Endothelial adherens junctions at a glance. J Cell Sci. 2013; 126: 2545. [DOI] [PubMed] [Google Scholar]
- 31. Gavard J. Endothelial permeability and VE-cadherin: a wacky comradeship. Cell Adh Migr. 2014; 8: 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wallez Y, Vilgrain I, Huber P. Angiogenesis: the VE-cadherin switch. Trends Cardiovasc Med. 2006; 16: 55–59. [DOI] [PubMed] [Google Scholar]
- 33. Gentek R, Ghigo C, Hoeffel G, et al.. Hemogenic endothelial fate mapping reveals dual developmental origin of mast cells. Immunity. 2018; 48: 1160–1171. e1165. [DOI] [PubMed] [Google Scholar]
- 34. Alva JA, Zovein AC, Monvoisin A, et al.. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Develop Dynamics. 2006; 235: 759–767. [DOI] [PubMed] [Google Scholar]
- 35. Madisen L, Zwingman TA, Sunkin SM, et al.. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010; 13: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abe T, Fujimori T. Reporter mouse lines for fluorescence imaging. Develop Growth Different. 2013; 55: 390–405. [DOI] [PubMed] [Google Scholar]
- 37. Milde F, Lauw S, Koumoutsakos P, Iruela-Arispe ML. The mouse retina in 3D: quantification of vascular growth and remodeling. Integr Biol (Camb). 2013; 5: 1426–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anderson SR, Roberts JM, Zhang J, et al.. Developmental apoptosis promotes a disease-related gene signature and independence from CSF1R signaling in retinal microglia. Cell Rep. 2019; 27: 2002–2013. e2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goldmann T, Wieghofer P, Muller PF, et al.. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat Neurosci. 2013; 16: 1618–1626. [DOI] [PubMed] [Google Scholar]
- 40. Paschalis EI, Lei F, Zhou C, et al.. Microglia regulate neuroglia remodeling in various ocular and retinal injuries. J Immunol. 2019; 202: 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paschalis EI, Lei F, Zhou C, et al.. Permanent neuroglial remodeling of the retina following infiltration of CSF1R inhibition-resistant peripheral monocytes. Proc Natl Acad Sci USA . 2018; 115: E11359–E11368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heuss ND, Pierson MJ, Roehrich H, et al.. Optic nerve as a source of activated retinal microglia post-injury. Acta Neuropathol Commun. 2018; 6: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kogata N, Arai Y, Pearson JT, et al.. Cardiac ischemia activates vascular endothelial cadherin promoter in both preexisting vascular cells and bone marrow cells involved in neovascularization. Circ Res. 2006; 98: 897–904. [DOI] [PubMed] [Google Scholar]
- 44. Nakano-Doi A, Sakuma R, Matsuyama T, Nakagomi T. Ischemic stroke activates the VE-cadherin promoter and increases VE-cadherin expression in adult mice. Histol Histopathol. 2018; 33: 507–521. [DOI] [PubMed] [Google Scholar]
- 45. Helmfors L, Boman A, Civitelli L, et al.. Protective properties of lysozyme on β-amyloid pathology: implications for Alzheimer disease. Neurobiol Dis. 2015; 83: 122–133. [DOI] [PubMed] [Google Scholar]
- 46. Yadav S, Surolia A. Lysozyme elicits pain during nerve injury by neuronal Toll-like receptor 4 activation and has therapeutic potential in neuropathic pain. Sci Translat Med. 2019; 11: eaav4176. [DOI] [PubMed] [Google Scholar]
- 47. Tan Z-J, Peng Y, Song H-L, Zheng J-J, Yu X. N-cadherin-dependent neuron–neuron interaction is required for the maintenance of activity-induced dendrite growth. Proc Natl Acad Sci. 2010; 107: 9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rahman AA, Lai NK, Albright JE, Urquhart PE, Webb AR, Morrison BE. Nigral dopaminergic neuron replenishment in adult mice through VE-cadherin-expressing neural progenitor cells. Neural Regener Res. 2017; 12: 1865–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gardner PJ, Liyanage SE, Cristante E, et al.. Hypoxia inducible factors are dispensable for myeloid cell migration into the inflamed mouse eye. Scientific Rep. 2017; 7: 40830–40830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Haimon Z, Volaski A, Orthgiess J, et al.. Re-evaluating microglia expression profiles using RiboTag and cell isolation strategies. Nat Immunol. 2018; 19: 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.