Abstract
Introduction:
Rare tumors account for one fourth of adult tumors; in children, rare tumors represent approximately 15–20% of childhood malignancies, thus accounting for a significant burden of disease. The rarity of these individual diseases creates many challenges, from developing a thorough understanding of the disease pathophysiology, clinical characterization, to the conduct of meaningful clinical trials and eventually the development of effective therapies.
Areas covered:
Despite these challenges, substantial advances have been made in recent years including the development of novel clinical trial designs and endpoints including molecularly driven treatment trials that have resulted in approval of novel therapies for rare diseases. Collaboration amongst basic and clinical researchers, patient advocacy groups, industry and regulatory agencies has proven successful in select cases and holds promise for future progress in the treatment of rare tumors. In this review, we will highlight several examples of trials for rare tumors, with a focus on examples from pediatric oncology, where strong, nationwide collaborative groups have existed for many years.
Expert opinion:
Future progress in developing therapies for rare tumors will depend not only on continued scientific advances, but also on collaboration between investigators from various disciplines, institutions, regulatory agencies and patient advocacy groups.
Keywords: BASKET trials, molecular subtypes, orphan drugs, umbrella trials
1. Defining rare tumors
More than 25 million Americans are affected by 7000 diseases classified as rare. The definition of a ‘rare disease’ varies in the literature. The US Orphan Drug Act of 1983 defines rare diseases as those affecting less than 200,000 people. A report of rare cancer incidence using the Cancer in North America data set applied the published definition of fewer than 15 cases per 100,000 per year; by this estimate, 60 of 71 cancer types classified in this report were considered rare, accounting for 25% of adult tumors.[1] RARECARE, a European group working to define the incidence of rare cancers in Europe, defines a rare cancer as one with an incidence <6 per 100,000.[2] Based on the definition of a rare disease as one affecting less than 200,000 people, all childhood cancers are considered rare, affecting 12,400 children and young adults yearly in the United States. Of these cancers, approximately 15–20% represent rare tumors within pediatric cancers, as identified by the Children’s Oncology Group Rare Tumors Committee.[3]
Thus, while each of the rare tumors affects relatively small numbers of individuals, the burden of rare tumors collectively is quite substantial. In Europe, rare cancers account for 22% of all cancer diagnoses with 541,000 new diagnoses every year.[2] According to an analysis of the Cancer in North America database, rare cancers were proportionally more common among young adults.[1] The same analysis also found that people of non-white race, and those of Hispanic ethnicity, were more likely than Caucasians to have a rare cancer diagnosis, compounding the issue of health disparities amongst ethnic groups.[4] Thus, despite the relatively small number of patients afflicted with each disease, the overall impact on the population is much larger and proportionally higher in minority groups.
Although rare tumors have been defined in the past based on distinct histologies, as greater knowledge of the molecular basis of cancer develops, the definition of rare tumors will likely extend to rare molecular subtypes of more common cancers that exhibit distinct clinical behavior. For example, between 2% and 7% of non-small cell lung cancers (NSCLC), one of the most common cancers, carry the fusion gene EML4-ALK, encoding a constitutively activated kinase. Based on Surveillance, Epidemiology and End Results (SEER) program-reported incidence of NSCLC being 41/100,000, the incidence of ALK-mutated lung cancer is between 0.8 and 2.8/100,000, meeting criteria for rare cancers.[5] The identification of this dysregulated molecular pathway in this subset of patients led to the study of crizotinib, an ALK tyrosine kinase inhibitor, in these patients after mutational screening. A targeted approach in this rare subtype has proven successful, with an estimated progression-free survival of 8.6 months on treatment with crizotinib and overall response rate of 61.2% based on a recent meta-analysis.[6] Multi-institutional trials in rare tumors, such as ALK-mutated lung cancer, provide a pathway for bringing together investigators across institutions to address clinically relevant issues in the treatment of rare tumors.
2. Challenges in diagnosis and development of disease expertise
Numerous challenges exist in the development of therapies for rare tumors. The difficulty begins at diagnosis: tumors that are relatively rare may be difficult to diagnose as many physicians and pathologists may not be familiar with the disease and need to seek expert opinion outside their institutions. Late diagnosis or initial misdiagnosis may prevent timely implementation of therapy, leading to worse prognosis for patients. An example of delayed diagnosis is found in pediatric medullary thyroid carcinoma (MTC), often associated with MEN 2A, 2B or familial MTC; diagnosed early, this slow growing cancer is easily treated or even prevented by prophylactic thyroidectomy.[7] Lack of recognition of the syndromic features that are associated with MTC in the pediatric population often delays diagnosis such that many patients present with advanced disease. Difficulty in diagnosis is also found in the example of nuclear protein of the testis (NUT) midline carcinoma, a very rare and highly lethal cancer. These cancers lack a distinctive histology, and diagnosis relies on identification of NUT rearrangement; previously, this was performed by fluorescence in situ hybridization or reverse transcriptase (RT)-PCR, but recently a NUT-specific monoclonal antibody has been developed, with excellent sensitivity and specificity. [8] Despite the availability of sensitive diagnostic testing, it is likely that many cases go unrecognized, as the testing requires a high index of suspicion.[9] These issues of late or misdiagnosis confound the understanding of disease course and therapeutic response. The rarity of each disease makes it less amenable to study; the pathophysiology, natural history and prognosis of the disease may not be well characterized. A certain center may not be able to accumulate sufficient experience with the disease to allow the development of expertise. In order to improve general knowledge of the disease and specifically improve treatment options, collaboration between multiple institutions is necessary.
The ability to improve disease expertise and patient outcomes in rare cancers through widespread collaboration is exemplified by collaborations in pediatric cancers. As pediatric cancer is relatively rare in comparison to adult cancers, investigators recognized early that collaboration between institutions was necessary to obtain sufficient experience. Such collaboration was implemented successfully by the Children’s Oncology Group (COG) and its predecessors, resulting in marked improvement in outcomes in many childhood cancers.[10] Between 1975 and 2006, mortality rates for all childhood cancers declined by more than 50%.[11] Five-year survival in acute lymphoblastic leukemia (ALL) in patients <15 years old improved from 61% in the years 1975–1978 to 88.5% in the years 1999–2002. Childhood ALL is a great success story, but even childhood AML demonstrated marked improvement during this time, with improvement in 5-year survival from <20% to 50% during the same time period.[11] Certainly, not all childhood cancers have seen the same improvements, but this demonstrates the power of cooperative group trials in relatively rare conditions. Currently, the COG Rare Tumor Committee has created an initiative to investigate the epidemiology and biology of rare pediatric cancers with the goal of identification of therapeutic targets and risk stratification for use in clinical trials.[3]
Despite the recent advances spurred by multi-institutional cooperation, the Infrequent Tumor subcommittee, a subset of the COG Rare Tumor Committee, has faced challenges in their attempt to study children with rare tumors, as elaborated by Pappo et al.[12] Enrollment in both registries and clinical trials for rare tumors fell far short of expected cases based on SEER data during the first years of the initiative, between 2002 and 2007. The authors suggested the increased prevalence of rare tumors in the young adult age group as a contributing factor: these patients are less likely to be seen at a pediatric center and, thus, a center which participates in the Children’s Oncology Group. Though the COG has an active protocol for tumor banking, in the years between 2003 and 2007, only 11% of samples represented a tumor-type considered rare, defined by Pappo et al as those tumors classified as ‘other malignant epithelial neoplasms’ and melanoma in the International Classification of Childhood Cancer subgroup XI for the SEER database.[12] The inability to collect tumor samples for future research is particularly unfortunate in these cancers for which new treatments are desperately needed. The ability of tumor registries and specimen banking to augment the understanding of rare diseases is demonstrated by the pleuropulmonary blastoma registry, an independently funded tumor registry. Research from this registry led to the discovery of familial association with DICER1 mutations.[13] In addition, expansion of this effort to a national natural history study of patients with DICER1-mutated tumors has allowed for the development of a much more comprehensive understanding of manifestations of DICER1 mutations.[14]
Cooperative group trials have the benefit of involving multiple centers, improving access to patients with rare diseases and thereby augment collective expertise, but of course require substantial effort in ensuring uniform practice and are complicated by logistics and regulatory concerns at each institution. In the case of international trials, both the benefits in terms of patient accrual and the logistical difficulties are further magnified, the latter due in part to different regulatory requirements between countries.[15,16]
3. Disease pathogenesis and molecular drivers as therapeutic targets
It is often difficult to collect sufficient samples of rare tumors to fully understand what molecular alterations are critical to tumor pathogenesis and progression. Tumors in general are often genetically unstable, and it takes large numbers of samples to determine statistically what changes are “drivers” and what changes are noise from passenger mutations or other molecular alterations. In the case of rare tumors, it may be difficult to impossible to collect enough samples for the necessary analyses. Fortunately, advances in technology are providing alternative approaches. As genome sequencing has become faster and more economical, the collective knowledge of genetic mutations in cancers has grown tremendously, expanding the knowledge of rare cancers and rare subtypes of common cancers. Sequencing of common cancers has identified mutations in genes that were previously associated with rare tumors, such as NF1 and VHL.[17] If cancer driver genes are the same in rare and common tumors, specific mutations found in common tumors can be tested in small numbers of rare tumor samples, resulting in less “noise.” The searchable database Catalog of Somatic Mutations in Cancer was launched in 2004 originally detailing 4 cancer genes. It is now the world’s largest and most comprehensive resource, describing 2,002,811 coding point mutations in over one million tumor samples. It also contains 6 million noncoding mutations, 10,534 gene fusions, 61,299 genome rearrangements, 695,504 abnormal copy number segments and 60,119,787 abnormal expression variants.[18] This wealth of information can be used to facilitate the identification of mutations in rare tumor samples that are more likely to be important in tumorigenesis, ultimately augmenting the understanding of rare tumor pathophysiology.
Another approach that has greatly benefited from advances in genome sequencing is direct comparison of tumor DNA with the patients’ own genomic DNA. This again reduces noise by removing naturally occurring variants in the patient’s genetic background from the analysis. In a recent example of this, a single neurofibromatosis type 1 patient was followed over 14 years, sampling the benign tumor, the malignant transformation of the tumor and the metastasis.[19] This type of analysis not only identifies potential driver mutations, but also gives information on the order with which they occur. Sequencing of relapsed tumors can shed light on the genetic evolution of relapsed tumors, and mechanisms of resistance, with potential therapeutic implications, as recently demonstrated by Maris et al in 23 paired samples of primary and relapsed neuroblastoma.[20] The comparison of whole genome sequencing of the paired tumor samples demonstrated clonal evolution from the primary tumor, with 18/23 relapsed tumors demonstrating RAS-MAPK pathway activating mutations, suggesting a potential therapeutic target. Two recent pediatric studies have explored the use of molecular profiling in assigning targeted therapy. The individualized cancer therapy study evaluated 100 patients with advanced solid tumors and provided a recommendation of treatment if an actionable mutation with available therapy existed; of 54 reviewed at the time of abstract publication, 16 were given a recommendation, though others had potentially actionable mutations without an available therapy.[21] The Baylor Advancing Sequencing in Childhood Cancer Care study also evaluated 80 tumors through whole exome sequencing and demonstrated clinically relevant mutations in 22/80 patients.[22] A larger adult study of 2000 patients found that 39% demonstrated a potentially actionable tumor mutation.[23] These studies reveal the feasibility of performing detailed molecular profiling in adult and pediatric patients.
Ultimately, the goal of identifying driver mutations is to find targets for therapeutic intervention. As the capacity grows to classify tumor drivers in rare tumors and molecular subtypes of common cancers with increasing speed and accuracy, so will the need to develop therapies targeted at these subtypes. A mutation or genetic aberration is considered actionable if it is oncogenic or if it is differentially expressed in tumor tissue, and there is an agent to target it.[24] Targeted therapies have met with success, such as the use of a CSF1 R inhibitor, which directly inhibits the mutated oncogenic target in tenosynovial giant-cell tumors.[25] The rationale for targeting a given mutation in a tumor varies depending upon the state of knowledge in a given disease type. Targeting the mutation may have proven clinical benefit, either in the tumor in question or another histology with the same mutation, as in the example of BRAFV600E – mutated melanoma and other cancers.[26] Conversely, the mutation might not be a direct target of a therapeutic agent, but represent a marker for responsiveness. For example, TSC1 mutations have been found to indicate sensitivity to everolimus in bladder cancer.[27] In cases without pre-existing clinical evidence of efficacy, preclinical evidence may suggest responsiveness to a drug. Knowledge of aberrations in a given pathway may also provide the rationale for targeting a particular pathway. However, such targeted therapies do not always elicit clinical benefit.[28,29] In the case of pediatric cancers, the Pediatric Preclinical Testing Program found that many agents which are active in adult cancers lack activity in pediatric cancers.[30] The presence of redundant tumor promoting pathways or upregulation of a compensatory pathway, as well as other mechanisms, contribute to mechanisms of resistance, as has been demonstrated in resistance to imatinib in chronic myeloid leukemia.[31] Alternatively, the drug may not sufficiently suppress the targeted pathway or it may not be optimally delivered to the target tissue. Performing tumor biopsies on treatment, when feasible, to evaluate for drug effect, may help elucidate mechanisms of response and resistance, with potential therapeutic implications.
4. Clinical trials for rare tumors
Once candidate therapeutics have been identified, rare tumors present daunting hurdles to be able to test the efficacy of a given agent. It is very difficult for any one center to recruit enough patients with a rare tumor to conduct a trial in a timely manner, thus multi-institutional cooperation is often critical to success. However, even collaborative groups may have difficulty recruiting adequate numbers of patients. An example of this was seen in two recent randomized treatment studies for pediatric melanoma patients over 10 years of age. Despite cooperation between the Children’s Oncology Group, Easter Cooperative Oncology Group and Southwest Oncology Group, only four patients were enrolled in these studies over a period of four years, illustrating the very real concern of trials failing to accrue adequate numbers of patient in rare diseases.
Trials which focus solely on rare diseases may be difficult to accrue and complete in a timely fashion; thus, several relatively novel trial designs which allow participation of patients with rare tumors in broader trials are being pro-posed. Basket trials are used to evaluate multiple tumor histologies with a common mutation, such as the ongoing study of LOXO-101, a selective tropomyosin receptor kinase inhibitor, in subjects with NTRK fusion-positive solid tumors, and VE-BASKET, a phase II study of vemurafenib in nonmelanoma tumors that are BRAFV600E-positive. In the LOXO-101 trial, 8 cohorts of patients with tumors bearing NTRK fusions will be enrolled, including patients with salivary gland cancer, a relatively rare neoplasm, and a mixed cohort of ‘other’ neoplasms, which will allow patients with other rare tumors carrying NTRK fusions to participate.[32] Vemurafenib is a selective oral inhibitor of mutated BRAF; it is approved by the FDA for first-line treatment of BRAFV600E-mutated melanoma that is unresectable or meta-static. In the VE-BASKET trial, six disease cohorts, as well as a seventh cohort of mixed histology, were enrolled with a primary endpoint of response at 8 weeks. Disease groups included NSCLC, cholangiocarcinoma and Erdheim-Chester disease/Langerhans Cell histiocytosis. In patients with Erdheim-Chester disease/Langerhans Cell histiocytosis, the response rate was 43%; the NSCLC group demonstrated a response rate of 42%, compared to a 7% response rate in standard second-line therapy, docetaxel.[26] The advantage of the ‘BASKET’ design is the ability to include either a cohort of patients with a rare disease carrying the mutation of interest, such as in the case of Erdheim-Chester disease/Langerhans Cell histiocytosis or to enroll patients with rare tumors in the ‘other solid tumor’ cohort, providing at least preliminary evidence of clinical activity that can inform future evaluation.
A second trial design, the umbrella trial, is designed to evaluate multiple agents targeting a variety of mutations, either within one tumor type such as in the Biomarker-Integrated Approaches of Targeted Therapy for Lung Cancer Elimination trial, or in different tumor types. This approach also allows the participation of patients with rare tumor within larger clinical trials. Another example of this is the WINTHER (Worldwide Innovate Networking Consortium for personalized cancer therapy) Trial, an ongoing precision medicine trial designed to match targeted therapies to tumors of different histologies. As recently reviewed by Rodon et al, opening this international multicenter study was tremendously challenging for multiple reasons.[33] Multiple therapeutic agents required different means of obtaining drug, which was compounded by varying regulations across international borders. In the US, the need for Clinical Laboratory Improvement Amendments-certified omics tools for diagnosis and the need for an investigational device exemption as mandated by the FDA precipitated delays in opening the trial in the US. This example is an illustration of the difficulty in carrying out multicenter international trials. National Cancer Institute (NCI)-Molecular Analysis for Therapy Choice trial is another molecularly driven umbrella trial which incorporates multiple, single-arm phase II studies; Molecular Analysis for Therapy Choice aims to enroll at least 25% of total patients with less common tumor types including rare tumors.[34]
Patients with rare tumors have also been treated in phase I trials which incorporate multiple histologies. For example, the activity of cabozantinib in MTC was first observed in a phase I trial with 25 adult patients, including 3 with MTC. [35] Preclinical studies had demonstrated in vivo efficacy in a xenograft model of MTC.[36] A subsequent phase I study with an expanded MTC cohort of 37 patients confirmed the activity of cabozantinib in this disease, with 50% of the 35 evaluable patients demonstrating partial response at the first radiologic response evaluation.[37] Due to the activity witnessed in this trial, a phase III randomized, placebo-controlled study of cabozantinib in progressive MTC was performed, which demonstrated improvement in progression-free survival for cabozantinib (11.2 months for cabozantinib arm vs. 4 months for placebo).[38] The ability to progress from a phase I mixed histology trial to a phase III randomized control trial relatively quickly was facilitated by a strong rationale in both preclinical studies and in early phase clinical development. A pediatric phase I trial of cabozantinib in children and adolescents with recurrent or refractory solid tumors is ongoing. Given the existing evidence of efficacy in MTC, this phase I trial for refractory solid tumors includes a separate stratum for pediatric MTC so that patients with this very rare tumor can be enrolled at any time on the trial. This allowed for enrollment of several patients with MTC and thus enrichment for this disease.[39] A pediatric phase I trial of crizotinib in patients with refractory solid tumor or anaplastic large cell lymphoma enrolled a dose-finding stratum in addition to strata for patients with neuroblastoma and patients with confirmed ALK translocations, mutations, or amplification.[40] Overall, responses were seen in 14 of 79 patients, with a greater response seen in patients with activating mutations in ALK, including 8 of 9 with anaplastic large cell lymphoma. Incorporating disease-specific subsets into phase I trials can facilitate and speed the development of therapeutics for rare malignancies.
5. Regulatory approval
Rare diseases, in general, and rare cancers, in particular, have benefitted from federal regulations that facilitate the development of treatments for rare conditions. The US Orphan Drug Act of 1983 provided incentives to industry for investing in development of therapeutics for rare diseases. The act facilitated clinical trials of orphan products by providing federal funding of grants and contracts; the act also provided tax credits for 50% of clinical testing costs and exclusive marketing rights for 7 years following marketing approval of orphan products.[41] As orphan drug applications have the potential to provide significant improvement for serious conditions, many orphan drugs may also receive priority review status based on the Prescription Drug User Fee Act of 1992.[42] A review of FDA drug approvals during the period following the implementation of the act revealed a marked increase in approvals for orphan drugs: 279 separate drugs were approved between 1983 and 2009, compared to 34 drugs that would have received orphan status between 1967 and 1983.[41] Some of this increase in the number of orphan drugs approved could be due to an overall increase in the number of drugs recently approved by the FDA for all indications. However, the initiatives of the FDA that highlight the need to develop drugs for rare diseases is likely contributing to the observed increase in the number of orphan drug approvals. Some of these drugs were approved for multiple indications, for a total of 347 approvals; an example of this is imatinib, with approval for seven indications including gastrointestinal stromal tumors, chronic myeloid leukemia, and Philadelphia chromosome-positive ALL. Twenty-eight percent of the orphan drug approvals during the period 1983–2009 were in the realm of oncology, including both chemotherapy and supportive care therapies. In combination with tremendous advancements in scientific knowledge over the past four decades driving drug development, greater federal support of orphan drugs has accelerated the approval process and led to more therapies for orphan indications reaching patients.
Historically, drug approvals have been based on demonstration of improvement in survival; however, in some diseases, use of this endpoint may lead to underestimation of drug benefit, or survival may not be an appropriate endpoint and alternative endpoints need to be developed. The approval of ruxolitinib in myeloproliferative neoplasms highlights the use of alternative endpoints. A phase I/II trial of ruxolitinib in myelofibrosis demonstrated rapid and sustained improvement in splenomegaly, with 52% of patients demonstrating ≥50% reduction in spleen size as determined by volumetric MRI.[43] Many patients also experienced resolution of constitutional symptoms, increased performance scores and weight gain; similar clinical benefit was seen in subsequent placebo-controlled trials. Ruxolitinib was approved for myeloproliferative neoplasms on the basis of these findings; approval was accelerated through priority review, utilized in cases of potential for significant improvement in a disease that lacked adequate existing therapies.[44] This approval demonstrates the power of both patient-reported outcomes and disease-specific measures in evaluating effective treatments, and the willingness of the FDA to consider such measures as alternative endpoints to survival in select diseases.
Caution is necessary, however, in utilizing alternative endpoints. In a recent study of 54 cancer drugs approved in the years 2008 through 2012, 36 were approved on the basis of surrogate endpoints. Thirty-one of these drugs (86%) have either failed to demonstrate an improvement in survival or lacked data to support an effect on overall survival.[45] Although use of surrogate endpoints has utility in rare diseases, care must be taken in appropriate follow-up to ensure these endpoints ultimately correlate with improved survival or improvement in quality of life in order to justify any potential toxicities.
An example of the rapid progression in a very rare disease from molecular understanding to small, successful clinical trials to drug approval is the FDA’s approval of everolimus for the treatment of subependymal giant cell astrocytomas (SEGA) in patients with tuberous sclerosis complex (TSC). TSC is an autosomal dominant syndrome due to mutation in TSC1 or TSC2; the loss of these tumor suppressor genes leads to activation of the mTOR pathway.[46] The syndrome is characterized by the formation of benign hamartomas in multiple organ systems. SEGAs develop in 20% of adult TSC patients.[47] Prior to the study of everolimus in SEGA, surgical resection was the only known effective therapy. For patients in whom surgical resection was not feasible, there was no alternative therapy. Everolimus was identified as a potential treatment due to the known upregulation of the mTOR pathway in this disease. Krueger et al published the results of their prospective, single-arm phase I/II study in 2010; they demonstrated ≥50% reduction in size in 9 of 28 patients.[47] Responses were sustained during the study period (6 months), and patients also demonstrated decreased seizure frequency and improvement in quality of life measures. These findings prompted the accelerated approval for patients with TSC and SEGAs needing treatment but who are not candidates for curative surgical resection. The approval of this study is notable for its small size, as accelerated approval was granted based on a study of only 28 patients; additionally, the study incorporated alternative endpoints in the form of seizure frequency and patient-reported outcomes in addition to decrease in tumor size. Subsequent larger studies have confirmed the safety and efficacy of everolimus in this population. Successful, well-designed small trials can thus lead to accelerated drug approval, a valuable approach in rare tumors.
6. Building networks
In recent years, there has been an increasing awareness of the challenges facing researchers who study rare tumors and the patients who are afflicted with these diseases. In the US, both the NCI and National Center for Advancing Translational Sciences Office of Rare Diseases have developed programs including the Rare Tumors Initiative and the Rare Diseases Clinical Research Network to address some of the challenges associated with rare tumors research. International collaboration in the form of the International Rare Cancer Initiative has formed between the National Institute for Health Research, Cancer Research Network and Cancer Research UK in the UK, the NCI in the US and the European Organization for Research and Treatment of Cancer. The RARECARE working group aims to better define the incidence and improve the quality of data on rare cancers in Europe. These collaborative organizations of clinicians, scientists and policy makers are raising awareness of rare tumors, the challenges facing researchers studying them, and solutions to accelerate progress.
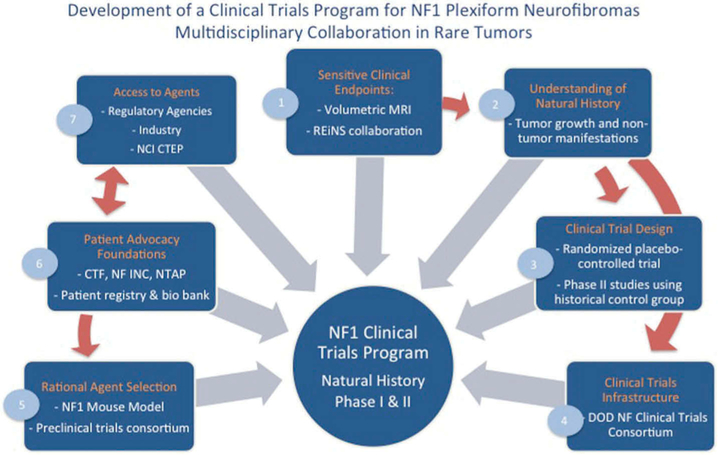
In addition, patient advocacy groups for these diseases can bring greater awareness and partner with physicians and researchers to connect patients to opportunities for novel therapies. The advantages of this network building was recently demonstrated with NCI’s Rare Tumor Initiative partnering with the Desmoid Tumor Research Foundation to recruit 17 patients within a short time period for a clinical trial in desmoid tumors, a very rare tumor diagnosed in only 900 people per year in the US.[48] More and more, patients are seen as critical partners in developing outcome measurements for clinical trials. The Patient-Centered Outcomes Research Institute has been a leader in this area across many diseases by encouraging the inclusion of patients as research partners in the development of clinical studies. By building networks not only between researchers and clinicians at different institutions, but also between different stakeholders, such as patient advocacy groups and regulatory agencies, developing therapies for rare tumors becomes not only possible but also quite promising. An example of the power of multidisciplinary collaboration is presented in Figure 1, a multidisciplinary collaboration for the development of clinical trials for plexiform neurofibromas in patients with neurofibromatosis type 1.
Figure 1. Development of a clinical trial program for NF1 plexiform neurofibromas: multidisciplinary collaboration in rare tumors.
- The development of volumetric MRI analysis of PN allowed sensitive and reproducible measurement of changes in PN size and is now recommended and used as a sensitive endpoint in most clinical trials directed at PN.[49,50] As more clinical trials for NF develop, the Response Evaluation in Neurofibromatosis and Schwannomatosis international working group was launched with the goal to develop standardized patient-reported and functional outcome measures to be used across clinical trials in NF.[51]
- Volumetric MRI analysis in conjunction with longitudinal imaging and clinical evaluation brought a greater understanding of the natural history of PN growth and of non-tumor manifestations in NF1 patients. Comparison of the characteristics of NF1 PN patients and pediatric cancer patients enrolling in clinical trials uncovered differences, which need to be considered in the design of NF1 PN trials.[52]
- In absence of a detailed knowledge of the natural history of PN, a randomized, placebo-controlled, flexible cross-over phase II study of tipifarnib in children and young adults with NF1 and PN provided early data on the natural history of PN while at the same time determining the activity of tipifarnib.[53] The placebo cohort was then used as a historical control group in subsequent treatment trials in PN utilizing identical eligibility criteria.[54-57]
- The development of a Department of Defense (DOD) sponsored Clinical Trials Consortium allowed acceleration of the conduct of multi-institutional trials for NF.[58]
- Development of mouse models of NF1 PN which recapitulate the disease resulted in preclinical testing and rational selections of agents to be evaluated in clinical trials.[59] For example, a mouse model of NF1 neurofibromas was predictive of the response to MEK inhibitors observed in a clinical trial.[60-62] A preclinical trials consortium sponsored by the Children’s Tumor Foundation (CTF) and the Neurofibromatosis Therapeutic Acceleration Program (NTAP) and promotes basic and translational discovery.
- Patient advocacy group involvement has contributed to patient education, fundraising and trial enrollment. CTF’s patient registry not only serves to gather information, but helps identify patients for clinical trials. CTF is also establishing a bio banking initiative.
- Regulatory agencies such as the FDA have contributed to clinical trial development in their willingness to discuss and consider alternative endpoints such as response by volumetric MRI, patient reported and functional outcomes. The support of NCI CTEP and of pharmaceutical companies to sponsor clinical trials for PN has been critical to the progress made.[53,55,63].
7. NCI rare tumor initiative
The NCI Center for Cancer Research recently established the NCI Rare Tumors Initiative, one of several such initiatives worldwide, which we review here in further detail. The goal of the initiative is to bring together basic scientists, clinicians, industry partners and patient advocacy groups. The insights fostered by collaboration between these different groups have the power to accelerate drug development, clinical trials and ultimately new therapies. A recent symposium of the Rare Tumors Initiative, sponsored by NCI and the National Center for Advancing Translational Sciences Office of Rare Diseases in Bethesda, MD in 2015, brought together experts in patient advocacy, epidemiology, genomics, drug discovery, clinical trial design, late stage drug development and regulatory issues to interact and discuss how to accelerate development of rare tumor therapies. Several themes emerged from the discussions.
It is clear that patients are a particularly important partner in developing rare tumor therapies, from donating tumor samples to participating in clinical trials to driving fundraising for research. Discussants emphasized the importance of increasing communication between patients and researchers, as well as educating patients and the general public about rare disease research. The use of social media for outreach and including patients in the development of patient-reported outcomes were discussed. Humanizing the scientific process by involving scientists in patient groups and opening scientific meeting to patients is also important to get patients more invested in what researchers need to successfully develop therapies. The availability of tissue samples for research is a major hurdle for scientists. The challenges of developing patient registries and tissue repositories were discussed, including the issues of developing high-quality data collection for repositories, enlisting community surgeons and pathologists to assist in sample collection, the costs of maintaining repositories and issues of streamlining informed consent and handling intellectual property arising from patient tissues.
In the area of preclinical research, the lack of identified driver mutations, particularly in rare sarcomas, was highlighted, as well as the difficulty in conducting epidemiological studies and replicating them. These issues are being addressed by greater access to samples as well as multi-institutional and international collaborations. The role of epigenomics and metabolomics in understanding tumor drivers was also emphasized, as some rare tumors show alterations in pathways that are not detected at the level of genetic mutations.[64] The advantages of having robust cell models for high-throughput drug screens and robust animal models for preclinical drug testing were demonstrated.[60] Issues of developing strong preclinical models were discussed, including the development of centralized mouse repositories, advances in genetic engineering of mice and the development of patient-derived xenografts.
The focus of clinical discussions centered on how to measure success in clinical trials of rare tumors. Beyond the challenge of accruing patients, it was clear that a better understanding of the natural history of the disease and robust, objective response criteria are key to success. New outcome measures can be developed, through collaboration with the FDA, allowing for meaningful measurement of response when standard response criteria are not applicable.[49] Issues raised included how to quantify improvements in quality of life when tumor shrinkage is not the best response criteria, as well as how to handle multiple criteria that differ in response to therapy, such as tumor shrinkage and functional measures.
The symposium highlighted many challenges in rare tumors research, but concluded that many could be overcome by increased communication and collaboration between stakeholders. Symposia such as this will allow people working on different rare tumors to learn from each other across different tumor types and disciplines.
8. Conclusion
Despite numerous challenges faced in the pursuit of developing therapies for rare tumors, significant progress has been made in recent years. Progress has been facilitated by advances in technology, greater scientific knowledge, novel trial design, progressive regulatory practices and large-scale cooperation. Disease registries and tissue banking can facilitate research into the causes of rare tumors. Cooperation between scientists at multiple institutions will accelerate understanding of rare tumors more than can be obtained by any individual center. Collaboration between patients, physicians and scientists, and industry has the potential to facilitate therapeutic development. Though clinical trials in rare tumors may be challenging, they represent opportunities for making substantial progress and developing therapies for areas of unmet needs; patient advocacy groups can enable enrollment in these trials through patient-oriented networks. Through these strategies and continued scientific advances, there is hope for continued progress in developing therapies for rare tumors.
9. Expert opinion
Rare tumors represent a significant disease burden and present unique challenges in diagnosis, understanding of disease course and pathogenesis, and development of effective therapies. Overcoming these challenges has the potential to benefit a substantial fraction of cancer patients by developing methods to identify therapies for rare tumors. In addition, as personalized medicine approaches focus in on narrower subsets of common tumors, lessons learned in rare tumors can be applied more broadly to develop therapy for cancer subtypes, with the potential to benefit all cancer patients.
This review highlights the biggest challenges in rare tumor research. Specifically, the limited number of patients with a given rare tumor (1) limits the development of expertise in the disease, slowing diagnosis and early intervention, (2) impedes understanding of molecular pathogenesis to identify actionable mutations and (3) increases the time, and thus cost, needed to complete clinical trials or makes successful accrual of patients nearly impossible. These challenges have been partially addressed by changes in regulations governing drug development for rare disease indications and by the founding of national and international initiatives to promote research and clinical development in the field of rare diseases.
Increased collaborations between researchers at different institutions and between patient advocacy groups and researchers, in part through the development of new initiatives, is increasing communication about and awareness of rare tumors; thus, helping to develop expertise needed to avoid delays in diagnosis. Patient advocacy groups are helping to connect patients to researchers to benefit all phases of rare tumor research. However, recent experience has shown that even with better networks, patient accrual is still a major challenge.
Understanding molecular pathogenesis of rare tumors has the power to identify potential targets and inform development of therapies for rare tumors. Tissue availability for research is a key limiting factor, and tissue banking initiatives have great potential to increase scientific understanding of primary and relapsed tumor biology, as well as identify potential therapeutic targets. These approaches are limited by the availability of targeted therapies to identified targets, and even when candidates exist, targeted therapies may not elicit clinical benefit for a variety of reasons, often poorly understood. While all cancer research faces similar struggles with targeted therapies not always exhibiting the predicted clinical benefit, rare tumors can prove more difficult when researching the reasons for resistance, due to low sample numbers. Future efforts to biopsy rare tumors on treatment with targeted agents have the potential to augment our understanding of mechanisms of response and resistance. Technological improvements and cost reductions are making the molecular analysis of rare tumor samples more feasible.
Clinical trials for these therapies can pose challenges in recruitment due to disease rarity; indeed, performing clinical trials in these rare populations may present the biggest challenge in the development of therapies for rare tumors. However, these challenges can be overcome through thoughtful trial design and collaborative efforts amongst institutions, with industry, regulatory agencies and disease-specific advocacy groups. Clinical researchers should consider novel clinical trial designs, such as the incorporation of rare histologies within a basket or umbrella trial, as a way to study diseases that might be difficult to study independently due to concerns related to patient accrual. The emphasis on large, multicenter ‘master’ protocols sponsored by industry or federal government (such as the NCI) hold tremendous promise of generating at least preliminary data of drug activity in rare tumors that can inform and expedite development of novel therapies for such diseases. In addition, novel endpoints, such as patient reported outcomes, are currently being tested in a number of different diseases, and alternative, validated endpoints can add strength to clinical trial designs on rare tumors.
Future directions in rare tumors research are likely to focus on further improvements in multi-institutional and multidisciplinary collaborations and networks, increasing interactions between stakeholders. Improved knowledge of molecular targets combined with innovative trial designs will allow the study of rare tumors within larger clinical trials. Rethinking endpoints in clinical trials, while requiring validation, will open up new possibilities to designing trials with same numbers of patients to look for effects. These advancements are expected to accelerate the development of rare tumor therapies in the future.
Article highlights.
Rare tumors represent a significant disease burden and present unique challenges in the development of effective therapies.
Improved knowledge of pathophysiology at the molecular level has the potential to identify new therapeutic targets.
Novel trial designs, such as BASKET or umbrella trials, may facilitate evaluation of novel agents in the treatment of rare diseases.
Collaborative efforts between industry, clinicians, scientists, government and patient advocacy groups can be advantageous in many phases of therapeutic development.
Expert opinion.
Defining rare tumors
Challenges in diagnosis and development of disease expertise
Disease pathogenesis and molecular drivers as therapeutic targets
Clinical trials for rare tumors
Regulatory approval
Building networks
NCI rare tumor initiative
Conclusion
Expert opinion
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Greenlee RT, Goodman MT, Lynch CF, et al. The occurrence of rare cancers in U.S. adults, 1995-2004. Public Health Rep. 2010;125(1):28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keat N, Law K, McConnell A, et al. International Rare Cancers Initiative (IRCI). Ecancermedicalscience. 2013;7(ed20). doi: 10.3332/ecancer.2013.ed20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Galindo C, Krailo M, Frazier L, et al. Children’s Oncology Group’s 2013 Blueprint for Research: Rare Tumors. Pediatr Blood Cancer. 2013;60(6):1016–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keppel K Ten largest racial and ethnic health disparities in the United States based on Healthy People 2010 objectives. Am J Epidemiol. 2007;166:97–103. [DOI] [PubMed] [Google Scholar]
- 5.Howlader N, Noone AM, Krapcho M, et al. (eds). SEER cancer statistics review, 1975–2012 [Internet]. Bethesda, MD: National Cancer Institute; 2015. [cited 2015 Sept 21]. http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015. [Google Scholar]
- 6.Qian H, Gao F, Wang H, et al. The efficacy and safety of crizotinib in the treatment of anaplastic lymphoma kinase-positive non-small cell lung cancer: a meta-analysis of clinical trials. BMC Cancer. 2014;14:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waguespack SG, Rich TA, Perrier N, et al. Management of medullary thyroid carcinoma and MEN2 syndromes in childhood. Nat Rev Endocrinol. 2011;7(10):596–607. [DOI] [PubMed] [Google Scholar]
- 8.French CA. The importance of diagnosing NUT midline carcinoma. Head Neck Pathol. 2013;7(1):11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.French CA. Demystified molecular pathology of NUT midline carcinomas. J Clin Pathol. 2010;63(6):492–496. [DOI] [PubMed] [Google Scholar]
- 10.Reaman GH. Successful integration of cooperative groups: the origin of the Children’s Oncology Group. Am Soc Clin Oncol Educ Book. 2012:149–151. doi: 10.14694/EdBook_AM.2012.32.149. [DOI] [PubMed] [Google Scholar]
- 11.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28(15):2625–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pappo AS, Krailo M, Chen Z, et al. Infrequent tumor initiative of the Children’s Oncology Group: initial lessons learned and their impact on future plans. J Clin Oncol. 2010;28(33):5011–5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill DA, Ivanovich J, Priest JR, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325(5943):965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart D DICER1-related pleuropulmonary blastoma cancer predisposition syndrome: a natural history study [Internet]. 2015. [cited 2015 Sep 17]. Available from: https://clinicaltrials.gov/ct2/show/NCT01247597?term=dicer&rank=1.
- 15.Vilas-Boas I The drug approval process in the US, Europe and Japan. J Manages Care Pharm. 1997;3:459–465. [Google Scholar]
- 16.Alqahtani SS-VE, Rodriguez-Monguio R, Eguale T. Priority review drugs approved by the FDA and the EMA: time for international regulatory harmonization of pharmaceuticals. Pharmacoepidemiol Drug Saf. 2015;24(7):709–715. [DOI] [PubMed] [Google Scholar]
- 17.Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455 (7216):1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes SA, Beare D, Gunasekaran P, et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43(D1): D805–D811.•• Discusses COSMIC, a comprehensive cataglog of tumor mutations, and its impact on cancer research.
- 19.Hirbe AC, Dahiya S, Miller CA, et al. Whole exome sequencing reveals the order of genetic changes during malignant transformation and metastasis in a single patient with nf1-plexiform neurofibroma. Clin Cancer Res. 2015;21(18):4201–4211. doi: 10.1158/1078-0432.CCR-14-3049. Epub 2015 Apr 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eleveld TF, Oldridge DA, Bernard V, et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat Genet. 2015;47(8):864–871.• Genome sequencing of primary and relapsed neuroblastoma samples reveals molecular evolution of relapsed tumors.
- 21.Janeway K, DuBois SG, Glade Bender JL, et al. Multicenter study assessing tumor molecular profiles in advanced pediatric solid tumors. J Clin Oncol. 2014;32(5s): abstr 10011. [Google Scholar]
- 22.Parsons D, Roy A, Monzon FA, et al. What’s in an exome? Diversity of diagnostic and incidental findings revealed by clinical tumor and germline sequencing of 100 children with solid tumors. J Clin Oncol. 2014;32(5s):abst 10012. [Google Scholar]
- 23.Meric-Bernstam F, Brusco L, Shaw K, et al. Feasibility of large-scale genomic testing to facilitate enrollment onto genomically matched clinical trials. J Clin Oncol. 2015;33(25):2753–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vidwans SJ, Turski ML, Janku F, et al. A framework for genomic biomarker actionability and its use in clinical decision making. Oncoscience. 2014;1(10):614–623.•• Defines types of molecular aberrations which may serve as a basis for targeted therapy.
- 25.Tap WD, Wainberg ZA, Anthony SP, et al. Structure-guided blockade of csf1r kinase in tenosynovial giant-cell tumor. N Engl J Med. 2015;373(5):428–437. [DOI] [PubMed] [Google Scholar]
- 26.Hyman DM, Blay JY, Chau I, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. New England J Med. 2015;373(8):726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iyer G, Hanrahan AJ, Milowsky MI, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338(6104):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? Lancet Oncol. 2013;14(2):e60–e69. [DOI] [PubMed] [Google Scholar]
- 29.Arnedos M, Vielh P, Soria JC, et al. The genetic complexity of common cancers and the promise of personalized medicine: is there any hope? J Pathol. 2014;232(2):274–282. [DOI] [PubMed] [Google Scholar]
- 30.Cancer Therapy Evaluation Program. Pediatric preclinical testing consortium [Internet]. 2015. [cited 2015 Oct 31]. Available from: http://ctep.cancer.gov/MajorInitiatives/Pediatric_Preclinical_Testing_Program.htm.
- 31.Balabanov SM, Braig M, Brummendorf TH. Current aspects in resistance against tyrosine kinase inhibitors in chronic mye-logenous leukemia. Drug Discov Today Technol. 2014;11:89–99. [DOI] [PubMed] [Google Scholar]
- 32.ClinicalTrials.gov. Study of LOXO-101 in subjects with NTRK fusion positive solid tumors, NCI identifier NCT02576431 [Internet]. 2015. [cited 2015 Oct 30]. Available from: https://clinicaltrials.gov/ct2/show/NCT02576431.
- 33.Rodon J, Soria JC, Berger R, et al. Challenges in initiating and conducting personalized cancer therapy trials: perspectives from WINTHER, a Worldwide Innovative Network (WIN) Consortium trial. Ann Oncol. 2015;26(8):1791–1798.•• Explores the difficulties encountered in initiating a large, international umbrella trial.
- 34.ClinicalTrials.gov. NCI-MATCH: targeted therapy directed by genetic testing in treating patients with advanced refractory solid tumors or lymphomas [Internet]. 2015. [cited 2015 Aug]. Available from: https://clinicaltrials.gov/show/NCT02465060.•• Ongoing large-scale clinical trial utilizing an umbrella design.
- 35.Salgia R, Hong DS, Camacho LH, et al. A phase I dose-escalation study of the safety and pharmacokinetics (PK) of XL184, a VEGFR and MET kinase inhibitor, administered orally to patients (pts) with advanced malignancies. J Clin Oncol. 2007;25(Suppl 18):14031. [Google Scholar]
- 36.Bentzien F, Zuzow M, Heald N, et al. In vitro and in vivo activity of cabozantinib (XL184), an inhibitor of RET, MET, and VEGFR2, in a model of medullary thyroid cancer. Thyroid. 2013;23(12):1569–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurzrock R, Sherman SI, Ball DW, et al. Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29(19):2660–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elisei R, Schlumberger MJ, Muller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chuk MK, Widemann BC, Ahern CH, et al. A phase I study of cabozantinib (XL184) in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: A Children’s Oncology Group phase I consortium trial. J Clin Oncol. 2015;32(5s), 2014 (suppl; abstr 10078). [Google Scholar]
- 40.Mossé YP, Lim MS, Voss SD, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children’s Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14(6):472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kesselheim A, Innovation and the Orphan Drug Act, 1983-2009: regulatory and clinical characteristics of approved orphan drugs. in Rare Diseases and Orphan Products: Accelerating Research and Development., B. T. Institute of Medicine (US) Committee on Accelerating Rare Diseases Research and Orphan Product Development, Field MJ, editor. 2010, Washington (DC): National Academies Press. [PubMed] [Google Scholar]
- 42.Food and Drug Administration. Guidance for industry, expedited programs for serous conditions [Internet]. 2014. [cited 2015 Oct 25]. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm358301.pdf.
- 43.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Food and Drug Administration. FDA approves first drug to treat a rare bone marrow disease [Internet]. 2011. [cited 2015 Oct 25]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm280102.htm.
- 45.Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: An analysis of 5 years of us food and drug administration approvals. JAMA Intern Med. 2015;1–2. doi: 10.1001/jamainternmed.2015.5868 [DOI] [PubMed] [Google Scholar]
- 46.Franz DN, Belousova E, Sparagana S, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381(9861):125–132. [DOI] [PubMed] [Google Scholar]
- 47.Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. New England J Med. 2010;363 (19):1801–1811. [DOI] [PubMed] [Google Scholar]
- 48.Desmoid Tumor Research Foundation [Internet]. 2015. [cited 2015 Sep 16]. Available from: http://www.dtrf.org/index.php.
- 49.Dombi E, Ardern-Holmes SL, Babovic-Vuksanovic D, et al. Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology. 2013;81(21 Suppl 1):S33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dombi E, Solomon J, Gillespie AJ, et al. NF1 plexiform neurofibroma growth rate by volumetric MRI: relationship to age and body weight. Neurology. 2007;68 (9):643–647. [DOI] [PubMed] [Google Scholar]
- 51.Plotkin SR, Blakeley JO, Dombi E, et al. Achieving consensus for clinical trials: the REiNS International Collaboration. Neurology. 2013;81(21 Suppl 1):S1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim A, Gillespie A, Dombi E, et al. Characteristics of children enrolled in treatment trials for NF1-related plexiform neurofibromas. Neurology. 2009;73(16):1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Widemann BC, Dombi E, Gillespie A, et al. Phase 2 randomized, flexible crossover, double-blinded, placebo-controlled trial of the farnesyltransferase inhibitor tipifarnib in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Neuro Oncol. 2014;16(5):707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Widemann BC, Babovic-Vuksanovic D, Dombi E, et al. Phase II trial of pirfeni-done in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Pediatr Blood Cancer. 2014;61(9):1598–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim A, Dombi E, Tepas K, et al. Phase I trial and pharmacokinetic study of sorafenib in children with neurofibromatosis type I and plexiform neurofibromas. Pediatr Blood Cancer. 2013;60(3):396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jakacki R, Dombi E, Potter DM, et al. Preliminary results of a phase II trial of pegylated interferon-alfa-2B (PI) in pediatric patients with documented progression of neurofibromatosis type 1-related unresectable plexiform neurofibromas (PNF). Neuro Oncol. 2012;14:16. [Google Scholar]
- 57.Weiss B, Widemann BC, Wolters P, et al. Sirolimus for progressive neurofibromatosis type 1-associated plexiform sneurofibromas: a Neurofibromatosis Clinical Trials Consortium phase II study. Neuro Oncol. 2015;17(4):596–603. doi: 10.1093/neuonc/nou235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gutmann DH, Blakeley JO, Korf BR, et al. Optimizing biologically targeted clinical trials for neurofibromatosis. Expert Opin Investig Drugs. 2013;22(4):443–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu J, Dombi E, Jousma E, et al. Preclincial testing of sorafenib and RAD001 in the Nf (fl/fl);DhhCre mouse model of plexiform neurofibroma using magnetic resonance imaging. Pediatr Blood Cancer. 2012;58(2):173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jousma E, Rizvi TA, Wu J, et al. Preclinical assessments of the MEK inhibitor PD-0325901 in a mouse model of neurofibromatosis type 1. Pediatr Blood Cancer. 2015;62(10):1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang FC, Ingram DA, Chen S, et al. Nf1-dependent tumors require a microenvironment containing Nf1+/− and c-kit-dependent bone marrow. Cell. 2008;135(3):437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jessen WJ, Miller SJ, Jousma E, et al. MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. J Clin Invest. 2013;123(1):340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Widemann BC, Marcus LJ, Fisher MJ, et al. Phase I study of the MEK 1/2 inhibitor selumetinib (AZD6244) hydrogen sulfate in children and young adults with neurofibromatosis type 1 (NF1) and inoperable plexiform neurofibromas (PNs). J Clin Oncol. 2014;32(5s):abstr 10018. [Google Scholar]
- 64.Killian JK, Miettinen M, Walker RL, et al. Recurrent epimutation of SDHC in gastrointestinal stromal tumors. Sci Transl Med. 2014;6(268):268ra177. [DOI] [PMC free article] [PubMed] [Google Scholar]